Left Ventricular Fibrosis by Cardiac Magnetic Resonance Tissue Characterization in Chronic Mitral Regurgitation Patients
Abstract
1. Introduction
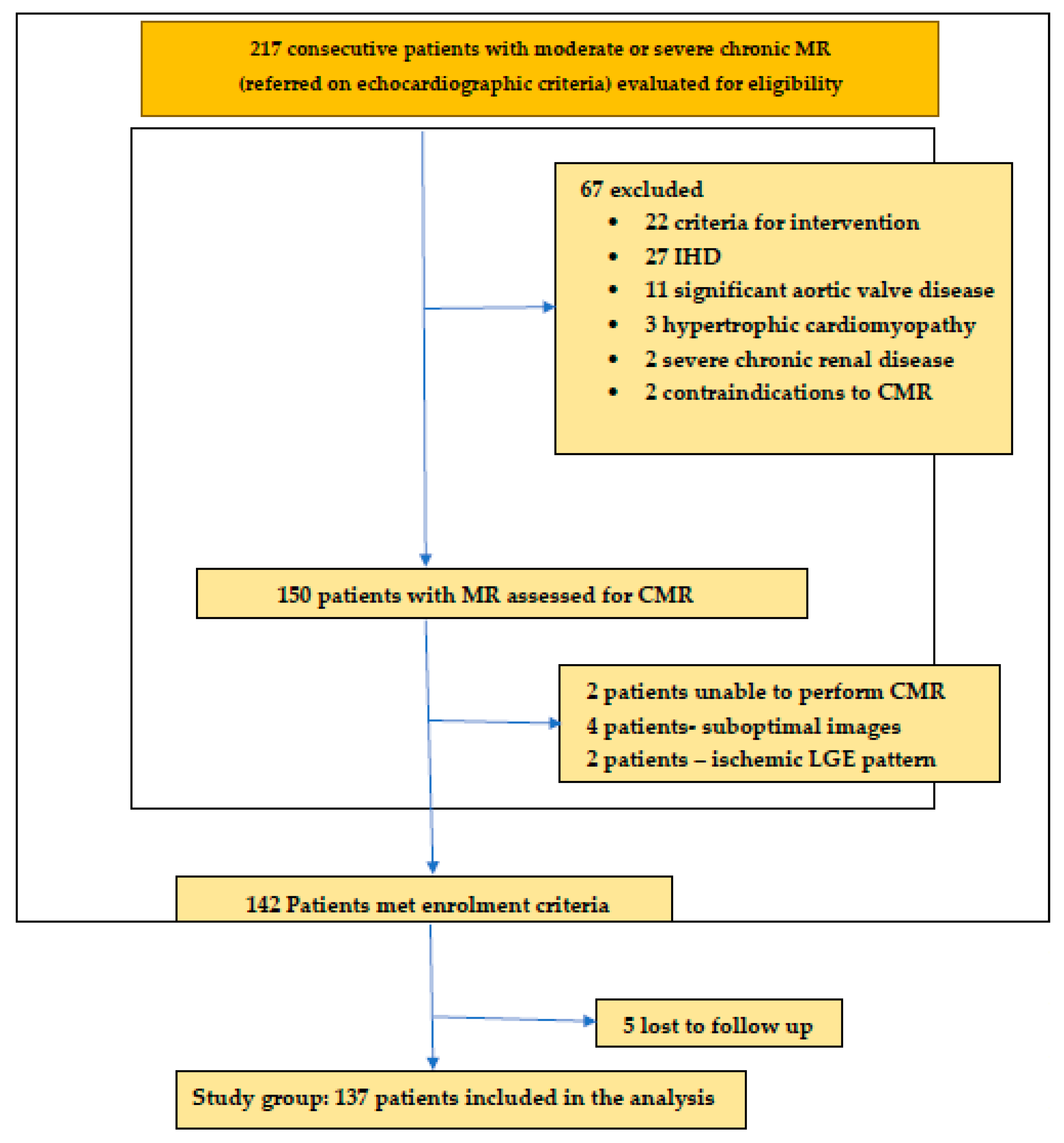
2. Patients and Methods
2.1. Study Population
2.2. Echocardiography
2.3. CMR Imaging Protocol
2.4. CMR Image Analysis
2.5. Follow up and Clinical Outcomes
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Ventricular Structure and Function
3.3. Replacement Fibrosis
3.4. Interstitial Fibrosis
3.5. Clinical Outcomes and Follow Up
3.5.1. Survival Analysis
3.5.2. Univariate and Multivariate Cox Analysis
3.5.3. Risk Stratification Scoring System
4. Discussion
4.1. Replacement Fibrosis by LGE
4.2. Diffuse Interstitial Fibrosis
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef]
- Kitkungvan, D.; Nabi, F.; Kim, R.J.; Bonow, R.O.; Khan, M.A.; Xu, J.; Little, S.H.; Quinones, M.A.; Lawrie, G.M.; Zoghbi, W.A.; et al. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J. Am. Coll. Cardiol. 2018, 72, 823–834. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for noninvasive evaluation of native valvular regurgitation: A report from the American Society of Echocardiography. Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Biner, S.; Rafique, A.; Rafii, F.; Tolstrup, K.; Noorani, O.; Shiota, T.; Gurudevan, S.; Siegel, R.J. Reproducibility of proximal isovelocity surface area, vena contracta, and regurgitant jet area for assessment of mitral regurgitation severity. J. Am. Coll. Cardiol. Imaging 2010, 3, 235–243. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Sinak, L.J.; Tajik, A.J.; Bailey, K.R.; Seward, J.B. Changes in effective regurgitant orifice throughout systole in patients with mitral valve prolapse. A clinical study using the proximal isovelocity surface area method. Circulation 1995, 92, 2951–2958. [Google Scholar] [CrossRef]
- Schwammenthal, E.; Chen, C.; Benning, F.; Block, M.; Breithardt, G.; Levine, R.A. Dynamics of mitral regurgitant flow and orifice area. Physiologic application of the proximal flow convergence method: Clinical data and experimental testing. Circulation 1994, 90, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Topilsky, Y.; Michelena, H.; Bichara, V.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Mitral valve prolapse with mid-late systolic mitral regurgitation: Pitfalls of evaluation and clinical outcome compared with holosystolic regurgitation. Circulation 2012, 125, 1643–1651. [Google Scholar] [CrossRef]
- Myerson, S.G. CMR in evaluating valvular heart disease: Diagnosis, severity, and outcomes. Cardiovasc. Imaging 2021, 14, 2020–2032. [Google Scholar]
- Penicka, M.; Vecera, J.; Mirica, D.C.; Kotrc, M.; Kockova, R.; Van Camp, G. Prognostic Implications of Magnetic Resonance–Derived Quantification in Asymptomatic Patients with Organic Mitral Regurgitation: Comparison with Doppler Echocardiography-Derived Integrative Approach. Circulation 2018, 137, 1349–1360. [Google Scholar] [CrossRef]
- Hunold, P.; Schlosser, T.; Vogt, F.M.; Eggebrecht, H.; Schmermund, A.; Bruder, O.; Schüler, W.O.; Barkhausen, J. Myocardial late enhancement in contrast-enhanced cardiac MRI: Distinction between infarction scar and non-infarction-related disease. AJR Am. J. Roentgenol. 2005, 184, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Podlesnikar, T.; Delgado, V.; Bax, J.J. Cardiovascular magnetic resonance imaging to assess myocardial fibrosis in valvular heart disease. Int. J. Cardiovasc. Imaging 2018, 34, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Constant Dit Beaufils, A.L.; Huttin, O.; Jobbe-Duval, A.; Senage, T.; Filippetti, L.; Piriou, N.; Cueff, C.; Venner, C.; Mandry, D.; Sellal, J.-M.; et al. Replacement myocardial fibrosis in patients with mitral valve prolapse: Relation to mitral regurgitation, ventricular remodeling, and Arrhythmia. Circulation 2021, 143, 1763–1774. [Google Scholar] [CrossRef]
- Park, M.H.; van Kampen, A.; Melnitchouk, S.; Wilkerson, R.J.; Nagata, Y.; Zhu, Y.; Wang, H.; Pandya, P.K.; Morningstar, J.E.; Borger, M.A.; et al. Native and post-repair residual mitral valve prolapse increases forces exerted on the papillary muscles: A possible mechanism for localized fibrosis? Circ. Cardiovasc. Interv. 2022, 15, e011928. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Koo, H.J.; Cho, M.S.; Nam, G.B.; Kang, J.W.; Yang, D.H. Late Gadolinium Enhancement of Left ventricular papillary muscles in patients with mitral regurgitation. Korean J. Radiol. 2021, 22, 1609–1618. [Google Scholar] [CrossRef]
- Scatteia, A.; Pascale, C.E.; Gallo, P.; Pezzullo, S.; America, R.; Cappelletti, A.M.; Vecchia, L.A.D.; Guarini, P.; Dellegrottaglie, S. Abnormal papillary Muscle Signal on Cine MRI as a typical feature of mitral valve prolapse. Sci. Rep. 2020, 10, 9166. [Google Scholar] [CrossRef]
- Taylor, A.J.; Salerno, M.; Dharmakumar, R.; Jerosch-Herold, M. T1 Mapping: Basic Techniques and Clinical Applications. JACC Cardiovasc. Imaging 2016, 9, 67–81. [Google Scholar] [CrossRef]
- Kitkungvan, D.; Yang, E.Y.; El Tallawi, K.C.; Nagueh, S.F.; Nabi, F.; Khan, M.A.; Nguyen, D.T.; Graviss, E.A.; Lawrie, G.M.; Zoghbi, W.A.; et al. Prognostic Implications of Diffuse Interstitial Fibrosis in Asymptomatic Primary Mitral Regurgitation. Circulation 2019, 140, 2122–2124. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; La Canna, G.; Pepi, M.; Dulgheru, R.; Dweck, M.; Delgado, V.; Garbi, M.; A Vannan, M.; et al. Multimodality imaging assessment of native valvular regurgitation: An EACVI and ESC council of valvular disease position paper. Eur. Heart J. Cardiovasc. Imaging. 2022, 23, e171–e232. [Google Scholar] [CrossRef]
- Kramer, C.; Barkhausen, J.; Flamm, S.; Kim, R.; Nagel, E. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J. Cardiovasc. Magn. Reson. 2013, 15, 91. [Google Scholar] [CrossRef]
- Kawel-Boehm, N.; Hetzel, S.J.; Ambale-Venkatesh, B.; Captur, G.; Francois, C.J.; Jerosch-Herold, M.; Salerno, M.; Teague, S.D.; Valsangiacomo-Buechel, E.; van der Geest, R.J.; et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J. Cardiovasc. Magn. Reason. 2020, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Aung, N.; Sanghvi, M.M.; Zemrak, F.; Fung, K.; Paiva, J.M.; Francis, J.M.; Khanji, M.Y.; Lukaschuk, E.; Lee, A.M.; et al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J. Cardiovasc. Magn. Reason. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Beek, A.; Hofman, M.; Kühl, H.; Twisk, J.; van Dockum, W.; Visser, C.; van Rossum, A. Standardizing the Definition of Hyperenhancement in the Quantitative Assessment of Infarct Size and Myocardial Viability Using Delayed Contrast-Enhanced CMR. J. Cardiovasc. Magn. Reason. 2005, 7, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Pavon, A.G.; Arangalage, D.; Pascale, P.; Hugelshofer, S.; Rutz, T.; Porretta, A.P.; Bloa, A.L.; Muller, O.; Pruvot, E.; Schwitter, J. Myocardial extracellular volume by T1 mapping: A new marker of arrhythmia in mitral valve prolapse. J. Cardiovasc. Magn. Reason. 2021, 23, 102. [Google Scholar] [CrossRef] [PubMed]
- Nordhues, B.D.; Siontis, K.C.; Scott, C.G.; Nkomo, V.T.; Ackerman, M.J.; Asirvatham, S.J.; Noseworthy, P.A. Bileaflt Mitral Valve Prolapse and Risk of Ventricular Dysrhythmias and Death. J. Cardiovasc. Electrophysiol. 2016, 27, 463. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Marra, M.P.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Uretsky, S.; Animashaun, I.B.; Sakul, S.; Aldaia, L.; Marcoff, L.; Koulogiannis, K.; Argulian, E.; Rosenthal, M.; Wolff, S.D.; Gillam, L.D. American Society of Echocardiography Algorithm for Degenerative Mitral Regurgitation: Comparison with CMR. J. Am. Coll. Cardiol. Imaging 2022, 15, 747–760. [Google Scholar] [CrossRef]
- Cawley, P.J.; Hamilton-Craig, C.; Owens, D.S.; Krieger, E.V.; Strugnell, W.E.; Mitsumori, L.; D’jang, C.L.; Schwaegler, R.G.; Nguyen, K.Q.; Nguyen, B.; et al. Prospective comparison of valve regurgitation quantitation by cardiac magnetic resonance imaging and transthoracic echocardiography. Circ. Cardiovasc. Imaging 2013, 6, 48–57. [Google Scholar] [CrossRef]
- Lopez-Mattei, J.C.; Ibrahim, H.; Shaikh, K.A.; Little, S.H.; Shah, D.J.; Maragiannis, D.; Zoghbi, W.A. Comparative assessment of mitral regurgitation severity by transthoracic echocardiography and cardiac magnetic resonance using an integrative and quantitative approach. Am. J. Cardiol. 2016, 117, 264–270. [Google Scholar] [CrossRef]
- Sachdev, V.; Hannoush, H.; Sidenko, S.; Saba, S.G.; Sears-Rogan, P.; Bandettini, W.P.; Brofferio, A.; Shanbhag, S.M.; Brenneman, C.L.; Horvath, K.A.; et al. Are echocardiography and CMR really discordant in mitral regurgitation? J. Am. Coll. Cardiol. Imaging 2017, 10, 823–824. [Google Scholar] [CrossRef]
- Uretsky, S.; Gillam, L.; Lang, R.; Chaudhry, F.A.; Argulian, E.; Supariwala, A.; Gurram, S.; Jain, K.; Subero, M.; Jang, J.J.; et al. Discordance between echocardiography and MRI in the assessment of mitral regurgitation severity: A prospective multicenter trial. J. Am. Coll. Cardiol. 2015, 65, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Pavon, A.G.; Guglielmo, M.; Mennilli, P.M.; Falcão, M.B.L.; Bergamaschi, L.; Costantin, D.F.; Vivaldo, M.; Leo, L.A.; Schlossbauer, S.; Roy, C.W.; et al. The Role of Cardiovascular Magnetic Resonance in Patients with Mitral Regurgitation. J. Cardiovasc. Dev. Dis. 2022, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Pavon, A.G.; Monney, P.; Schwitter, J. Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality Imaging to Detect High-Risk Features. Diagnostics 2021, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Neil, D.A.; Bhabra, M.; Patel, R.; Barker, T.A.; Nikolaidis, N.; Billing, J.S.; Hayer, M.; Baig, S.; Price, A.M.; et al. Reverse myocardial remodeling following valve repair in patients with chronic severe primary degenerative mitral regurgitation. J. Am. Coll. Cardiol. Imaging 2022, 15, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Tsunamoto, H.; Yamamoto, H.; Masumoto, A.; Taniguchi, Y.; Takahashi, N.; Onishi, T.; Takaya, T.; Kawai, H.; Hirata, K.-I.; Tanaka, H. Efficacy of Native T1 Mapping for Patients with Non-Ischemic Cardiomyopathy and Ventricular Functional Mitral Regurgitation Undergoing Transcatheter Edge-to-Edge Repair. Circ. J. 2024, 88, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Bull, S.; White, S.K.; Piechnik, S.K.; Flett, A.S.; Ferreira, V.M.; Loudon, M.; Francis, J.M.; Karamitsos, T.D.; Prendergast, B.D.; Robson, M.D.; et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart 2013, 99, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Kawel, N.; Nacif, M.; Zavodni, A.; Jones, J.; Liu, S.; Sibley, C.T.; A Bluemke, D. TT1 mapping of the myocardium: Intra-individual assessment of the effect of field strength, cardiac cycle and variation by myocardial region. J. Cardiovasc. Magn. Reson. 2012, 14, 27. [Google Scholar] [CrossRef]
- de Meester de Ravenstein, C.; Bouzin, C.; Lazam, S.; Boulif, J.; Amzulescu, M.; Melchior, J.; Pasquet, A.; Vancraeynest, D.; Pouleur, A.-C.; Vanoverschelde, J.-L.J.; et al. Histological validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look-Locker Imaging (MOLLI) T1 mapping at 3 T. J. Cardiovasc. Magn. Reson. 2015, 17, 48. [Google Scholar] [CrossRef]





| Clinical Data | Study Group (n = 137) | Control Group (n = 130) | p Value |
|---|---|---|---|
| Age (years), mean, SD | 65.6 ± 7.1 | 63.2 ± 11.6 | NS |
| Male sex, n (%) | 77 (56.2) | 69 (53.07) | NS |
| Body mas index (kg/m2), SD | 25.5, 4.8 | 24.7, 4.4 | NS |
| NYHA Class I/II/III | 39/98/0 | 33/97/0 | NS |
| Comorbidities | |||
| Hypertension, n (%) | 78 (56.9) | 70 (53.8) | NS |
| Diabetes | 45 (32.8) | 41 (31.5) | NS |
| Atrial fibrillation | 24 (17.51) | 12 (9.23) | 0.0271 |
| Chronic kidney disease | 19 (13.86) | 5 (11.53) | NS |
| Blood tests | |||
| Hemoglobin (g/dL) | 12.7 ± 1.9 | 12.9 ± 1.8 | NS |
| NT-proBNP (pg/mL) | 327 [171–729] | 72 [31–157] | 0.0037 |
| eGFR (mL/min/1.73 m2), SD | 77 ± 13.8 | 79 ± 12.5 | NS |
| Medications | |||
| ACEIs/ARBs/ARNI, n (%) | 98 (71.53) | 92 (70.76) | NS |
| β-blockers, n (%) | 96 (70.07) | 90 (69.23) | NS |
| SGLT2 inhibitors | 22 (16.05) | 9 (6.92) | 0.0191 |
| MRAs | 21 (15.32) | 12 (9.23) | 0.0346 |
| Diuretics | 29 (21.16) | 25 (19.23) | NS |
| Parameters Transthoracic Echocardiography | Study Group | Control Group | p Value |
|---|---|---|---|
| LVEDVI (mL/m2) | 112 ± 36.2 | 75.3 ± 19.7 | 0.0275 |
| LVESVI (mL/m2) | 39 ± 18.3 | 23 ± 11.3 | 0.0414 |
| LVEF (%) | 65 ± 8 | 68 ± 8 | NS |
| LVSVI (mL/m2) | 33.5 ± 14.4 | 23.7 ± 11.4 | 0.0195 |
| GLS (%) | 17 ± 3.1 | 20 ± 3.7 | 0.0360 |
| LAVolI (mL/m2) | 73.4 ± 25.7 | 32.2 ± 26.2 | 0.0073 |
| TAPSE (mm) | 28 ± 7 | 29 ± 8 | NS |
| MR RVol (mL) | 41.8 ± 12.7 | 13.5 ± 8.1 | 0.0023 |
| MR RF (%) | 39.8 ± 11.3 | 7.8 ± 6.4 | 0.0017 |
| CMR parameters | |||
| Native T1 values (ms) | 1167 ± 58.5 | 971 ± 51.4 | 0.0211 |
| Postcontrast T1 values (ms) | 381 ± 26.5 | 437 ± 38.2 | 0.0192 |
| ECV | 32.3 ± 3.5 | 23.9 ± 2.2 | 0.0226 |
| LVEDVI (mL/m2) | 123 ± 39.5 | 79.1 ± 18.3 | 0.0061 |
| LVESVI (mL/m2) | 45 ± 17.8 | 27 ± 12.1 | 0.0085 |
| LVEF (%) | 61 ± 5 | 69 ± 4 | NS |
| LVSVI (mL/m2) | 30.2 ± 11.5 | 23.5 ± 12.2 | 0.0191 |
| LV mass index (g/m2) | 77.3 ± 24.8 | 69.9 ± 17.1 | 0.0388 |
| RVEDVI (mL/m2) | 76.1 ± 20.1 | 66.9 ± 19.0 | 0.0267 |
| RVESVI (mL/m2) | 33.0 ± 13.7 | 31 ± 14.4 | 0.0419 |
| RVEF (%) | 51.8 ± 13.5 | 56.0 ± 12.4 | NS |
| RVSVI (mL/m2) | 34.0 ± 15.7 | 25.7 ± 11.8 | 0.0381 |
| RVol (mL) | 48.7 ± 11.3 | 12.5 ± 7.4 | 0.0106 |
| RF (%) | 46.9 ± 10.2 | 8.5 ± 5.1 | 0.0043 |
| LGE+ | 39 | 10 | 0.0029 |
| PM LGE | 9 | 1 | 0.0011 |
| LAVolI (mL/m2) | 81.5 ± 19.4 | 34.3 ± 18.1 | 0.0051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badau Riebel, C.I.; Agoston-Coldea, L. Left Ventricular Fibrosis by Cardiac Magnetic Resonance Tissue Characterization in Chronic Mitral Regurgitation Patients. J. Clin. Med. 2024, 13, 3877. https://doi.org/10.3390/jcm13133877
Badau Riebel CI, Agoston-Coldea L. Left Ventricular Fibrosis by Cardiac Magnetic Resonance Tissue Characterization in Chronic Mitral Regurgitation Patients. Journal of Clinical Medicine. 2024; 13(13):3877. https://doi.org/10.3390/jcm13133877
Chicago/Turabian StyleBadau Riebel, Catalina Ileana, and Lucia Agoston-Coldea. 2024. "Left Ventricular Fibrosis by Cardiac Magnetic Resonance Tissue Characterization in Chronic Mitral Regurgitation Patients" Journal of Clinical Medicine 13, no. 13: 3877. https://doi.org/10.3390/jcm13133877
APA StyleBadau Riebel, C. I., & Agoston-Coldea, L. (2024). Left Ventricular Fibrosis by Cardiac Magnetic Resonance Tissue Characterization in Chronic Mitral Regurgitation Patients. Journal of Clinical Medicine, 13(13), 3877. https://doi.org/10.3390/jcm13133877





