Surgical Outcomes in Chiari 1 and Chiari 1.5 Malformation Treated by Posterior Fossa Reconstruction: A Comprehensive Analysis of 110 Pediatric Cases and Literature Review
Abstract
1. Introduction
2. Patients and Methods
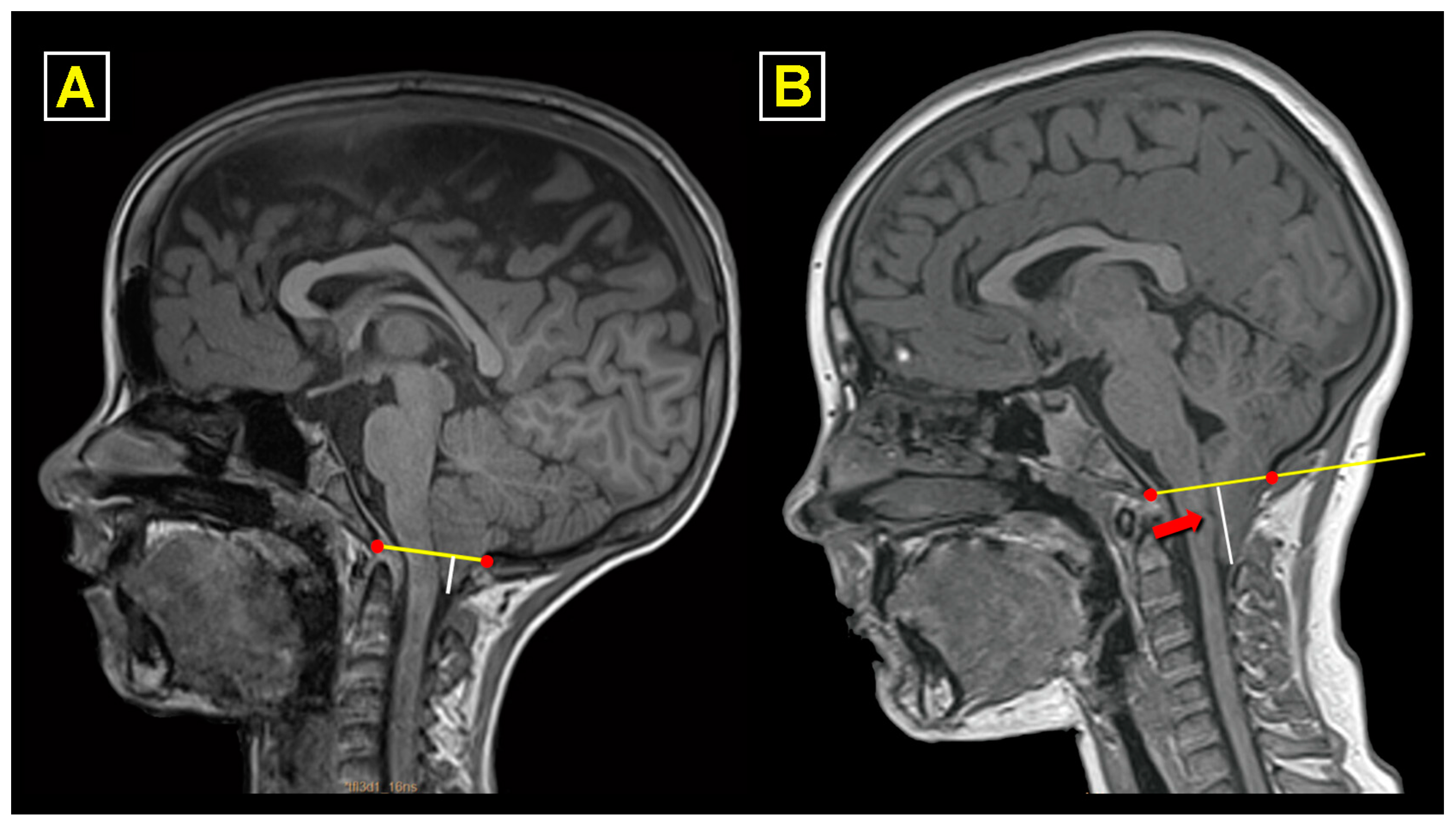
2.1. Study Protocol and Neuroradiological Workup
2.2. Indications for Surgery
2.3. Anesthesia Protocol
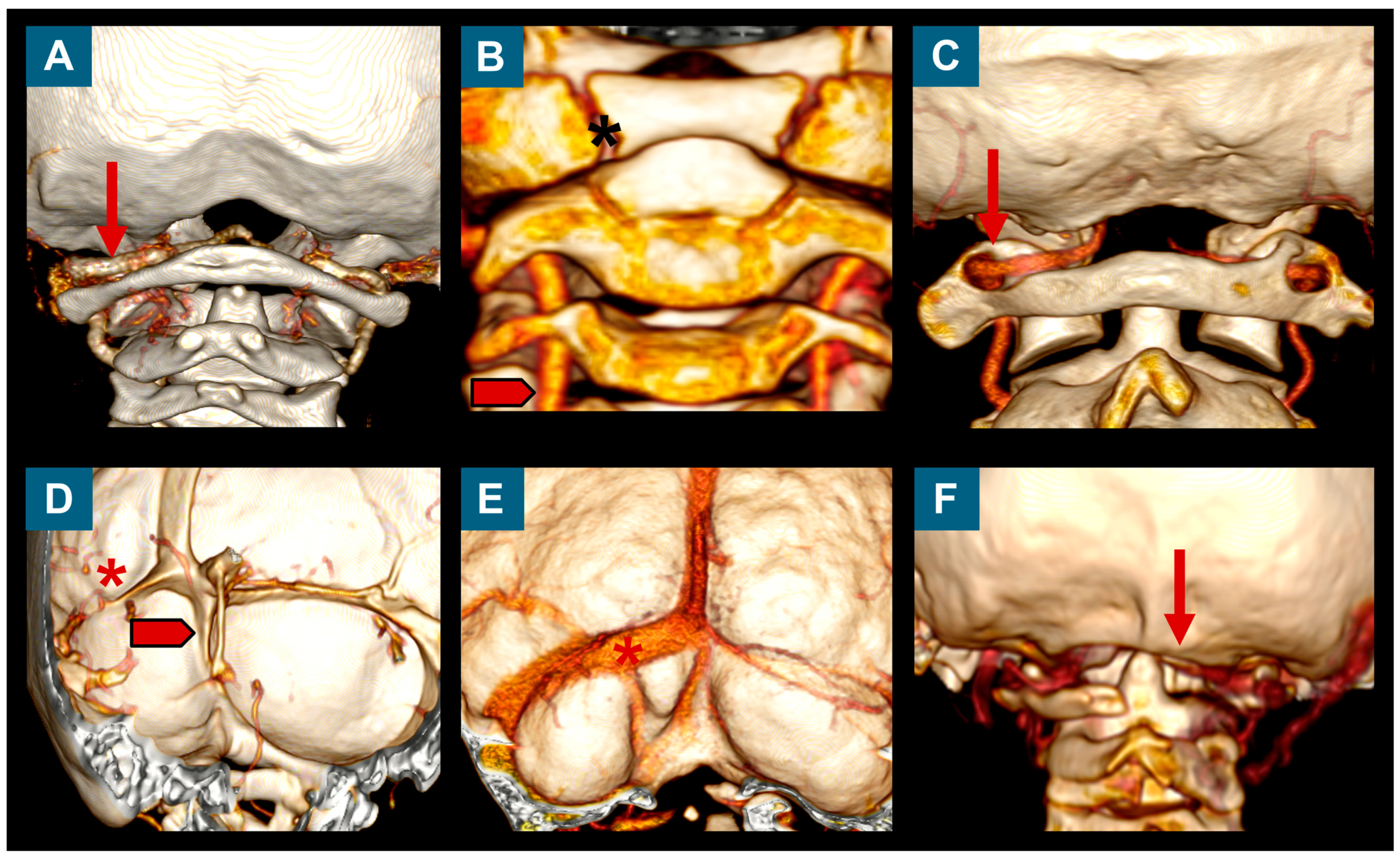
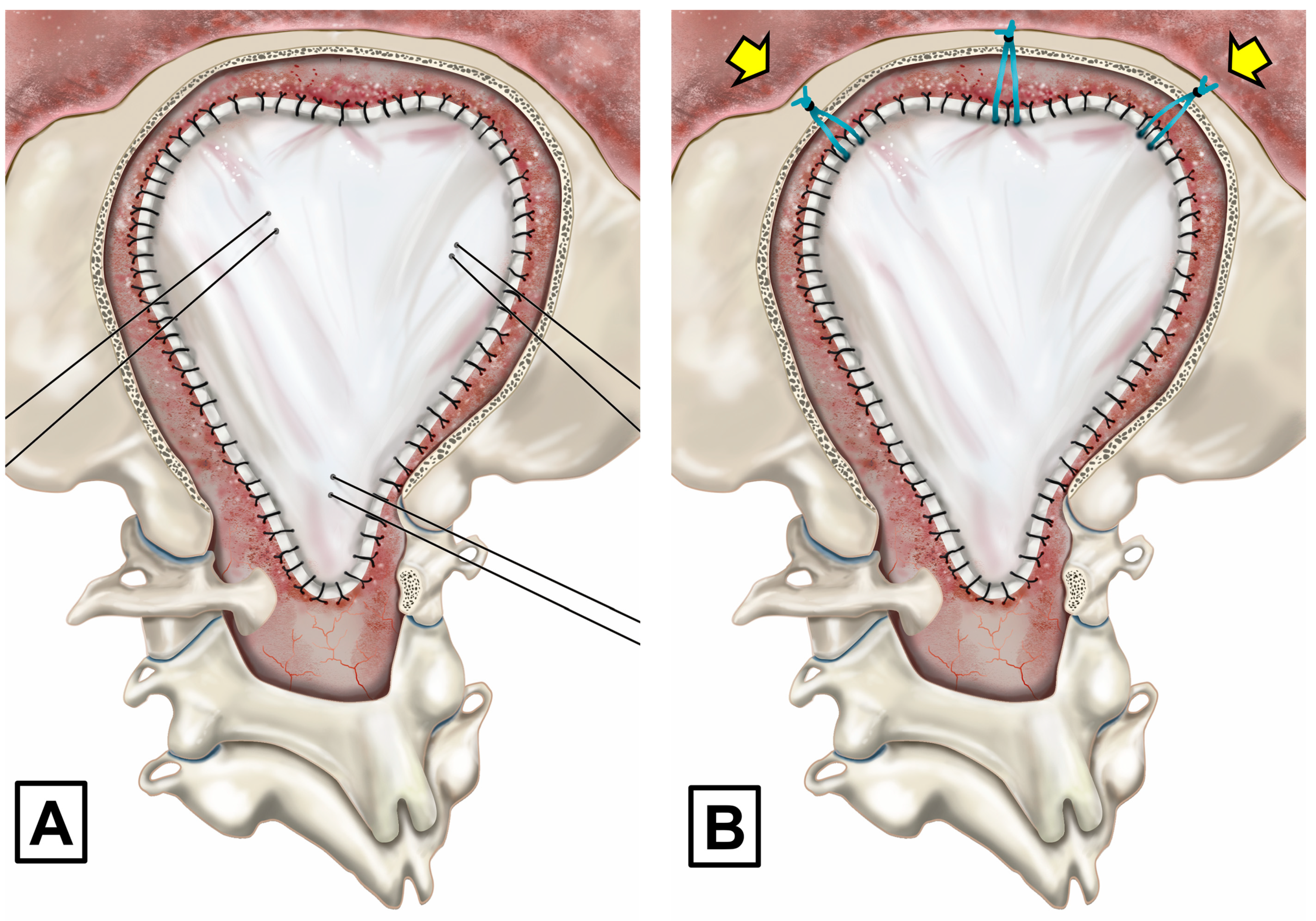
2.4. Posterior Fossa Reconstruction/Surgical Technique
Extended Surgery in CM-1 with Arachnoid Scarring and in Patients with CM-1.5
2.5. Postoperative Adverse Events and Clinical Outcomes
2.6. Statistical Analysis
Data Management
3. Clinical Results
3.1. Patient Population
3.2. Clinical Symptoms
3.3. Results of Neurophysiological Studies
3.4. Surgical Findings
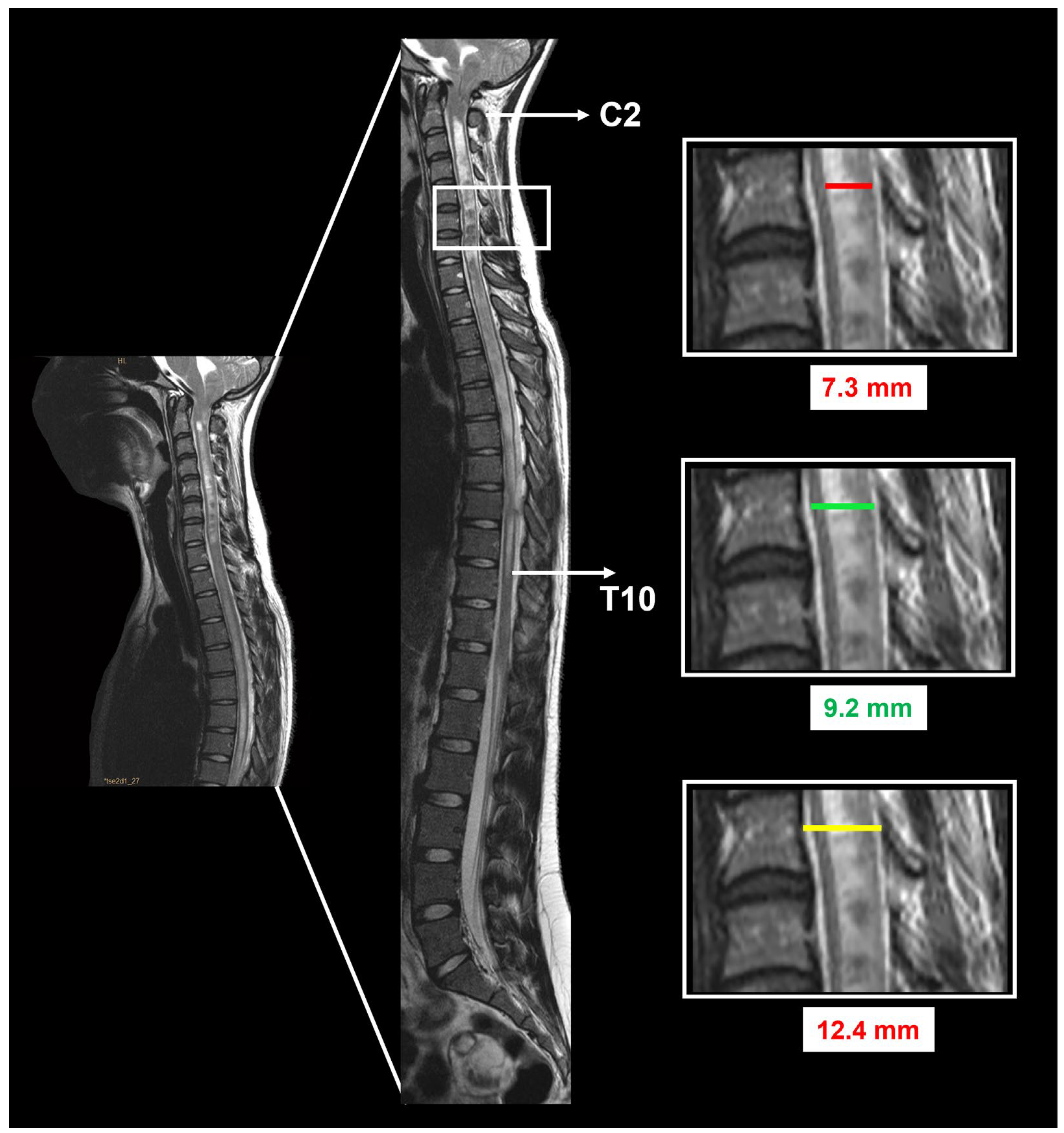
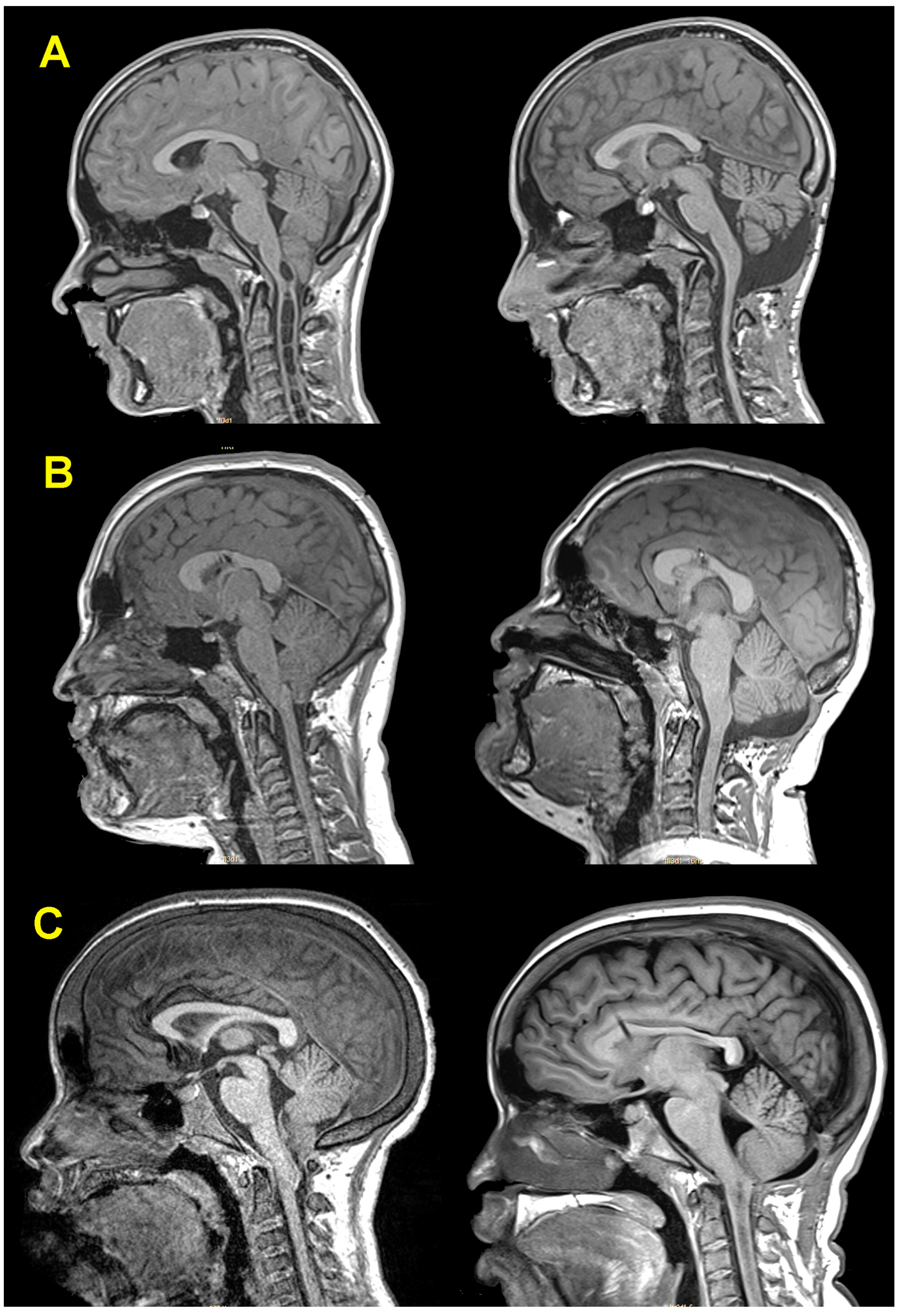
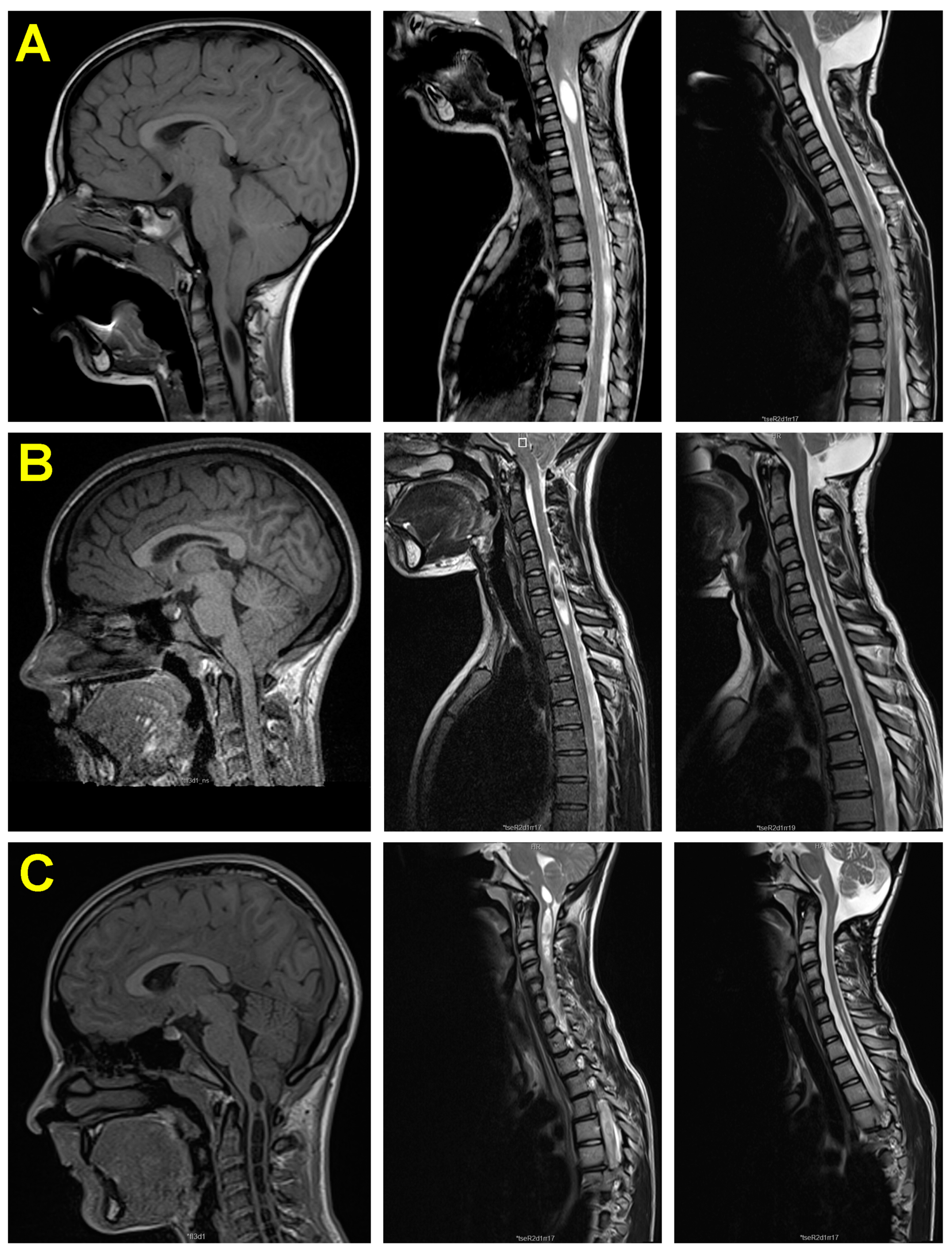
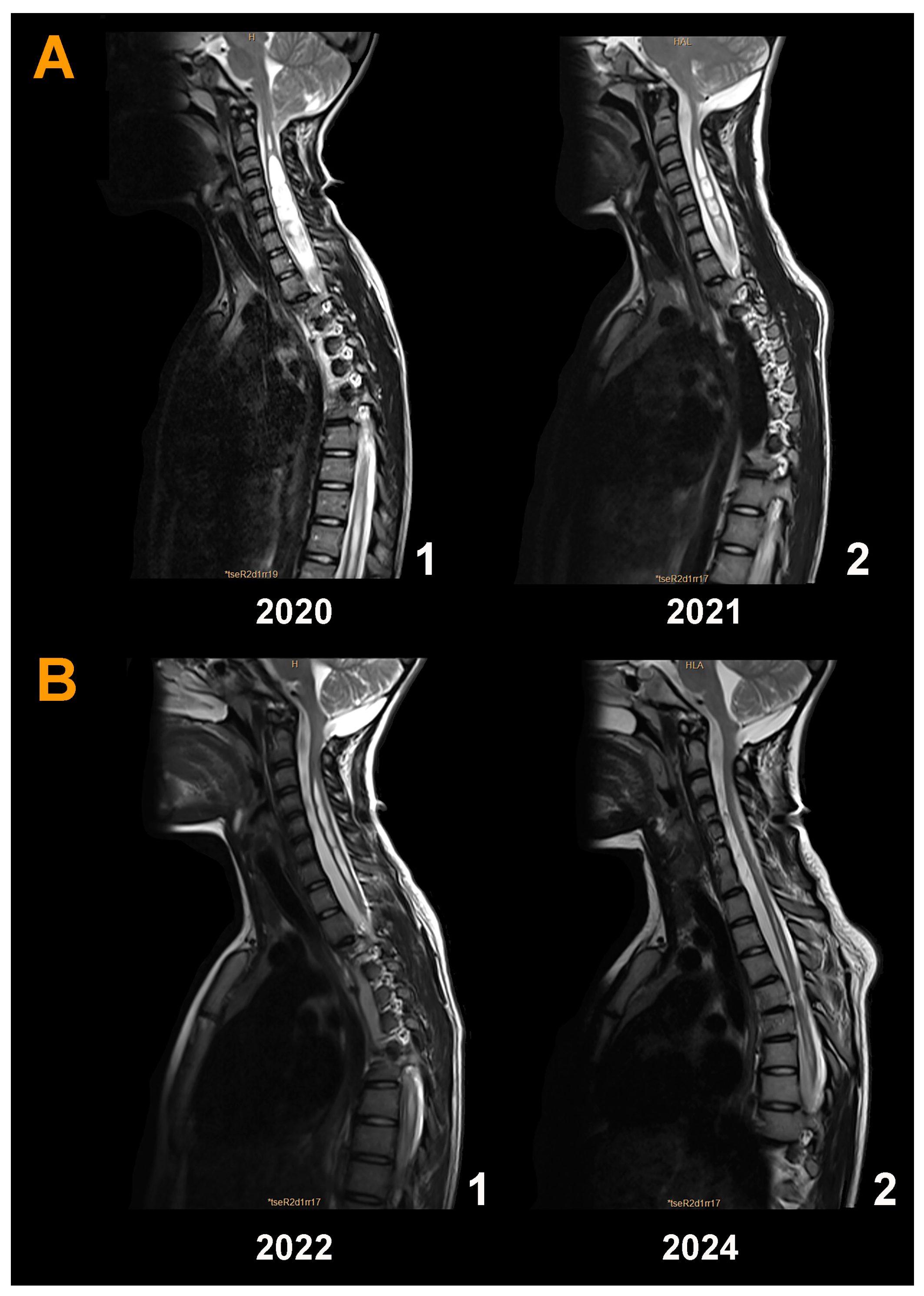
3.5. Neuroimaging Findings before and after Surgery
3.6. Postoperative Adverse Events (AEs)
3.7. Effectiveness of Surgery and Outcomes
3.8. Brief Description and Analysis of the Surgical Failures
4. Discussion
4.1. Should Asymptomatic Children with Syr Have Surgery?
4.2. Decompressing versus Reconstructing the Posterior Fossa
4.3. The Buoyant Brain: A Neglected but Fundamental Property of Neural Tissue
4.4. What Constitutes a Successful Surgical Outcome in CM?
4.5. Does Cerebellar Slumping Become More Likely after Large Craniectomies?
4.6. Postoperative Adverse Events
4.6.1. Aseptic Meningitis Syndrome (AMS)
4.6.2. CSF Leaks and Pseudomeningocele
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chiari, H. Über Veränderungen des Kleinhirns Infolge von Hydrocephalie des Grosshirns. In Denkschriften der Kaiserlichen Akademie der Wissenschaften/Mathematisch-Naturwissenschaftliche Classe; Österreichische Akademie der Wissenschaften: Wien, Austria, 1861; Volume 17, pp. 1172–1175. [Google Scholar]
- Chiari, H. Über Veränderungen des Kleinhirns, des Pons und der Medulla Oblongata in Folge von Congenitaler Hydrocephalie; F. Tempsky: Wien, Austria, 1895; Volume 63, pp. 71–116. [Google Scholar]
- Sahuquillo, J.; Moncho, D.; Ferré, A.; López-Bermeo, D.; Sahuquillo-Muxi, A.; Poca, M.A. A Critical Update of the Classification of Chiari and Chiari-like Malformations. J. Clin. Med. 2023, 12, 4626. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.; Tsering, D.; Myseros, J.S.; Magge, S.N.; Oluigbo, C.; Sanchez, C.E.; Keating, R.F. Management of Chiari I malformations: A paradigm in evolution. Childs Nerv. Syst. 2019, 35, 1809–1826. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.; O’Connell, A.; Kiermer, V. How can we ensure visibility and diversity in research contributions? How the Contributor Role Taxonomy (CRediT) is helping the shift from authorship to contributorship. Learn. Publ. 2019, 32, 71–74. [Google Scholar] [CrossRef]
- Moncho, D.; Poca, M.A.; Minoves, T.; Ferre, A.; Canas, V.; Sahuquillo, J. Are evoked potentials clinically useful in the study of patients with Chiari malformation Type 1? J. Neurosurg. 2017, 126, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Ferre, A.; Poca, M.A.; de la Calzada, M.D.; Moncho, D.; Romero, O.; Sampol, G.; Sahuquillo, J. Sleep-Related Breathing Disorders in Chiari Malformation Type 1: A Prospective Study of 90 Patients. Sleep 2017, 40, zsx069. [Google Scholar] [CrossRef] [PubMed]
- Milhorat, T.H.; Chou, M.W.; Trinidad, E.M.; Kula, R.W.; Mandell, M.; Wolpert, C.; Speer, M.C. Chiari I malformation redefined: Clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 1999, 44, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Klekamp, J. How Should Syringomyelia be Defined and Diagnosed? World Neurosurg. 2018, 111, e729–e745. [Google Scholar] [CrossRef]
- Giannakaki, V.; Wildman, J.; Thejasvin, K.; Pexas, G.; Nissen, J.; Ross, N.; Mitchell, P. Foramen Magnum Decompression for Chiari Malformation Type 1: Is There a Superior Surgical Technique? World Neurosurg. 2023, 170, e784–e790. [Google Scholar] [CrossRef]
- Saletti, V.; Farinotti, M.; Peretta, P.; Massimi, L.; Ciaramitaro, P.; Motta, S.; Solari, A.; Valentini, L.G. The management of Chiari malformation type 1 and syringomyelia in children: A review of the literature. Neurol. Sci. 2021, 42, 4965–4995. [Google Scholar] [CrossRef]
- Ciaramitaro, P.; Massimi, L.; Bertuccio, A.; Solari, A.; Farinotti, M.; Peretta, P.; Saletti, V.; Chiapparini, L.; Barbanera, A.; Garbossa, D.; et al. Diagnosis and treatment of Chiari malformation and syringomyelia in adults: International consensus document. Neurol. Sci. 2022, 43, 1327–1342. [Google Scholar] [CrossRef]
- Massimi, L.; Peretta, P.; Erbetta, A.; Solari, A.; Farinotti, M.; Ciaramitaro, P.; Saletti, V.; Caldarelli, M.; Canheu, A.C.; Celada, C.; et al. Diagnosis and treatment of Chiari malformation type 1 in children: The International Consensus Document. Neurol. Sci. 2021, 43, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Pattisapu, J.V.; Ackerman, L.L.; Infinger, L.K.; Maher, C.O.; Quinsey, C.; Rocque, B.G.; Silberstein, H.; Jackson, E.M.; Jernigan, S.; Niazi, T.; et al. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines for Patients with Chiari Malformation: Surgical Interventions. Neurosurgery 2023, 93, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Sahuquillo, J.; Rubio, E.; Poca, M.A.; Rovira, A.; Rodriguez-Baeza, A.; Cervera, C. Posterior fossa reconstruction: A surgical technique for the treatment of Chiari I malformation and Chiari I/syringomyelia complex--preliminary results and magnetic resonance imaging quantitative assessment of hindbrain migration. Neurosurgery 1994, 35, 874–884; discussion 884–875. [Google Scholar] [CrossRef]
- Duddy, M.J.; Williams, B. Hindbrain migration after decompression for hindbrain hernia: A quantitative assessment using MRI. Br. J. Neurosurg. 1991, 5, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Giannakaki, V.; Nissen, J. Foramen magnum decompression for Chiari malformation type I—UK surgical practice. Br. J. Neurosurg. 2022, 36, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Caldarelli, M.; Novegno, F.; Vassimi, L.; Romani, R.; Tamburrini, G.; Di Rocco, C. The role of limited posterior fossa craniectomy in the surgical treatment of Chiari malformation Type I: Experience with a pediatric series. J. Neurosurg. 2007, 106, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Buell, T.J.; Heiss, J.D.; Oldfield, E.H. Pathogenesis and Cerebrospinal Fluid Hydrodynamics of the Chiari I Malformation. Neurosurg. Clin. N. Am. 2015, 26, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Sadler, T.W. Langman’s Medical Embryology, 14th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2019; p. xxii. 432p. [Google Scholar]
- Marin-Padilla, M. Cephalic axial skeletal-neural dysraphic disorders: Embryology and pathology. Can. J. Neurol. Sci. 1991, 18, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Marin-Padilla, M.; Marin-Padilla, T.M. Morphogenesis of experimentally induced Arnold—Chiari malformation. J. Neurol. Sci. 1981, 50, 29–55. [Google Scholar] [CrossRef] [PubMed]
- Schijman, E. History, anatomic forms, and pathogenesis of Chiari I malformations. Childs Nerv. Syst. 2004, 20, 323–328. [Google Scholar] [CrossRef]
- Nyland, H.; Krogness, K.G. Size of posterior fossa in Chiari type 1 malformation in adults. Acta Neurochir. 1978, 40, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Schady, W.; Metcalfe, R.A.; Butler, P. The incidence of craniocervical bony anomalies in the adult Chiari malformation. J. Neurol. Sci. 1987, 82, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Stovner, L.J.; Bergan, U.; Nilsen, G.; Sjaastad, O. Posterior cranial fossa dimensions in the Chiari I malformation: Relation to pathogenesis and clinical presentation. Neuroradiology 1993, 35, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Quintana, F.; Berciano, J. Basichondrocranium anomalies in adult Chiari type I malformation: A morphometric study. J. Neurol. Sci. 1990, 99, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Batzdorf, U. Chiari I malformation with syringomyelia. Evaluation of surgical therapy by magnetic resonance imaging. J. Neurosurg. 1988, 68, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Sahuquillo, J.; Poca, M.A.; Rovira, A.; Raspall, G.; Chasampi, A. A new surgical technique for the treatment of Chiari I malformation and Chiari I/syringomyelia complex: Preliminary results in 10 patients. In Skull Base Surgery: Anatomy, Diagnosis and Treatment; Samii, M., Ed.; Karger: Basel, Switzerland, 1994; pp. 1126–1129. [Google Scholar]
- Batzdorf, U. Posterior fossa reconstruction: A surgical technique for the treatment of Chiari I malformation and Chiari I syringomyelia complex—Preliminary results and magnetic resonance imaging quantitative assessment of hindbrain migration (Comment). Neurosurgery 1994, 35, 884–885. [Google Scholar]
- Langridge, B.; Phillips, E.; Choi, D. Chiari Malformation Type 1: A Systematic Review of Natural History and Conservative Management. World Neurosurg. 2017, 104, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ciaramitaro, P.; Garbossa, D.; Peretta, P.; Piatelli, G.; Massimi, L.; Valentini, L.; Migliaretti, G.; Baldovino, S.; Roccatello, D.; Kodra, Y.; et al. Syringomyelia and Chiari Syndrome Registry: Advances in epidemiology, clinical phenotypes and natural history based on a North Western Italy cohort. Ann. Ist. Super. Sanita 2020, 56, 48–58. [Google Scholar] [CrossRef]
- Lawrence, B.J.; Urbizu, A.; Allen, P.A.; Loth, F.; Tubbs, R.S.; Bunck, A.C.; Kroger, J.R.; Rocque, B.G.; Madura, C.; Chen, J.A.; et al. Cerebellar tonsil ectopia measurement in type I Chiari malformation patients show poor inter-operator reliability. Fluids Barriers CNS 2018, 15, 33. [Google Scholar] [CrossRef]
- Urbizu, A.; Martin, B.A.; Moncho, D.; Rovira, A.; Poca, M.A.; Sahuquillo, J.; Macaya, A.; Espanol, M.I. Machine learning applied to neuroimaging for diagnosis of adult classic Chiari malformation: Role of the basion as a key morphometric indicator. J. Neurosurg. 2017, 129, 779–791. [Google Scholar] [CrossRef]
- Barkovich, A.J.; Wippold, F.J.; Sherman, J.L.; Citrin, C.M. Significance of cerebellar tonsillar position on MR. AJNR Am. J. Neuroradiol. 1986, 7, 795–799. [Google Scholar]
- Tubbs, R.S.; Iskandar, B.J.; Bartolucci, A.A.; Oakes, W.J. A critical analysis of the Chiari 1.5 malformation. J. Neurosurg. 2004, 101, 179–183. [Google Scholar] [CrossRef]
- Ferre, A.; Poca, M.A.; de la Calzada, M.D.; Moncho, D.; Urbizu, A.; Romero, O.; Sampol, G.; Sahuquillo, J. A Conditional Inference Tree Model for Predicting Sleep-Related Breathing Disorders in Patients with Chiari Malformation Type 1: Description and External Validation. J. Clin. Sleep Med. 2019, 15, 89–99. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, A.; Rhoton, A.L., Jr.; Lawton, M.T. Segmental anatomy of cerebellar arteries: A proposed nomenclature. Laboratory investigation. J. Neurosurg. 2011, 115, 387–397. [Google Scholar] [CrossRef]
- Currarino, G.; Rollins, N.; Diehl, J.T. Congenital defects of the posterior arch of the atlas: A report of seven cases including an affected mother and son. AJNR Am. J. Neuroradiol. 1994, 15, 249–254. [Google Scholar]
- Evans, J.W.A. An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch. Neurol. Psychiatry 1942, 47, 931–937. [Google Scholar] [CrossRef]
- Botelho, R.V.; Bittencourt, L.R.; Rotta, J.M.; Tufik, S. A prospective controlled study of sleep respiratory events in patients with craniovertebral junction malformation. J. Neurosurg. 2003, 99, 1004–1009. [Google Scholar] [CrossRef]
- Fujii, K.; Natori, Y.; Nakagaki, H.; Fukui, M. Management of syringomyelia associated with Chiari malformation: Comparative study of syrinx size and symptoms by magnetic resonance imaging. Surg. Neurol. 1991, 36, 281–285. [Google Scholar] [CrossRef]
- Vaquero, J.; Martinez, R.; Arias, A. Syringomyelia-Chiari complex: Magnetic resonance imaging and clinical evaluation of surgical treatment. J. Neurosurg. 1990, 73, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Sahuquillo, J.; Rubio, E.; Codina, A.; Molins, A.; Guitart, J.M.; Poca, M.A.; Chasampi, A. Reappraisal of the intracranial pressure and cerebrospinal fluid dynamics in patients with the so-called “normal pressure hydrocephalus” syndrome. Acta Neurochir. 1991, 112, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Ae, R. Update: Dura mater graft–associated Creutzfeldt-Jakob disease—Japan, 1975–2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 274–278. [Google Scholar] [CrossRef]
- George, B.; Matula, C.; Kihlstrom, L.; Ferrer, E.; Tetens, V. Safety and Efficacy of TachoSil (Absorbable Fibrin Sealant Patch) Compared with Current Practice for the Prevention of Cerebrospinal Fluid Leaks in Patients Undergoing Skull Base Surgery: A Randomized Controlled Trial. Neurosurgery 2017, 80, 847–853. [Google Scholar] [CrossRef]
- Dao Trong, P.; Olivares, A.; El Damaty, A.; Unterberg, A. Adverse events in neurosurgery: A comprehensive single-center analysis of a prospectively compiled database. Acta Neurochir. 2023, 165, 585–593. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Team, P. RStudio: Integrated Development Environment for R. Available online: http://www.posit.co/ (accessed on 21 January 2024).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Berry, R.; Brooks, R.; Gamaldo, C.; Harding, S.; Lloyd, R.; Marcus, C.; Vaughn, B.; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (Version 2.2); American Academy of Sleep Medicine: Darien, IL, USA, 2015. [Google Scholar]
- Oldfield, E.H.; Muraszko, K.; Shawker, T.H.; Patronas, N.J. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J. Neurosurg. 1994, 80, 3–15. [Google Scholar] [CrossRef]
- Heiss, J.D.; Jarvis, K.; Smith, R.K.; Eskioglu, E.; Gierthmuehlen, M.; Patronas, N.J.; Butman, J.A.; Argersinger, D.P.; Lonser, R.R.; Oldfield, E.H. Origin of Syrinx Fluid in Syringomyelia: A Physiological Study. Neurosurgery 2018, 84, 457–468. [Google Scholar] [CrossRef]
- Heiss, J.D.; Patronas, N.; DeVroom, H.L.; Shawker, T.; Ennis, R.; Kammerer, W.; Eidsath, A.; Talbot, T.; Morris, J.; Eskioglu, E.; et al. Elucidating the pathophysiology of syringomyelia. J. Neurosurg. 1999, 91, 553–562. [Google Scholar] [CrossRef]
- Heiss, J.D.; Suffredini, G.; Smith, R.; Devroom, H.L.; Patronas, N.J.; Butman, J.A.; Thomas, F.; Oldfield, E.H. Pathophysiology of persistent syringomyelia after decompressive craniocervical surgery. J. Neurosurg. Spine 2010, 13, 729–742. [Google Scholar] [CrossRef]
- Gardner, W.J.; Angel, J. The mechanism of syringomyelia and its surgical correction. Clin. Neurosurg. 1958, 6, 131–140. [Google Scholar] [CrossRef]
- Williams, B. Syringomyelia. Neurosurg. Clin. N. Am. 1990, 1, 653–685. [Google Scholar] [CrossRef]
- Heiss, J.D. Cerebrospinal Fluid Hydrodynamics in Chiari I Malformation and Syringomyelia: Modeling Pathophysiology. Neurosurg. Clin. N. Am. 2023, 34, 81–90. [Google Scholar] [CrossRef]
- Singhal, A.; Bowen-Roberts, T.; Steinbok, P.; Cochrane, D.; Byrne, A.T.; Kerr, J.M. Natural history of untreated syringomyelia in pediatric patients. Neurosurg. Focus. FOC 2011, 31, E13. [Google Scholar] [CrossRef]
- Cahan, L.D.; Bentson, J.R. Considerations in the diagnosis and treatment of syringomyelia and the Chiari malformation. J. Neurosurg. 1982, 57, 24–31. [Google Scholar] [CrossRef]
- Hertel, G.; Kramer, S.; Placzek, E. Syringomyelia. Clinical follow-up studies in 323 patients. Nervenarzt 1973, 44, 1–13. [Google Scholar]
- Klekamp, J.; Batzdorf, U.; Samii, M.; Bothe, H.W. The surgical treatment of Chiari I malformation. Acta Neurochir. 1996, 138, 788–801. [Google Scholar] [CrossRef]
- Aghakhani, N.; Parker, F.; David, P.; Morar, S.; Lacroix, C.; Benoudiba, F.; Tadie, M. Long-term follow-up of Chiari-related syringomyelia in adults: Analysis of 157 surgically treated cases. Neurosurgery 2009, 64, 308–315; discussion 315. [Google Scholar] [CrossRef]
- Wang, B.; Wang, C.; Zhang, Y.W.; Liang, Y.C.; Liu, W.H.; Yang, J.; Xu, Y.L.; Wang, Y.Z.; Jia, W.Q. Long-term outcomes of foramen magnum decompression with duraplasty for Chiari malformation type I in adults: A series of 297 patients. Neurosurg. Focus 2023, 54, E5. [Google Scholar] [CrossRef]
- Ciaramitaro, P.; Migliaretti, G.; Ferraris, M.; Garnero, A.; Morana, G.; Carucci, P.; Stura, I.; Massaro, F.; Garbossa, D. Syringomyelia Associated with Chiari 1 Malformation in Adults: Positive Outcome Predictors after Posterior Fossa Decompression with Duraplasty. J. Clin. Med. 2023, 12, 3019. [Google Scholar] [CrossRef]
- Heiss, J.D.; Eidsath, A.; Talbot, T.; Armonda, R.; Patronas, N.; Oldfield, E.H. Craniospinal decompression increases intracranial compliance and reduces subarachnoid pressure in patients with Chiari I syringomyelia (Abstracts). Neurosurgery 1994, 35, 567. [Google Scholar]
- Wetjen, N.M.; Heiss, J.D.; Oldfield, E.H. Time course of syringomyelia resolution following decompression of Chiari malformation Type I. J. Neurosurg. Pediatr. 2008, 1, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Urbizu, A.; Poca, M.A.; Vidal, X.; Rovira, A.; Sahuquillo, J.; Macaya, A. MRI-based morphometric analysis of posterior cranial fossa in the diagnosis of chiari malformation type I. J. Neuroimaging 2014, 24, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Holzner, S.; Wohns, D. Physics I for Dummies, 2nd ed.; Wiley Publishing, Inc.: Hoboken, NJ, USA, 2015; p. xii. 413p. [Google Scholar]
- Levin, E.; Muravchick, S.; Gold, M.I. Density of normal human cerebrospinal fluid and tetracaine solutions. Anesth. Analg. 1981, 60, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Lescot, T.; Bonnet, M.-P.; Zouaoui, A.; Muller, J.-C.; Fetita, C.; Coriat, P.; Puybasset, L. A quantitative computed tomography assessment of brain weight, volume, and specific gravity in severe head trauma. Intensive Care Med. 2005, 31, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.K.; Griffith, J.F.; Leung, J.H.; Chu, W.C.; Lam, T.P.; Ng, B.K.; Cheng, J.C. Effect of upright position on tonsillar level in adolescent idiopathic scoliosis. Eur. Radiol. 2015, 25, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Yamada, Y.; Kosugi, K.; Yamada, M.; Narita, K.; Nakahara, T.; Fujiwara, H.; Toda, M.; Jinzaki, M. Effect of gravity on brain structure as indicated on upright computed tomography. Sci. Rep. 2021, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.R.; Johnson, P.L. Sinking brain syndrome: Craniotomy can precipitate brainstem herniation in CSF hypovolemia. Neurology 2004, 62, 157. [Google Scholar] [CrossRef] [PubMed]
- Fiaschi, P.; Morana, G.; Anania, P.; Rossi, A.; Consales, A.; Piatelli, G.; Cama, A.; Pavanello, M. Tonsillar herniation spectrum: More than just Chiari I. Update and controversies on classification and management. Neurosurg. Rev. 2020, 43, 1473–1492. [Google Scholar] [CrossRef] [PubMed]
- Schievink, W.I.; Meyer, F.B.; Atkinson, J.L.D.; Mokri, B. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. J. Neurosurg. 1996, 84, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Samii, C.; Mobius, E.; Weber, W.; Heienbrok, H.W.; Berlit, P. Pseudo Chiari type I malformation secondary to cerebrospinal fluid leakage. J. Neurol. 1999, 246, 162–164. [Google Scholar] [CrossRef]
- Sugrue, P.A.; Hsieh, P.C.; Getch, C.C.; Batjer, H.H. Acute symptomatic cerebellar tonsillar herniation following intraoperative lumbar drainage. J. Neurosurg. 2009, 110, 800–803. [Google Scholar] [CrossRef]
- Sporns, P.B.; Schwindt, W.; Cnyrim, C.D.; Heindel, W.; Zoubi, T.; Zimmer, S.; Hanning, U.; Niederstadt, T.U. Undetected Dural Leaks Complicated by Accidental Drainage of Cerebrospinal Fluid (CSF) can Lead to Severe Neurological Deficits. RöFo 2016, 188, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Munshi, I.; Frim, D.; Stine-Reyes, R.; Weir, B.K.A.; Hekmatpanah, J.; Brown, F. Effects of posterior fossa decompression with and without duraplasty on Chiari malformation-associated hydromyelia. Neurosurgery 2000, 46, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Genitori, L.; Peretta, P.; Nurisso, C.; Macinante, L.; Mussa, F. Chiari type I anomalies in children and adolescents: Minimally invasive management in a series of 53 cases. Childs Nerv. Syst. 2000, 16, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Talamonti, G.; Picano, M.; Fragale, M.; Marcati, E.; Meccariello, G.; Boeris, D.; Cenzato, M. Reoperation in Chiari-1 Malformations. J. Clin. Med. 2023, 12, 2853. [Google Scholar] [CrossRef] [PubMed]
- Williams, B. A critical appraisal of posterior fossa surgery for communicating syringomyelia. Brain 1978, 101, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Holly, L.T.; Batzdorf, U. Management of cerebellar ptosis following craniovertebral decompression for Chiari I malformation. J. Neurosurg. 2001, 94, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Zarrin, D.; Goel, K.; Kim, W.J.; Holly, L.T.; Batzdorf, U. Chiari Type I Revision Decompressive Surgery Indications and Operative Technique: Experience in A Large Adult Cohort. World Neurosurg. 2024, 185, e1074–e1085. [Google Scholar] [CrossRef] [PubMed]
- Durham, S.R.; Fjeld-Olenec, K. Comparison of posterior fossa decompression with and without duraplasty for the surgical treatment of Chiari malformation Type I in pediatric patients: A meta-analysis. J. Neurosurg. Pediatr. 2008, 2, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Forander, P.; Sjavik, K.; Solheim, O.; Riphagen, I.; Gulati, S.; Salvesen, O.; Jakola, A.S. The case for duraplasty in adults undergoing posterior fossa decompression for Chiari I malformation: A systematic review and meta-analysis of observational studies. Clin. Neurol. Neurosurg. 2014, 125, 58–64. [Google Scholar] [CrossRef]
- Lu, V.M.; Phan, K.; Crowley, S.P.; Daniels, D.J. The addition of duraplasty to posterior fossa decompression in the surgical treatment of pediatric Chiari malformation Type I: A systematic review and meta-analysis of surgical and performance outcomes. J. Neurosurg. Pediatr. 2017, 20, 439–449. [Google Scholar] [CrossRef]
- Arnautovic, A.; Splavski, B.; Boop, F.A.; Arnautovic, K.I. Pediatric and adult Chiari malformation Type I surgical series 1965-2013: A review of demographics, operative treatment, and outcomes. J. Neurosurg. Pediatr. 2015, 15, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, A.I.; Penfield, W. Acute postoperative aseptic leptomeningitis: Review of cases and discussion of pathogenesis. Arch. Neurol. Psychiatry 1941, 46, 250–276. [Google Scholar] [CrossRef]
- Ross, D.; Rosegay, H.; Pons, V. Differentiation of aseptic and bacterial meningitis in postoperative neurosurgical patients. J. Neurosurg. 1988, 69, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Carmel, P.W.; Greif, L.K. The aseptic meningitis syndrome: A complication of posterior fossa surgery. Pediatr. Neurosurg. 1993, 19, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Carmel, P.W.; Fraser, R.A.; Stein, B.M. Aseptic meningitis following posterior fossa surgery in children. J. Neurosurg. 1974, 41, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Wang, T.; Gao, J.; Xu, J.; Lai, R.; Tan, D. Comparison of posterior fossa decompression with and without duraplasty for the surgical treatment of Chiari malformation type I in adult patients: A retrospective analysis of 103 patients. Medicine 2017, 96, e5945. [Google Scholar] [CrossRef] [PubMed]
- Farber, H.; McDowell, M.M.; Alhourani, A.; Agarwal, N.; Friedlander, R.M. Duraplasty Type as a Predictor of Meningitis and Shunting After Chiari I Decompression. World Neurosurg. 2018, 118, e778–e783. [Google Scholar] [CrossRef]
- Lee, C.K.; Mokhtari, T.; Connolly, I.D.; Li, G.; Shuer, L.M.; Chang, S.D.; Steinberg, G.K.; Hayden Gephart, M. Comparison of Porcine and Bovine Collagen Dural Substitutes in Posterior Fossa Decompression for Chiari I Malformation in Adults. World Neurosurg. 2017, 108, 33–40. [Google Scholar] [CrossRef]


| CM Subtypes | ||||
|---|---|---|---|---|
| Total Cohort (n = 110) | CM-1 (n = 66) | CM-1.5 (n = 44) | p | |
| Sex, boys (n/%) | 56 (51%) | 34 (51.5%) | 22 (50%) | 0.876 |
| Age, years (mean ± SD) | 9.9 ± 4.7 | 9.8 ± 4.9 | 10 ± 4.4 | 0.843 |
| Tonsillar herniation, mm * | 12.5 (3–32) | 10 (3–30) | 17 (7–32) | <0.001 † |
| Isolated CM | 84 (76.4%) | 46 (69.7%) | 38 (86.4%) | |
| CM and other medical conditions (n) | 26 (23.6%) | 20 (30.3%) | 6 (13.6%) | 0.044 † |
| - PIK3CA-related overgrowth | 4 | 2 | 2 | |
| - Autism spectrum disorder | 4 | 3 | 1 | |
| - Sickle cell syndromes | 4 | 3 | 1 | |
| - Hypophosphatemia rickets | 3 | 3 | ||
| - RASopathy (MAPK syndrome) | 3 | 2 | 1 | |
| - Epilepsy | 2 | 2 | - | |
| - Other ** | 6 | 5 | 1 | |
| Associated problems | ||||
| Syringomyelia | 48 (43.6%) | 28 (42.4%) | 20 (45.5%) | 0.754 |
| Hydrocephalus | 14 (12.7%) | 10 (15%) | 4 (9%) | 0.350 |
| Intracranial hypertension *** | 8 (7.3%) | 7 (10.6%) | 1 (2.3%) | 0.198 |
| Retroflexed odontoid | 24 (21.8%) | 13 (19.7%) | 11 (25%) | 0.509 |
| Clinical symptoms and signs | ||||
| Symptomatic patients | 92 (83.6%) | 55 (83.3%) | 37 (84.1%) | 0.916 |
| Time of evolution, months * | 24 (1–156) | 24 (4–132) | 12 (1–156) | 0.010 † |
| - Headache | 71 (77.2%) | 44 (80%) | 27 (73%) | 0.566 |
| - Dizziness | 24 (26.1%) | 17 (31%) | 7 (19%) | 0.320 |
| - Neck pain | 14 (15.2%) | 9 (16.4%) | 5 (13.5%) | 0.466 |
| - Fatigue | 14 (15.2%) | 9 (16.4%) | 5 (13.5%) | 0.664 |
| - Paresthesia | 14 (15.2%) | 8 (14.5%) | 6 (16.2) | 0.500 |
| Abnormal neurological examination | 28 (25.5%) | 19 (28.8%) | 9 (20.5%) | 0.299 |
| - Sensory disturbances | 25 (89.3%) | 16 (84.2%) | 9 (100%) | 0.642 |
| - Reflex abnormalities | 8 (28.6%) | 6 (31.6%) | 2 (22.2%) | 0.368 |
| - Kyphoscoliosis | 8 (28.6%) | 6 (31.6%) | 2 (22.2%) | 0.667 |
| - Motor weakness | 3 (10.7%) | 1 (5.2%) | 2 (22.2%) | 0.563 |
| CM Subtypes | ||||
|---|---|---|---|---|
| Total Cohort (n = 110) | CM-1 (n = 66) | CM-1.5 (n = 44) | p | |
| Surgical findings | ||||
| Arachnoid scarring | ||||
| - No/mild (scores = 0 or 1) | 75 (68.1%) | 50 (75.8%) | 25 (56.8%) | 0.037 † |
| - Moderate/severe (scores = 2–4) | 35 (31.9%) | 16 (24.2%) | 19 (43.2%) | 0.037 † |
| Tonsil coagulation or subpial resection | ||||
| - Yes | 52 (47.3%) | 18 (27.3%) | 34 (77.3%) | 0.001 † |
| Postoperative adverse events (PAE) | ||||
| Patients without no PAE | 77 (70%) | 47 (71.2%) | 30 (68.2%) | 0.734 |
| Patients with one or more PAE | 33 (30%) | 19 (28.8%) | 14 (31.8%) | 0.734 |
| Adverse events | ||||
| - Fever syndrome related to PFR | 25 (22.7%) | 15 (22.7%) | 10 (22.7%) | 1.000 |
| - CSF leaks | 5 (4.5%) | 2 (3%) | 3 (6.8%) | |
| - Hydrocephalus | 6 (5.5%) | 3 (4.5%) | 3 (6.8%) | |
| - Wound infection | 1 (0.9%) | 1 (1.5%) | - | |
| - Meningitis | 1 (0.9%) | - | 1 (2.3%) | |
| - Others | 4 (3.6%) | 3 (4.5%) | 1 (2.3%) | |
| Effectiveness of surgery (surgical outcome, time of follow-up in years) * | ||||
| - Very good (6.5, 0.8 to 17.5) | 101 (91.9%) | 61 (92.4%) | 40 (91%) | 0.776 |
| - Good (12.7, 0.7 to 15.7) | 5 (4.5%) | 3 (4.5%) | 2 (4.5%) | |
| - Bad (12.2, 8.9 to 14.5) | 4 (3.6%) | 2 (3.1%) | 2 4.5%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poca, M.A.; Lopez-Bermeo, D.; Moncho, D.; Ferre, A.; Sanchez-Montañez, A.; Mestres, O.; Galve, S.; Sahuquillo, J. Surgical Outcomes in Chiari 1 and Chiari 1.5 Malformation Treated by Posterior Fossa Reconstruction: A Comprehensive Analysis of 110 Pediatric Cases and Literature Review. J. Clin. Med. 2024, 13, 3852. https://doi.org/10.3390/jcm13133852
Poca MA, Lopez-Bermeo D, Moncho D, Ferre A, Sanchez-Montañez A, Mestres O, Galve S, Sahuquillo J. Surgical Outcomes in Chiari 1 and Chiari 1.5 Malformation Treated by Posterior Fossa Reconstruction: A Comprehensive Analysis of 110 Pediatric Cases and Literature Review. Journal of Clinical Medicine. 2024; 13(13):3852. https://doi.org/10.3390/jcm13133852
Chicago/Turabian StylePoca, Maria A., Diego Lopez-Bermeo, Dulce Moncho, Alex Ferre, Angel Sanchez-Montañez, Olga Mestres, Sandra Galve, and Juan Sahuquillo. 2024. "Surgical Outcomes in Chiari 1 and Chiari 1.5 Malformation Treated by Posterior Fossa Reconstruction: A Comprehensive Analysis of 110 Pediatric Cases and Literature Review" Journal of Clinical Medicine 13, no. 13: 3852. https://doi.org/10.3390/jcm13133852
APA StylePoca, M. A., Lopez-Bermeo, D., Moncho, D., Ferre, A., Sanchez-Montañez, A., Mestres, O., Galve, S., & Sahuquillo, J. (2024). Surgical Outcomes in Chiari 1 and Chiari 1.5 Malformation Treated by Posterior Fossa Reconstruction: A Comprehensive Analysis of 110 Pediatric Cases and Literature Review. Journal of Clinical Medicine, 13(13), 3852. https://doi.org/10.3390/jcm13133852








