The Role of Heat Shock Protein 70 (HSP70) in the Pathogenesis of Ocular Diseases—Current Literature Review
Abstract
1. Introduction
2. Materials and Methods
3. Discussion
3.1. HSP70 in the Normal Eye
3.2. The Role of HSP70 in the Pathogenesis of Ocular Diseases
3.2.1. Cornea
3.2.2. Lens
3.2.3. Retina
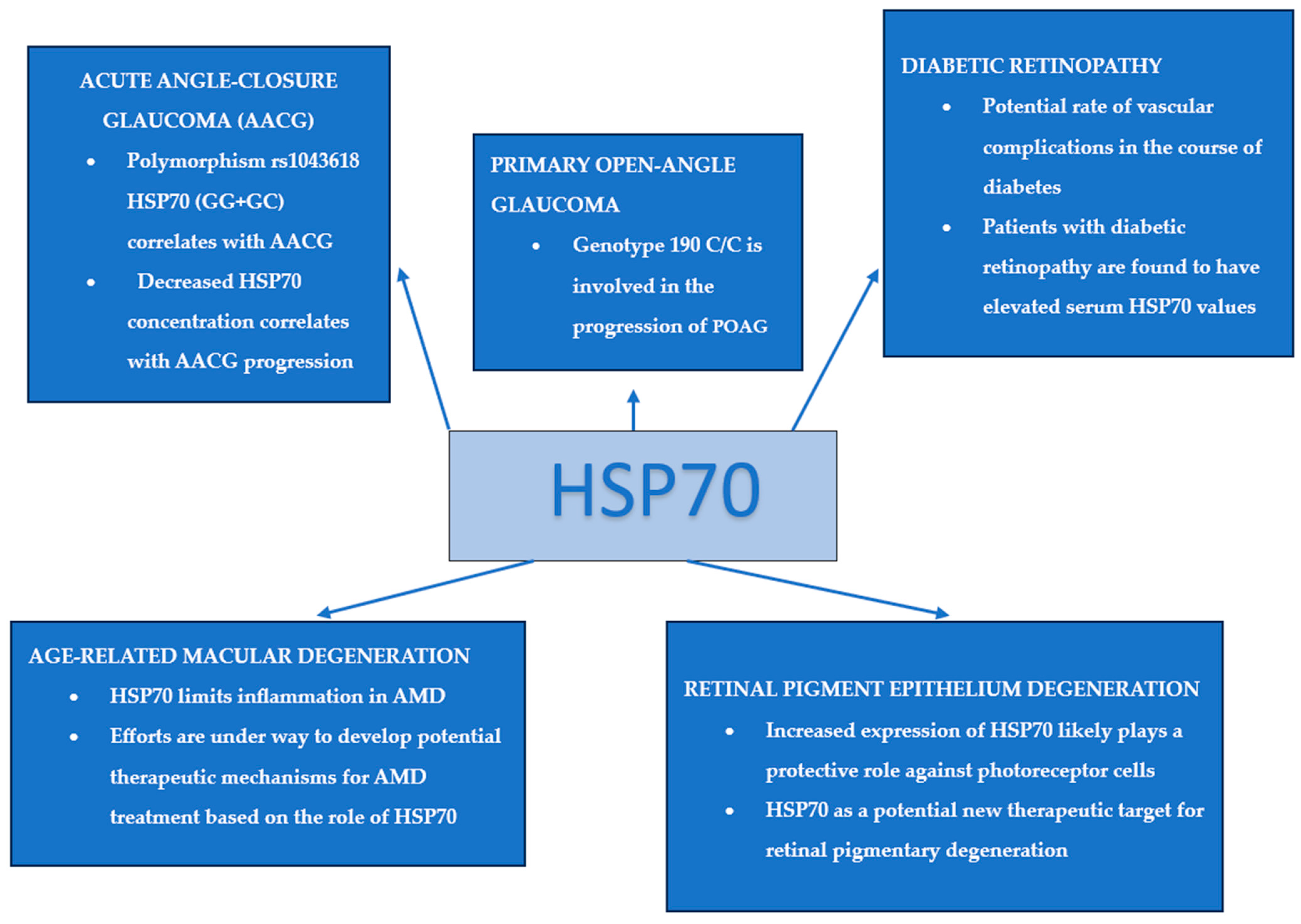
3.2.4. Diabetic Retinopathy
3.2.5. Retinal Pigment Epithelium (RPE) Degeneration
3.2.6. Age-Related Macular Degeneration (AMD)
- The hypothesised protective function of the HSP70 co-inducers paeoniflorin, celastrol, leucynostatin, and arimoclomol, which facilitate the transcriptional activation of HSF1.
- Exogenous enhancement of HSP70 levels by delivery of rhHSP70 (recombinant human HSP70), with evidence supporting its antioxidant function reported in the literature [52].
- HSP70-inducing retinal laser therapies [47].
3.2.7. Glaucoma
Primary Open-Angle Glaucoma (POAG)
Acute ANGLE-closure Glaucoma (AACG)
3.2.8. Pseudoexfoliation Syndrome
3.2.9. Eye Cancers and Autoimmune Diseases
3.2.10. Ocular Toxoplasmosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartl, F.U.; Hayer-Hartl, M. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science 2002, 295, 1852–1858. [Google Scholar] [CrossRef]
- Al-Zuhaeri, A.A.; Al-Shakour, A.A.; Mansour, A.A. Serum Level of Heat Shock Protein 70 in Patients with Type 2 Diabetes Mellitus in Basrah, Iraq. Arch. Razi Inst. 2022, 77, 1837–1844. [Google Scholar] [CrossRef]
- Yanai, R.; Thanos, A.; Connor, K.M. Complement involvement in neovascular ocular diseases. Adv. Exp. Med. Biol. 2012, 946, 161–183. [Google Scholar] [CrossRef]
- Gomez-Pastor, R.; Burchfiel, E.T.; Thiele, D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4–19. [Google Scholar] [CrossRef]
- Morimoto, R.I.; Santoro, M. Stress–inducible responses and heat shock proteins: New pharmacologic targets for cytoprotection. Nat. Biotechnol. 1998, 16, 833–838. [Google Scholar] [CrossRef]
- Pirkkala, L.; Nykänen, P.; Sistonen, L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001, 15, 1118–1131. [Google Scholar] [CrossRef]
- Voellmy, R. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones 2004, 9, 122–133. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Han, J.-A.; Cheong, T.-B.; Ryu, J.-C.; Kim, J.-C. Protective effect of heat shock protein 70 against oxidative stresses in human corneal fibroblasts. J. Korean Med. Sci. 2004, 19, 591–597. [Google Scholar] [CrossRef]
- Ghazaei, C. Role and mechanism of the Hsp70 molecular chaperone machines in bacterial pathogens. J. Med. Microbiol. 2017, 66, 259–265. [Google Scholar] [CrossRef]
- Bellini, S.; Barutta, F.; Mastrocola, R.; Imperatore, L.; Bruno, G.; Gruden, G. Heat Shock Proteins in Vascular Diabetic Complications: Review and Future Perspective. Int. J. Mol. Sci. 2017, 18, 2709. [Google Scholar] [CrossRef]
- Binder, M.J.; Pedley, A.M. The roles of molecular chaperones in regulating cell metabolism. FEBS Lett. 2023, 597, 1681–1701. [Google Scholar] [CrossRef] [PubMed]
- Tavaria, M.; Gabriele, T.; Kola, I.; Anderson, R.L. A hitchhiker’s guide to the human Hsp70 family. Cell Stress Chaperones 1996, 1, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Young, J.C. Mechanisms of the Hsp70 chaperone system. Biochem. Cell Biol. 2010, 88, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Vostakolaei, M.A.; Hatami-Baroogh, L.; Babaei, G.; Molavi, O.; Kordi, S.; Abdolalizadeh, J. Hsp70 in cancer: A double agent in the battle between survival and death. J. Cell. Physiol. 2021, 236, 3420–3444. [Google Scholar] [CrossRef] [PubMed]
- Penke, B.; Bogár, F.; Crul, T.; Sántha, M.; Tóth, M.E.; Vígh, L. Heat Shock Proteins and Autophagy Pathways in Neuroprotection: From Molecular Bases to Pharmacological Interventions. Int. J. Mol. Sci. 2018, 19, 325. [Google Scholar] [CrossRef] [PubMed]
- Tytell, M.; Barbe, M.F.; Brown, I.R. Induction of heat shock (stress) protein 70 and its mRNA in the normal and light-damaged rat retina after whole body hyperthermia. J. Neurosci. Res. 1994, 38, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, M.; Katar, M.; Maisel, H. Heat shock proteins of adult and embryonic human ocular lenses. J. Cell. Biochem. 2001, 84, 278–284. [Google Scholar] [CrossRef]
- Shanbagh, S.; Matalia, J.; Kannan, R.; Shetty, R.; Panmand, P.; Muthu, S.O.; Chaurasia, S.S.; Deshpande, V.; Bhattacharya, S.S.; Gopalakrishnan, A.V.; et al. Distinct gene expression profiles underlie morphological and etiological differences in pediatric cataracts. Indian J. Ophthalmol. 2023, 71, 2143–2151. [Google Scholar] [CrossRef]
- Cui, X.; Xie, P.P.; Jia, P.P.; Lou, Q.; Dun, G.; Li, S.; Liu, G.; Zhang, J.; Dong, Z.; Ma, Y.; et al. Hsf4 counteracts Hsf1 transcription activities and increases lens epithelial cell survival in vitro. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 746–755. [Google Scholar] [CrossRef]
- Kim, J.H.; Yu, Y.S.; Chung, H.; Heo, J.W.; Seo, J.S. Effect of the absence of heat shock protein 70.1 (hsp70.1) on retinal photic injury. Korean J. Ophthalmol. 2003, 17, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, J.H.; Yu, Y.S.; Jeong, S.M.; Kim, K.-W. Protective effect of heat shock proteins 70.1 and 70.3 on retinal photic injury after systemic hyperthermia. Korean J. Ophthalmol. 2005, 19, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Urbak, L.; Vorum, H. Heat Shock Proteins in the Human Eye. Int. J. Proteom. 2010, 2010, 479571. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-A.; Yanai, R.; Quan, W.-Y.; Morishige, N.; Nishida, T. Up-regulation of HSP70 by the fibronectin-derived peptide PHSRN in human corneal epithelial cells. Biochem. Biophys. Res. Commun. 2008, 370, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Rouhollahi, E.; Hajrezaie, M.; Karimian, H.; Abdulla, M.A.; Kadir, H.A. Annona muricata leaves accelerate wound healing in rats via involvement of Hsp70 and antioxidant defence. Int. J. Surg. 2015, 18, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Naqvi, Z.A.; Siddiqui, A.A.; Palmberg, C.; Shafqat, J.; Ahmed, N. Changes in albumin precursor and heat shock protein 70 expression and their potential role in response to corneal epithelial wound repair. Proteomics 2007, 7, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Banh, A.; Deschamps, P.A.; Vijayan, M.M.; Sivak, J.G.; West-Mays, J.A. The role of Hsp70 and Hsp90 in TGF-beta-induced epithelial-to-mesenchymal transition in rat lens epithelial explants. Mol. Vis. 2007, 13, 2248–2262. [Google Scholar] [PubMed]
- Dwivedi, D.J.; Pino, G.; Banh, A.; Nathu, Z.; Howchin, D.; Margetts, P.; Sivak, J.G.; West-Mays, J.A. Matrix metalloproteinase inhibitors suppress transforming growth factor-beta-induced subcapsular cataract formation. Am. J. Pathol. 2006, 168, 69–79. [Google Scholar] [CrossRef]
- Dzialoszynski, T.M.; Milne, K.J.; Trevithick, J.R.; Noble, E.G. Heat shock protein concentration and clarity of porcine lenses incubated at elevated temperatures. Mol. Vis. 2016, 22, 1309–1317. [Google Scholar] [CrossRef]
- Osborne, N.N.; Casson, R.J.; Wood, J.P.; Chidlow, G.; Graham, M.; Melena, J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004, 23, 91–147. [Google Scholar] [CrossRef]
- Shosha, E.; Xu, Z.; Yokota, H.; Saul, A.; Rojas, M.; Caldwell, R.W.; Narayanan, S.P. Arginase 2 promotes neurovascular degeneration during ischemia/reperfusion injury. Cell Death Dis. 2016, 7, e2483. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Zimmerman, M.B.; Kimura, A.; Sanon, A. Central retinal artery occlusion. Retinal survival time. Exp. Eye Res. 2004, 78, 723–736. [Google Scholar] [CrossRef]
- Mukaida, Y.; Machida, S.; Masuda, T.; Tazawa, Y. Correlation of retinal function with retinal histopathology following ischemia-reperfusion in rat eyes. Curr. Eye Res. 2004, 28, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Anders, F.; Liu, A.; Mann, C.; Teister, J.; Lauzi, J.; Thanos, S.; Grus, F.H.; Pfeiffer, N.; Prokosch, V. The small heat shock protein alpha-crystallin B shows neuroprotective properties in a glaucoma animal model. Int. J. Mol. Sci. 2017, 18, 2418. [Google Scholar] [CrossRef]
- Piri, N.; Kwong, J.M.; Gu, L.; Caprioli, J. Heat shock proteins in the retina: Focus on HSP70 and alpha crystallins in ganglion cell survival. Prog. Retin. Eye Res. 2016, 52, 22–46. [Google Scholar] [CrossRef]
- Kuo, Y.; Ren, S.; Lao, U.; Edgar, B.A.; Wang, T. Suppression of polyglutamine protein toxicity by co-expression of a heat-shock protein 40 and a heat-shock protein 110. Cell Death Dis. 2013, 4, e833. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.A.; Saraswathy, S.; Pararajasegaram, G.; Bhat, S.P. Small heat shock protein alphaA-crystallin prevents photoreceptor degeneration in experimental autoimmune uveitis. PLoS ONE 2012, 7, e33582. [Google Scholar] [CrossRef]
- Rao, N.A.; Saraswathy, S.; Wu, G.S.; Katselis, G.S.; Wawrousek, E.F.; Bhat, S. Elevated retina-specific expression of the small heat shock protein, alphaA-crystallin, is associated with photoreceptor protection in experimental uveitis. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Kayama, M.; Nakazawa, T.; Thanos, A.; Morizane, Y.; Murakami, Y.; Theodoropoulou, S.; Abe, T.; Vavvas, D.; Miller, J.W. Heat shock protein 70 (HSP70) is critical for the photoreceptor stress response after retinal detachment via modulating anti-apoptotic akt kinase. Am. J. Pathol. 2011, 178, 1080–1091. [Google Scholar] [CrossRef]
- Liu, W.; Xia, F.; Ha, Y.; Zhu, S.; Li, Y.; Folorunso, O.; Pashaei-Marandi, A.; Lin, P.-Y.; Tilton, R.G.; Pierce, A.P.; et al. Neuroprotective Effects of HSF1 in Retinal Ischemia-Reperfusion Injury. Investig. Ophthalmol. Vis. Sci. 2019, 60, 965–977. [Google Scholar] [CrossRef]
- Chidlow, G.; Wood, J.P.M.; Casson, R.J. Expression of inducible heat shock proteins Hsp27 and Hsp70 in the visual pathway of rats subjected to various models of retinal ganglion cell injury. PLoS ONE 2014, 9, e114838. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Mohammad, G.; dos Santos, J.M.; Zhong, Q. Erratum. Abrogation of MMP-9 Gene Protects Against the Development of Retinopathy in Diabetic Mice by Preventing Mitochondrial Damage. Diabetes 2011, 60, 3023–3033, Erratum in Diabetes 2020, 70, 301. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, A.; Koriyama, Y. A role of Heat Shock Protein 70 in Photoreceptor Cell Death: Potential as a Novel Therapeutic Target in Retinal Degeneration. CNS Neurosci. Ther. 2016, 22, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, K.; Fan, P.; Chen, C.; Hu, J.; Huang, J.; Lu, W.; Xu, J.; Xu, S.; Ran, J.; et al. Geranylgeranylacetone-induced heat shock protein70 expression reduces retinal ischemia-reperfusion injury through PI3K/AKT/mTOR signaling. Exp. Eye Res. 2023, 229, 109416. [Google Scholar] [CrossRef]
- Jiang, K.; Fairless, E.; Kanda, A.; Gotoh, N.; Cogliati, T.; Li, T.; Swaroop, A. Divergent Effects of HSP70 Overexpression in Photoreceptors During Inherited Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 25. [Google Scholar] [CrossRef]
- Lyu, Q.; Ludwig, I.S.; Kooten, P.J.S.; Sijts, A.J.A.M.; Rutten, V.P.M.G.; van Eden, W.; Broere, F. Leucinostatin acts as a co-inducer for heat shock protein 70 in cultured canine retinal pigment epithelial cells. Cell Stress Chaperones 2020, 25, 235–243. [Google Scholar] [CrossRef]
- Kern, K.; Mertineit, C.-L.; Brinkmann, R.; Miura, Y. Expression of heat shock protein 70 and cell death kinetics after different thermal impacts on cultured retinal pigment epithelial cells. Exp. Eye Res. 2018, 170, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Sánchez, L.; Calado, S.M.; de la Cerda, B.; Aramburu, A.; García-Delgado, A.B.; Massalini, S.; Montero-Sánchez, A.; Bhatia, V.; Rodríguez-Bocanegra, E.; Diez-Lloret, A.; et al. Retinal pigment epithelium degeneration caused by aggregation of PRPF31 and the role of HSP70 family of proteins. Mol. Med. 2019, 26, 1. [Google Scholar] [CrossRef]
- Yu, Y.; Xia, X.; Li, H.; Zhang, Y.; Zhou, X.; Jiang, H. A new rhodopsin R135W mutation induces endoplasmic reticulum stress and apoptosis in retinal pigment epithelial cells. J. Cell. Physiol. 2019, 234, 14100–14108. [Google Scholar] [CrossRef]
- Ghaderi, S.; Ahmadian, S.; Soheili, Z.S.; Ahmadieh, H.; Samiei, S.; Kheitan, S.; Pirmardan, E.R. AAV delivery of GRP78/BiP promotes adaptation of human RPE cell to ER stress. J. Cell. Biochem. 2017, 119, 1355–1367. [Google Scholar] [CrossRef]
- Kang, K.; Yu, M. Protective effect of sulforaphane against retinal degeneration in the Pde6rd10 mouse model of retinitis pigmentosa. Curr. Eye Res. 2017, 42, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Subrizi, A.; Toropainen, E.; Ramsay, E.; Airaksinen, A.J.; Kaarniranta, K.; Urtti, A. Oxidative stress protection by exogenous delivery of rhHsp70 chaperone to the retinal pigment epithelium (RPE), a possible therapeutic strategy against rpe degeneration. Pharm. Res. 2015, 32, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-J.; Hu, R.; Sun, H.; Chen, B.; Li, X.; Chen, J.-B. 4-HNE induces proinflammatory cytokines of human retinal pigment epithelial cells by promoting extracellular efflux of HSP70. Exp. Eye Res. 2019, 188, 107792. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Soni, R.; Heindl, S.E.; Wiltshire, D.A.; Khan, S. Unravelling the Role of HSP70 as the Unexplored Molecular Target in Age-Related Macular Degeneration. Cureus 2020, 12, e8960. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gao, L.; Tang, R.; Zhang, W. Hsp70 protects human trabecular meshwork cells injury induced by UVB through Smad pathway. Pharmazie 2017, 72, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Hernandez, M.R.; Wax, M.B. Immunostaining of heat shock proteins in the retina and optic nerve head of normal and glaucomatous eyes. Arch. Ophthalmol. 2000, 118, 511–518. [Google Scholar] [CrossRef]
- Chen, H.; Cho, K.-S.; Vu, T.H.K.; Shen, C.-H.; Kaur, M.; Chen, G.; Mathew, R.; McHam, M.L.; Fazelat, A.; Lashkari, K.; et al. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat. Commun. 2018, 9, 3209. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Szaflik, J.P.; Gacek, M.; Przybylowska-Sygut, K.; Kamińska, A.; Szaflik, J.; Majsterek, I. BDNF and HSP gene polymorphisms and their influence on the progression of primary open-angle glaucoma in a Polish population. Arch. Med. Sci. 2014, 6, 1206–1213. [Google Scholar] [CrossRef]
- Nowak, A.; Majsterek, I.; Przybyłowska-Sygut, K.; Pytel, D.; Szymanek, K.; Szaflik, J.; Szaflik, J.P. Analysis of the expression and polymorphism of APOE, HSP, BDNF, AND GRIN2B genes associated with the neurodegeneration process in the pathogenesis of primary open angle glaucoma. BioMed Res. Int. 2015, 2015, 258281. [Google Scholar] [CrossRef]
- Nowak, A.; Rozpędek, W.; Cuchra, M.; Wojtczak, R.; Siwak, M.; Szymanek, K.; Szaflik, M.; Szaflik, J.; Szaflik, J.; Majsterek, I. Association of the expression level of the neurodegeneration-related proteins with the risk of development and progression of primary open-angle glaucoma. Acta Ophthalmol. 2018, 96, e97–e98. [Google Scholar] [CrossRef]
- Rong, S.S.; Tang, F.Y.; Chu, W.K.; Ma, L.; Yam, J.C.; Tang, S.M.; Li, J.; Gu, H.; Young, A.L.; Tham, C.C.; et al. Genetic associations of primary angle-closure disease. Ophthalmology 2016, 123, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Ayub, H.; Khan, M.I.; Micheal, S.; Akhtar, F.; Ajmal, M.; Shafique, S.; Ali, S.H.B.; Hollander, A.I.D.; Ahmed, A.; Qamar, R. Association of eNOS and HSP70 gene polymorphisms with glaucoma in Pakistani cohorts. Mol. Vis. 2010, 16, 18–25. [Google Scholar] [PubMed]
- Chen, H.; Tian, A.; Wu, Y.; Li, R.; Han, R.; Xu, X.; Cheng, S. HSP70 expression before and after treatment and its clinical value in patients with acute angle closure glaucoma. Exp. Ther. Med. 2021, 21, 253. [Google Scholar] [CrossRef] [PubMed]
- Ludwisiak-Kocerba, L.; Hevelke, A.; Kecik, D. Pseudoexfoliation syndrome—Etiopatogenesis and clinical course. Klin. Ocz./Acta Ophthalmol. Pol. 2006, 108, 82–86. [Google Scholar]
- Güler, M.; Aydın, S.; Urfalıoğlu, S.; Yardım, M. Aqueous humor heat-shock protein 70, periostin, and irisin levels in patients with pseudoexfoliation syndrome. Arq. Bras. Oftalmol. 2020, 83, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Hayat, B.; Kapuganti, R.S.; Padhy, B.; Mohanty, P.P.; Alone, D.P. Epigenetic silencing of heat shock protein 70 through DNA hypermethylation in pseudoexfoliation syndrome and glaucoma. J. Hum. Genet. 2020, 65, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.B.; Liu, X.Q.; Li, B.; He, X.J.; Jin, Y.L.; Li, L.Q.; Wang, N.L. Heat shock proteins and survivin: Relationship and effects on proliferation index of retinoblastoma cells. Histol. Histopathol. 2008, 23, 827–831. [Google Scholar] [CrossRef]
- de Smet, M.D.; Ramadan, A. Circulating antibodies to inducible heat shock protein 70 in patients with uveitis. Ocul. Immunol. Inflamm. 2001, 9, 85–92. [Google Scholar] [CrossRef]
- Karadağ, R.; Koca, C.; Totan, Y.; Yağci, R.; Aydin, M.; Karadağ, A.S.; Akbay, C.G.; Ekşioğlu, H.M.; Yiğitoğlu, M.R. Comparison of serum levels of IL-6, IL-8, TNF-α, C reactive protein and heat shock protein 70 in patients with active or inactive Behçet’s disease. Turk. J. Med. Sci. 2010, 40, 57–62. [Google Scholar] [CrossRef]
- Sahebari, M.; Hashemzadeh, K.; Mahmoudi, M.; Saremi, Z.; Mirfeizi, Z. Diagnostic yield of heat shock protein 70 (HSP-70) and anti-HSP-70 in Behcet-induced uveitis. Scand. J. Immunol. 2013, 77, 476–481. [Google Scholar] [CrossRef]
- Balkan, E.; Bilen, H.; Eyerci, N.; Keleş, S.; Kara, A.; Akdeniz, N.; Dogan, H. Cytokine, C-Reactive Protein, and Heat Shock Protein mRNA Expression Levels in Patients with Active Behçet’s Uveitis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Chiquet, C.; Chumpitazi, B.; Vilgrain, I.; Lesoin, A.; Fricker-Hidalgo, H.; Brenier-Pinchart, M.; Vasseneix, C.; Savy, O.; Campolmi, N.; Gain, P.; et al. Prospective study of serum and aqueous humour anti-Hsp70.1 IgG antibody levels in ocular toxoplasmosis. Parasite Immunol. 2020, 42, e12771. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.; Deshmukh, A.S.; Choudhury, C. Molecular chaperone function of stress inducible Hsp70 is critical for intracellular multiplication of Toxoplasma gondii. Biochim. Biophys. Acta-Mol. Cell Res. 2021, 1868, 118898. [Google Scholar] [CrossRef] [PubMed]
- Sayed, K.M.; Mahmoud, A.A. Heat shock protein-70 and hypoxia inducible factor-1α in type 2 diabetes mellitus patients complicated with retinopathy. Acta Ophtalmol. 2016, 94, 361–366. [Google Scholar] [CrossRef]
| Gene | Encoded Protein | Synonyms | Intracellular Location |
|---|---|---|---|
| HSPA1L | Hsp70-1L | HSP70-1L, HSP70-HOM, HSP70T, hum70t | cytosol, nucleus |
| HSPA1A | Hsp70-1 | HEL-S-103, HSP70, HSP70-1, HSP70-1A, HSP70-2, HSP70.1, HSP70.2, HSP70I, HSP72, HSPA1 | cytosol, nucleus |
| HSPA1B | Hsp70-2 | HSP70-1, HSP70-1B, HSP70-2, HSP70.1, HSP70.2, HSP72, HSPA1, HSX70 | cytosol, nucleus |
| HSPA2 | HSPA2 | HSP70-2, HSP70-3 | cytosol, nucleus |
| HSPA5 | Hsp70-5 | BIP, GRP78, HEL-S-89n | endoplasmic reticulum |
| HSPA6 | Hsp70-6 | HSP70B’ | cytosol |
| HSPA7 | Hsp70-7 | Hsp70B | _ |
| HSPA8 | Hsp70-8 | HEL-33, HEL-S-72p, HSC54, HSC70, HSC71, HSP71, HSP73, HSPA10, LAP-1, LAP1, NIP71 | cytosol, lysosomes |
| HSPA9 | Hsp70-9 | CSA, MOT; MOT2, SAAN, CRP40, EVPLS, GRP75, PBP74, GRP-75, HSPA9B, SIDBA4, MTHSP75, HEL-S-124m | mitochondria |
| HSPA12A | Hsp70-12A | _ | _ |
| HSPA12B | Hsp70-12B | _ | _ |
| HSPA13 | Hsp70-13 | STCH | endoplasmic reticulum |
| HSPA14 | Hsp70-14 | HSP70-4, HSP70L1, MSANTD7 | cytosol |
| Disease | Authors | Year of Publication | Main Conclusions |
|---|---|---|---|
| Keratopathy | Ko et al. [24] | 2008 | HSP70 plays a role in human corneal wound healing. |
| Cataract | Dzialoszynski et al. [29] | 2016 | HSP70 may be responsible for protecting the transparency of the lens. |
| Shanbagh et al. [19] | 2023 | HspA4/Hsp70 expression varies depending on the morphological type of paediatric cataracts. | |
| Diabetic retinopathy | Sayed et al. [74] | 2016 | The determined serum level of HSP70 in patients with diabetic retinopathy was significantly higher than in healthy patients. The increase in HSP70 levels was consistent, independent of the stage of retinopathy. |
| Kowluru et al. [42] | 2021 | MMP-9 inhibitors may have a potential preventive effect in the development of diabetic retinopathy, preventing the decline of mitochondrial HSPs. | |
| Al-Zuhaeri et al. [2] | 2022 | The level of HSP70 was significantly higher in patients with type 2 diabetes compared to controls; with the presence of vascular complications such as proliferative retinopathy, HSP70 levels reached even higher values. | |
| Retinal pigment epithelium (RPE) degeneration | Furukawa et al. [43] | 2016 | Increasing HSP70 expression likely protects photoreceptor cells. Inducers of HSP70 expression, such as valproic acid and geranylgeranylacetone, may serve as potential therapeutic agents for the prevention of retinal degenerative diseases. |
| Kern et al. [47] | 2018 | HSP70 is an important therapeutic factor in hyperthermia treatment; HSP70 plays a key role in apoptosis and wound healing in RPE cells. | |
| Valdés-Sánchez et al. [48] | 2019 | HSP70 is a potential new therapeutic target for the treatment of retinal degeneration caused by PRPF31 mutations. | |
| Yu et al. [49] | 2019 | HSP70 is a potential new therapeutic target for an autosomal dominant form of retinal pigmentary degeneration associated with a novel R135W rhodopsin mutation. HSP70 alleviates RPE stress and prevents R135W rhodopsin-induced apoptosis. | |
| Jiang et al. [45] | 2020 | Overexpression of HSP70 has been shown to have varying effects on photoreceptors, depending on the type of mutant protein; it can either improve photoreceptor survival or exacerbate retinal degeneration. | |
| Lyu et al. [46] | 2020 | Leucinostat increases HSP70 expression in canine RPE cells in response to stress and could potentially serve as a novel co-inducer of HSP70 expression. | |
| Zhang et al. [44] | 2023 | Geranylgeranylacetone-induced expression of HSP70 significantly reduces gliosis, autophagosome accumulation, and apoptosis in retinal ischemia-reperfusion injury. | |
| Age-related macular degeneration (AMD) | Yang et al. [53] | 2019 | The study confirmed the roles of chaperone proteins in reducing inflammation in AMD and investigated the mechanisms of action of three HSP-70 inducers: Arimoclomol, Paeoniflorin, and methyl-β-cyclodextrin, all of which exhibited potential anti-inflammatory effects. |
| Kumar et al. [54] | 2020 | In their review, the authors described potential therapeutic mechanisms based on the role of HSP70 in the treatment of AMD. | |
| Primary open-angle glaucoma (POAG) | Nowak et al. [58] | 2014 | The correlation of the 190G/C polymorphism of the HSP70-1 gene with the occurrence of POAG was described. |
| Nowak et al. [59] | 2015 | The study noted the involvement of the 190 C/C genotype in the progression of POAG. The 190 G/C genotype of the HSP70-1 gene occurred in this study with comparable frequency in both healthy and POAG patients. | |
| Nowak et al. [60] | 2018 | There was no difference in HSP70 levels between patients and controls, indicating no correlation between HSP70 and the development of POAG. | |
| Acute angle-closure glaucoma (AACG) | Rong et al. [61] | 2016 | The rs1043618 HSP70 (GG + GC) polymorphism correlates with primary angle-closure glaucoma. |
| Chen et al. [63] | 2021 | The correlation of reduced HSP70 levels with the progression of AACG was proven. | |
| Pseudoexfoliation syndrome (PEX) | Güler et al. [65] | 2020 | In the study, an increase in HSP70 levels was noted in patients with PEX detected in the aqueous humour. |
| Hayat et al. [66] | 2020 | The study observed a decrease in HSP70 expression in the lens capsule of PEX patients; this reduction in expression was associated with hypermethylation of CpG islands. | |
| Retinoblastoma | Jiang et al. [67] | 2008 | HSP70 was detected in cancer cells, suggesting its involvement in inhibiting apoptosis in retinoblastoma. |
| Behçet’s Uveitis | de Smet et al. [68] | 2001 | Elevated levels of anti-HSP70 antibodies were detected in patients with Behçet’s Uveitis compared to healthy controls. |
| Karadağ et al. [69] | 2010 | Regardless of disease activity, elevated levels of HSP70 were detected in the serum of patients with Behcet’s disease. | |
| Sahebari et al. [70] | 2013 | In the study, patients with Behçet’s disease had higher levels of HSP70 compared to the control group, with the highest values recorded in patients with Behçet’s Uveitis. | |
| Balkan et al. [71] | 2018 | There was no difference in HSP70 levels between patients with Behçet’s Uveitis and healthy controls. | |
| Ocular toxoplasmosis | Chiquet et al. [72] | 2020 | There was no difference in anti-HSP70 antibody levels between patients with toxoplasmosis uveitis and healthy controls. A correlation between anti-HSP70 antibody levels and retinal damage in patients with ocular tuberculosis was observed. |
| Mitra et al. [73] | 2021 | It was concluded that T. gondii infection increases host HSP70 expression, and the HSP70 inhibitor, 2-phenylethylsulfonamide (PES), was suggested as a potential therapy for toxoplasmosis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modrzejewska, M.; Zdanowska, O. The Role of Heat Shock Protein 70 (HSP70) in the Pathogenesis of Ocular Diseases—Current Literature Review. J. Clin. Med. 2024, 13, 3851. https://doi.org/10.3390/jcm13133851
Modrzejewska M, Zdanowska O. The Role of Heat Shock Protein 70 (HSP70) in the Pathogenesis of Ocular Diseases—Current Literature Review. Journal of Clinical Medicine. 2024; 13(13):3851. https://doi.org/10.3390/jcm13133851
Chicago/Turabian StyleModrzejewska, Monika, and Oliwia Zdanowska. 2024. "The Role of Heat Shock Protein 70 (HSP70) in the Pathogenesis of Ocular Diseases—Current Literature Review" Journal of Clinical Medicine 13, no. 13: 3851. https://doi.org/10.3390/jcm13133851
APA StyleModrzejewska, M., & Zdanowska, O. (2024). The Role of Heat Shock Protein 70 (HSP70) in the Pathogenesis of Ocular Diseases—Current Literature Review. Journal of Clinical Medicine, 13(13), 3851. https://doi.org/10.3390/jcm13133851






