1. Introduction
Corneal neovascularization (cNV) entails the growth of blood vessels into the physiologically avascular cornea and is provoked by various factors including inflammation, infection, trauma, or underlying systemic diseases [
1]. This can interfere with the cornea’s transparency due to light scattering, scarring, and lipid deposition and is a known risk factor for corneal graft rejection.
In an avascular state, the cornea is characterized by its unique immune privilege, which allows for remarkably low rates of rejection after transplantation compared to other organs [
2,
3]. While the rate of graft survival at 5 years in a low-risk recipient is 90%, high-risk recipients experience survival rates under 50% at one year and ≤10% after two years [
4]. Features that contribute to the qualification of a recipient as being at high risk for rejection include a history of graft rejection, significant cNV, and active inflammation [
1]. Since cNV signifies a considerable risk for rejection, preconditioning treatment appears to be indicated prior to keratoplasty. However, all possible treatment options (diathermy, argon laser coagulation, Mitomycin-C chemoembolization) may lead to inflammation and an increased risk of rejection in the early postoperative phase. Thus, careful documentation and follow-up is imperative. Currently, no data on the risk of rejection are known for any of these treatment options, and further, there are no available clinical comparisons between the listed treatments.
Various treatments are available to combat cNV, often in a big-picture goal to support corneal graft acceptance and survival. The choice of treatment depends on the underlying cause, severity of the condition, and other factors such as the patient’s age and general health. Among possible treatments are topical or systemic medications, including anti-inflammatory drugs (such as corticosteroids), fine needle diathermy, laser treatment, injection of Mitomycin C (MICE), as well as vascular endothelial growth factor (VEGF) inhibitors including bevacizumab, which can help reduce inflammation and slow the growth of new blood and lymph vessels, which appear to correlate [
1,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14]. Recently, peripheral corneal crosslinking (pCXL) has been gaining interest in this indication profile due to its ability to induce the apoptosis of vascular endothelial cells [
15,
16]. Further, a marked advantage to crosslinking is its ability to not only achieve damage to unwanted blood vessels but to simultaneously damage the associated lymph vessels in the treated area [
15]. Due to lymph vessels’ significant role in rejection reactions, prohibiting excessive growth is likely to have a beneficial effect on high-risk recipient beds.
Due to the dynamic nature of vessel development and the tendency for an amplifying feedback loop, treatment combating cNV is a demanding task. Currently, there is a lack of quantifiable, high-quality imaging techniques for cNV [
17]. While slit lamp photography is a useful aid in general anterior segment imaging, it does not suffice as a sole imaging device for cNV due to its inability to clearly visualize arteries, vessels being masked by opacities (such as scarring), as well as difficulties depicting small capillaries. Fluorescein angiography (FA) and indocyanine green angiography (ICGA) offer a more precise depiction of vessels, but they always represent invasive and resource-consuming procedures, where evaluation is dependent on subjective grading. Optical coherence tomography angiography (OCTA) is an already established technique for retinal imaging, and early applications to the anterior segment show positive results [
18,
19]. Using its ability to quantify vascular activity in the cornea could signify a considerable improvement in the quality of cNV documentation, which may influence future decision-making regarding treatment protocols essentially.
Therefore, the central objective of this pilot study is twofold: to demonstrate the effectiveness and short-term safety of pCXL with high-fluence settings for the treatment of cNV and to quantitatively analyze any given induction of angioregression with pCXL using OCTA imaging compared to slit lamp photography.
2. Methods
This retrospective pilot study was approved by the Ethics Committee of the Medical University of Vienna (EK 1295/2022) and included six patients with significant cNV in at least one corneal quadrant of one affected eye, who’s minimal pachymetry exceeded 350 µm at the cNV location of interest, and who showed no indication of active herpetic or other infectious corneal disease, uncontrolled atopic disease, or uncontrolled glaucoma. Only patients with cNV that were non-responsive to topical steroid therapy were offered pCXL.
Six eyes of six patients (five right eyes, one left eye) were included. Two patients were female and four were male, aged between 49 and 77 (mean 58 ± 9.9 years). Visual acuity prior to treatment was 0.76 logMAR on average (min 1.0 logMAR; max 0.3 logMAR) and showed no change after treatment. The underlying etiology of the cNV included postherpetic neovascularization (
n = 2) and neovascularization after corneal transplantation (
n = 4) (
Table 1). Patients’ informed consent was obtained before imaging and treatment.
Swept-source OCTA of the cornea (Plex Elite 9000, Carl Zeiss Meditec, Dublin, CA, USA) was performed with a 10-diopter adaptor lens prior to pCXL and as a part of the 1 week and 1 month follow-up. At each session, 3 × 3 mm as well as 6 × 6 mm scan patterns were performed centered on the corneal apex. Segmentation needed to be performed manually on each B scan, delineating the epithelial and endothelial surfaces. The resulting images allowed for an interpretation of the cNV maximal depth (posterior border of the deepest flow signal from epithelial surface, µm) and the cNV depth (=ratio between cNV thickness and total corneal thickness, %) per software available with the OCTA device. FIJI (ImageJ 2.0.0, imagej.net) was then used to delineate the limbus. Any image details beyond this border as well as corneal background flow noise were masked in black to allow for accurate vessel measurements. Further analysis was performed using Angiotool (0.6a, National Cancer Institute, Bethesda, MD, USA), which is able to identify the total area of the vascular complex (CV lesion size, mm
2) [
6,
7,
8]. This tool is further able to calculate vessel characteristics, including total vessel length (mm), number of vessel junctions, and vessel junction density (n/mm
2).
Slit lamp color photography (SL 800, Carl Zeiss Meditec, Dublin, CA, USA) was additionally performed at each visit, with a focus on the corneal vascularization/scar/opacity, using 10- and 16-fold magnifications with diffuse illumination. Quality grading was performed for each acquired image. Only images graded to be of a high quality regarding the focus on the region of interest and resolution of the vessel were included in the data analysis as a measure of quality control.
CXL was performed epi-off in the area of the vascular net using a 0.1% riboflavin solution in 20% dextran phosphate sodium (Peschke D, PESCHKE Trade GmbH, Huenenberg, Switzerland) per a well-recognized procedure (one drop every 2 min for 20 min). An accelerated protocol using 9 mW with a total energy dosage of 7.2 J/cm2 was performed for all patients with UV-impermeable protection of the limbus and cornea outside of the treated area by use of a custom-made stencil for each eye in place during treatment. Postoperative care followed a standardized procedure including steroid (Prednifluid, Dermapharm GmbH, Vienna, Austria) and antibiotic eye drops (Gentax, AGEPHA Pharma, Senec, Slovakia), as well as bandage lenses. Follow-up visits were planned according to standard clinical procedure at the 1- and 4-week marks, at which imaging was repeated in addition to standard ophthalmic examination.
3. Results
None of the patients reported significant pain following pCXL, and there were no adverse events after treatment such as persistent peri- and postoperative bleeding, delayed healing patterns with persistent epithelial defects, or signs of limbal stem cell deficiency.
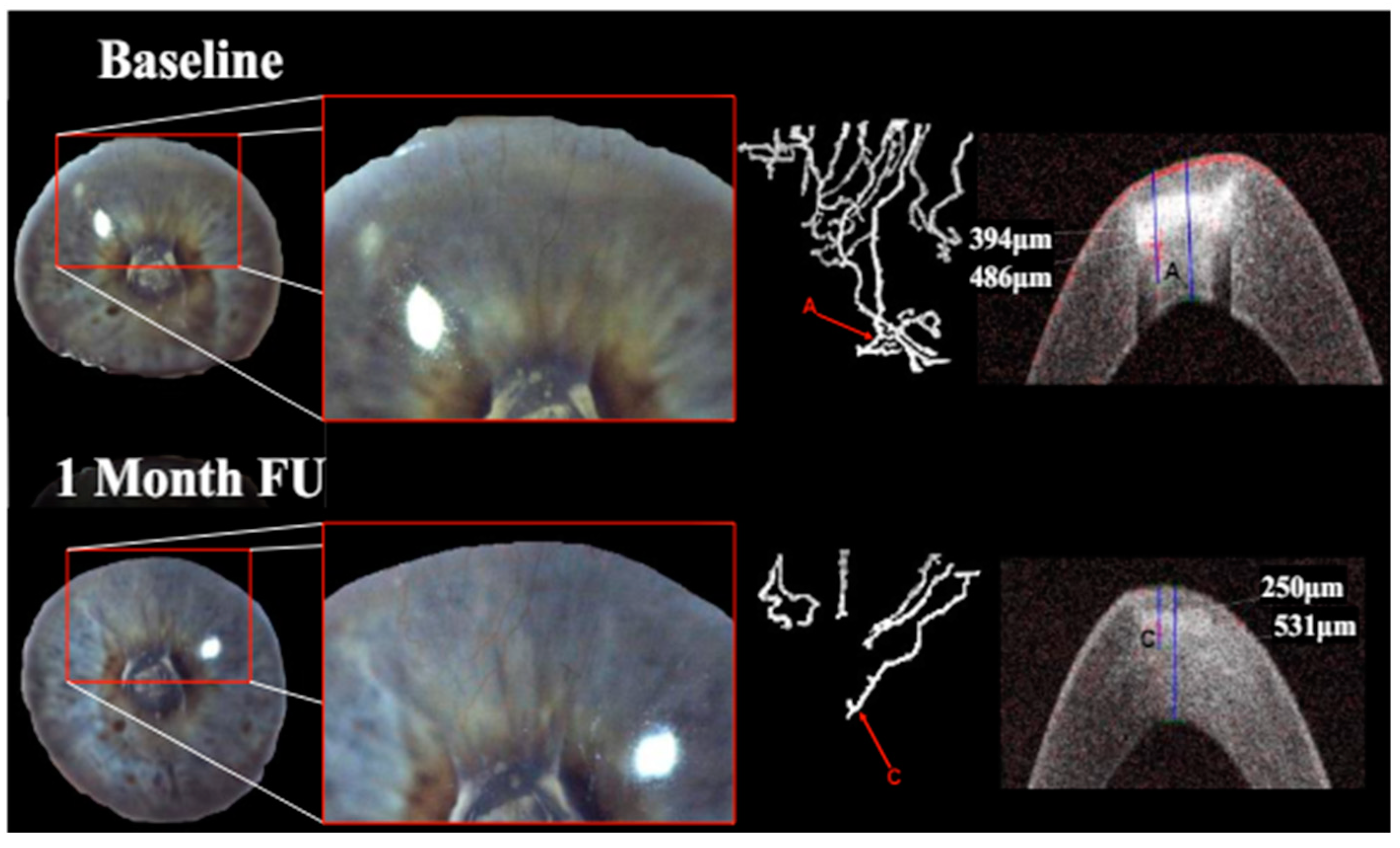
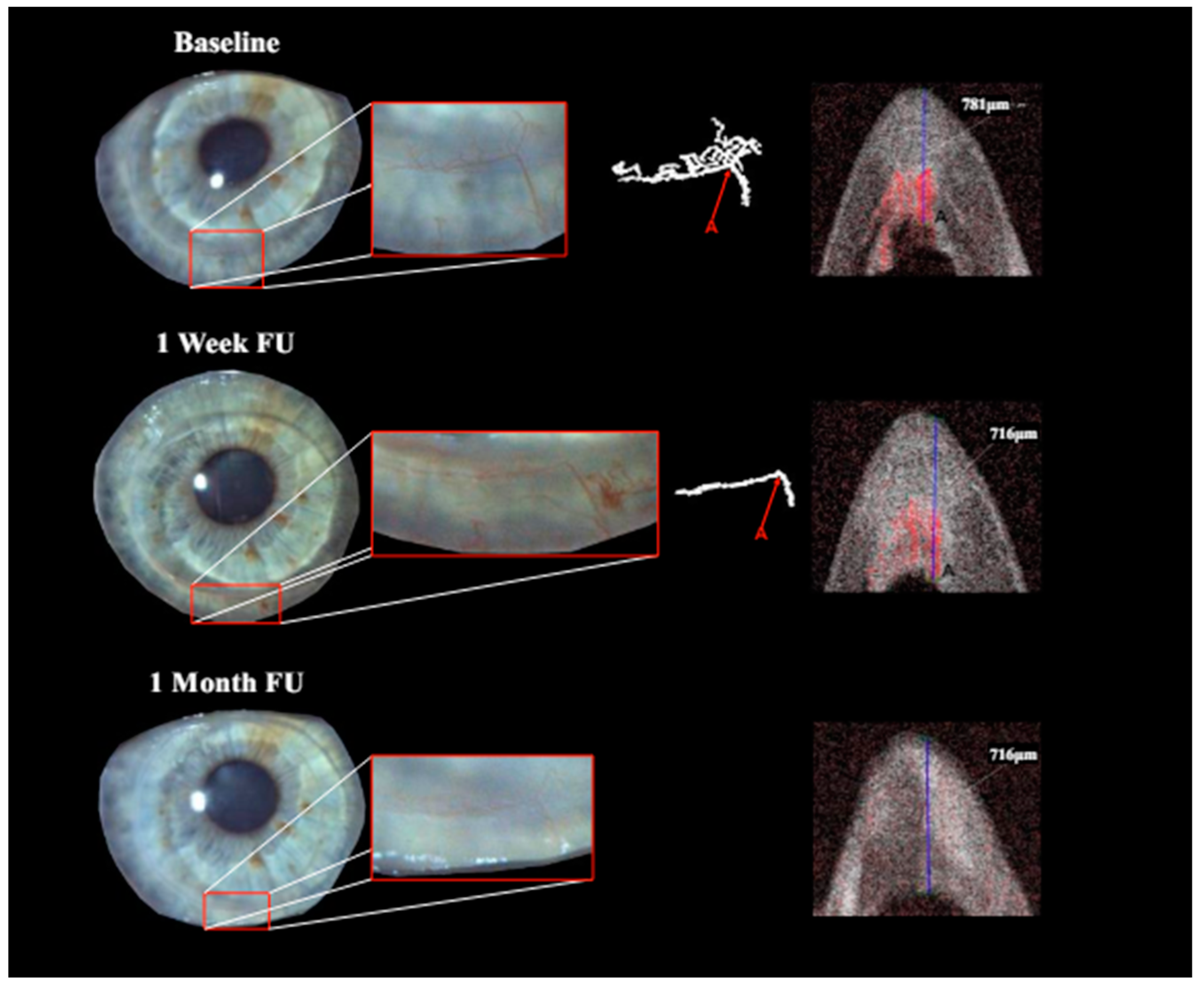
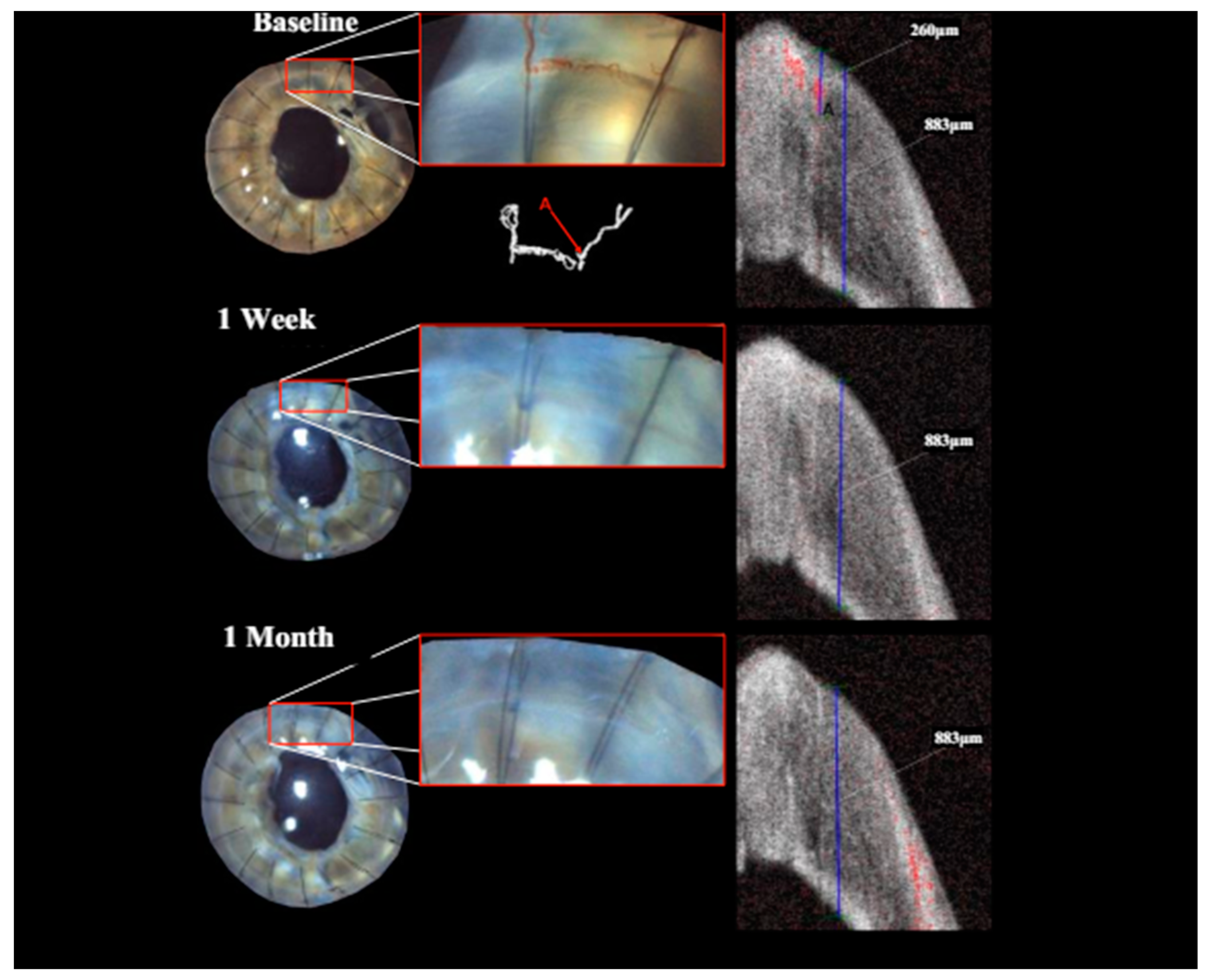
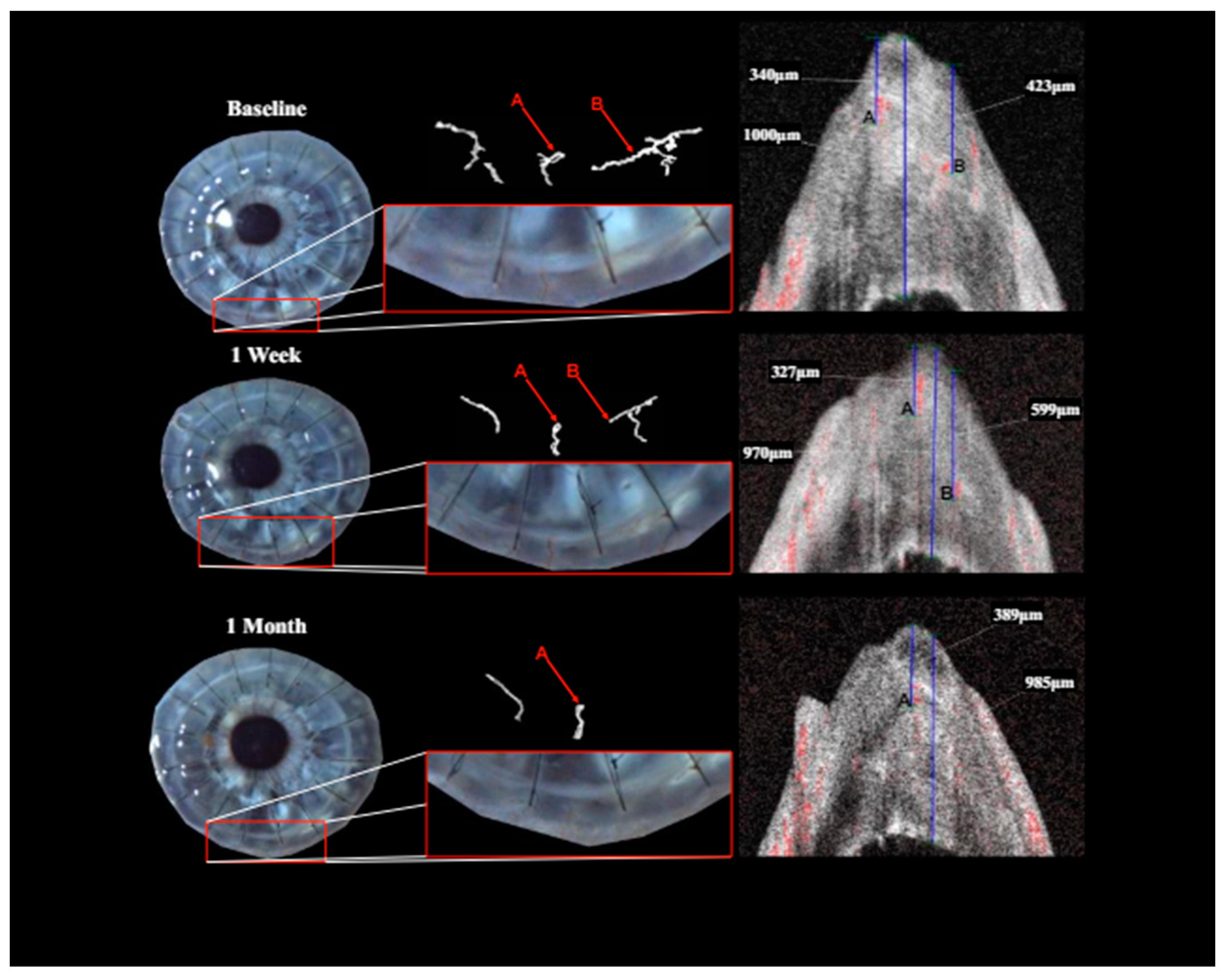
Qualitatively, the identification and follow-up of vessels were considerably more difficult in images obtained with slit lamp photography rather than the isolated vessels depicted via OCTA.
Quantitative image analysis showed a decline in the total vessel area from an average of 1025.4 mm2 (min: 0.13 mm2; max: 3637 mm2) at the baseline evaluation to 382.4 mm2 (min: 0.08 mm2; max: 1528 mm2) at the one-month follow-up (p = 0.096). Total vessel length was reduced from an average of 107.1 mm (min: 2.8 mm; max: 321.1 mm) to 47 mm (min: 2.6 mm; max: 156.5 mm) (p = 0.27). The average number of junctions at baseline was 46.67 (min: 3; max: 166) and 26.5 (min: 0; max: 50) after one month (p = 0.23), and junction density was reduced from an average of 10.75/mm2 (min: 0.0002/mm2; max: 36.5056/mm2) to an average of 7.37/mm2 (min: 0; max 18.7356/mm2) (p = 0.24).
Treatment with pCXL achieved a visible angioregressive effect in the targeted areas in all corneas, as could be observed in processed OCTA images as well as in slit lamp photography. Quantitatively, vessel area was reduced from an average of 31.7% (min 7.7%, max 60.4%) to an average of 21.2% (min 6.7%, max 34.1%) (
p = 0.069). The level of detail regarding vessel conformity, location, and depth given by OCTA imaging stands out in comparison to photography, as can be seen in
Figure 1,
Figure 2 and
Figure 3. Also, imaging could clearly demonstrate that the effect achievable with the applied protocol led to complete and immediate angioregression in several cases (ass illustrated exemplarily by
Figure 3 and
Figure 4). In some eyes though, complete angioregression could only be observed with some delay (see
Figure 2), whilst in further cases, the observed effect of vessel regression was incomplete until month 1 (see
Figure 1).
4. Discussion
Prior to high-risk corneal transplantation, optimal preconditioning of the recipient by mitigating risk factors for rejection is a central concern. However, the treatment of cNV is a difficult and complex task. Finding an effective treatment is made even more difficult by suboptimal available documentation possibilities. Verbal description is subject to immense levels of subjectivity and while slit lamp photography offers an objective method of documentation, it lacks the ability for quantitative analysis and does not always allow for visualization of deep stromal cNV, nor does it mark activity. While accurate and objective, fluorescein and indocyanine green angiography techniques are time-consuming and invasive, requiring a somewhat strict indication for repeated and frequent imaging. Thus, imaging via OCTA represents a leap in the quality of documentation for cNV. Further, being able to quantify vasculature is an opportunity for very high degrees of objectivity and comparability.
This retrospective pilot study shows that OCTA imaging and analysis is uniquely capable of providing detailed objective imaging of and quantitative data for monitoring cNV. Seeing as this trial is also the first to apply high-fluence CXL to the peripheral cornea, documenting its effectiveness as well as possible side effects was a major objective. While no adverse effects could be observed, several patients showed incomplete vessel regression of treated vessels within the follow-up period.
The achieved treatment effect could be shown very well visually as well as in quantitative data. Repeated measurements during the follow-up period showed that this imaging technique is replicable and user-friendly. A welcome benefit to this technique is that a technician can perform imaging independent of a physician’s availability (compared to FLA/ICGA), making it a particularly time-saving tool for standard clinical practice.
While OCTA images clearly offered a more advanced analysis of vessel dynamics due to the isolation from overshadowing structures and the ability to quantify vessel characteristics compared to slit lamp photography, segmentation and analysis of raw OCTA images to achieve images suited for further interpretation is time- and skill-intensive. Currently, this is the main barrier to its routine clinical use for cNV analysis. With advances in interpretation software for corneal imaging, OCTA may become a viable clinical tool.
With increasing use of artificial intelligence (AI) in clinical ophthalmology, the quality of imaging found with OCTA could allow AI to become a viable and valuable tool for the segmentation and analysis of cNV.
Also, despite promising results, it is imperative to consider the limitations of this trial. The current results are based on a fairly short follow-up period. Further analyses will offer insight into the long-term effectiveness of pCXL for this indication profile. The small number of included patients further makes it unlikely to reach significant statistical results. However, this is the first trial to show that high-fluence pCXL is an effective treatment option for cNV via non-invasive OCTA imaging, and short-term effectiveness is a crucial marker when angioregressive treatment is used as a preconditioning measure prior to keratoplasty. At least for the immediate postoperative phase, this treatment proved safe in this pilot study. These results pave the way for future larger-scale, longitudinal studies to investigate the optimal fluence effective for treating corneal lymph and blood vessels as well as the identification of the most ideal vascular parameters for monitoring the effectiveness of treatment.
Several explanations could account for the insufficient effect that was observed after pCXL in some patients. One possible explanation may be that the CXL effect may not have reached the depth of some of the targeted deeper vessels. The results from an earlier laboratory study suggest that an increase in fluence under consistent intensity (9 mW) could be an appropriate adjustment to reach vessels in the deep stroma [
20]. Another hurdle in effective angioregression could be vessel size—large established vessels showing a high level of resistance to treatment. To increase effectiveness, combining pCXL with other treatments such as fine needle diathermy or MICE (to close the lumen of primary large feeder vessels) may be useful. Lastly, in order to avoid reperfusion after an initially satisfactory effect, one may consider following pCXL up with anti-VEGF therapy to combat any VEGF upregulation that may promote reperfusion.
This trial evaluated the effectiveness of high-fluence accelerated pCXL with non-invasive monitoring using innovative anterior segment OCTA. The level of detail in the documentation of cNV, extending to its pro- and regression, that is achievable with OCTA imaging signifies much needed progress for anterior segment imaging. Being able to accurately document cNV allows for a more sophisticated and nuanced evaluation of treatment effect. As became apparent, the applied protocol showed promising angioregression without adverse effects, but it was insufficient in achieving complete angioregression in some cases, indicating the need for further adjustment in the treatment protocol. The ability to perform reliable and non-invasive cNV imaging bears the potential to play an essential role in future development and decisions regarding angioregressive therapy of the cornea.
Author Contributions
Conceptualization, R.D. and G.S.; validation, G.S.; formal analysis, M.K.; investigation, R.D.; data curation, M.K.; writing—original draft, R.D.; writing—review & editing, G.S. and J.A.; visualization, J.A.; supervision, J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study involves human participants and was approved by the Ethics Committee of the Medical University of Vienna (EK 1295/2022) on 3 May 2022.
Informed Consent Statement
Informed written consent was obtained from patients.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to patient privacy interests.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hos, D.; Schlereth, S.L.; Bock, F.; Heindl, L.M.; Cursiefen, C. Antilymphangiogenic therapy to promote transplant survival and to reduce cancer metastasis: What can we learn from the eye? Semin. Cell Dev. Biol. 2015, 38, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Hos, D.; Matthaei, M.; Bock, F.; Maruyama, K.; Notara, M.; Clahsen, T.; Hou, Y.; Le, V.N.H.; Salabarria, A.-C.; Horstmann, J.; et al. Immune reactions after modern lamellar (DALK, DSAEK, DMEK) versus conventional penetrating corneal transplantation. Prog. Retin. Eye Res. 2019, 73, 100768. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, B.; Taylor, R.S.; Cursiefen, C. Corneal Neovascularization as a Risk Factor for Graft Failure and Rejection after Keratoplasty: An evidence-based meta-analysis. Ophthalmology 2010, 117, 1300–1305.e7. [Google Scholar] [CrossRef] [PubMed]
- Schönberg, A.; Hamdorf, M.; Bock, F. Immunomodulatory Strategies Targeting Dendritic Cells to Improve Corneal Graft Survival. J. Clin. Med. 2020, 9, 1280. [Google Scholar] [CrossRef] [PubMed]
- Hos, D.; Saban, D.R.; Bock, F.; Regenfuss, B.; Onderka, J.; Masli, S.; Cursiefen, C. Suppression of Inflammatory Corneal Lymphangiogenesis by Application of Topical Corticosteroids. Arch. Ophthalmol. 2011, 129, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Dastjerdi, M.H.; Saban, D.R.; Okanobo, A.; Nallasamy, N.; Sadrai, Z.; Chauhan, S.K.; Hajrasouliha, A.R.; Dana, R. Effects of Topical and Subconjunctival Bevacizumab in High-Risk Corneal Transplant Survival. Investig. Opthalmol. Vis. Sci. 2010, 51, 2411–2417. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, P.I.; Hergeldzhieva, T.G. Avastin use in high risk corneal transplantation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Harooni, H.; Reddy, V.; Root, T.; Ambati, B. Bevacizumab for Graft Rejection. Ophthalmology 2007, 114, 1950. [Google Scholar] [CrossRef] [PubMed]
- Dekaris, I.; Gabrić, N.; Drača, N.; Pauk-Gulić, M.; Miličić, N. Three-year corneal graft survival rate in high-risk cases treated with subconjunctival and topical bevacizumab. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Cao, J.; Chen, L.; Liu, Y.; Maruyama, K.; Jackson, D.; Kruse, F.E.; Wiegand, S.J.; Dana, M.R.; Streilein, J.W. Inhibition of Hemangiogenesis and Lymphangiogenesis after Normal-Risk Corneal Transplantation by Neutralizing VEGF Promotes Graft Survival. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2666–2673. [Google Scholar] [CrossRef] [PubMed]
- Salabarria, A.C.; Braun, G.; Heykants, M.; Koch, M.; Reuten, R.; Mahabir, E.; Cursiefen, C.; Bock, F. Local VEGF—A blockade modulates the microenvironment of the corneal graft bed. Arab. Archaeol. Epigr. 2019, 19, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.T.; Dua, H.S.; Hossain, P. Fine needle diathermy occlusion of corneal vessels. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2148–2153. [Google Scholar]
- Le, V.N.H.; Schneider, A.-C.; Scholz, R.; Bock, F.; Cursiefen, C. Fine Needle-Diathermy Regresses Pathological Corneal (Lymph)Angiogenesis and Promotes High-Risk Corneal Transplant Survival. Sci. Rep. 2018, 8, 5707. [Google Scholar] [CrossRef] [PubMed]
- Bennis, A.; Chraibi, F.; Abdellaoui, M.; Benatiya, I. Invasive squamous cell carcinoma of the cornea treated with mitomycin eye drops. J. Francais D Ophtalmol. 2018, 41, E367–E370. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Le, V.N.H.; Tóth, G.; Siebelmann, S.; Horstmann, J.; Gabriel, T.; Bock, F.; Cursiefen, C. UV light crosslinking regresses mature corneal blood and lymphatic vessels and promotes subsequent high-risk corneal transplant survival. Am. J. Transplant. 2018, 18, 2873–2884. [Google Scholar] [CrossRef] [PubMed]
- Schaub, F.; Hou, Y.; Zhang, W.; Bock, F.; Hos, D.; Cursiefen, C. Corneal Crosslinking to Regress Pathologic Corneal Neovascularization Before High-Risk Keratoplasty. Cornea 2021, 40, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF—A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Sim, D.A.; Keane, P.A.; Sng, C.C.A.; Egan, C.A.; Tufail, A.; Wilkins, M.R. Optical Coherence Tomography Angiography for Anterior Segment Vasculature Imaging. Ophthalmology 2015, 122, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Oie, Y.; Nishida, K. Evaluation of Corneal Neovascularization Using Optical Coherence Tomography Angiography in Patients with Limbal Stem Cell Deficiency. Cornea 2017, 36 (Suppl. S1), S72–S75. [Google Scholar] [CrossRef] [PubMed]
- Donner, R.; Laggner, M.; Aschauer, J.; Lammer, J.; Schmidinger, G. Identification of Treatment Protocols for Effective Cross-Linking of the Peripheral Cornea: An Experimental Study. Ophthalmol. Ther. 2022, 11, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).