VAC-Stent in the Treatment of Post-Esophagectomy Anastomotic Leaks: A New “Kid on the Block” Who Marries the Best of Old Techniques—A Review
Abstract
1. Introduction
2. Main Endoscopic Treatments of Anastomotic Leaks
2.1. Self-Expandable Metal Stents (SEMSs)
2.2. Endoscopic Vacuum Therapy (EVT)
2.3. Comparison between SEMSs and EVT
3. VAC-Stent
3.1. Design of the VAC-Stent
3.2. Indications
3.3. Outcomes
3.3.1. Efficacy
3.3.2. Safety
3.4. VAC-Stent versus Current Techniques: Advantages and Disadvantages
3.4.1. VAC-Stent vs. SEMS
Advantages
Disadvantages
3.4.2. VAC-Stent vs. EVT
Advantages
Disadvantages
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Barbaro, A.; Eldredge, T.A.; Shenfine, J. Diagnosing anastomotic leak post-esophagectomy: A systematic review. Dis. Esophagus 2021, 34, 1–15. [Google Scholar] [CrossRef]
- Fabbi, M.; Hagens, E.R.C.; van Berge Henegouwen, M.I.; Gisbertz, S.S. Anastomotic leakage after esophagectomy for esophageal cancer: Definitions, diagnostics, and treatment. Dis. Esophagus 2020, 34, 1–14. [Google Scholar] [CrossRef]
- Kassis, E.S.; Kosinski, S.; Ross, P.; Koppes, K.E.; Donahue, J.M.; Daniel, V.C. Predictors of anastomotic leak after esophagectomy: An analysis of the society of thoracic surgeons general thoracic database. Ann. Thorac. Surg. 2013, 96, 1919–1926. [Google Scholar] [CrossRef]
- Low, D.E.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.E.; D’Journo, X.B.; Griffin, S.M.; Hölscher, A.H.; Hofstetter, W.L.; Jobe, B.; et al. International Consensus on Standardization of Data Collection for Complications Associated with Esophagectomy. Ann. Surg. 2015, 262, 286–294. [Google Scholar] [CrossRef]
- Busweiler, L.A.; Wijnhoven, B.P.; van Berge Henegouwen, M.I.; Henneman, D.; van Grieken, N.C.; Wouters, M.W.; van Hillegersberg, R.; van Sandick, J.W.; Dutch Upper Gastrointestinal Cancer Audit (DUCA) Group. Early outcomes from the Dutch Upper Gastrointestinal Cancer Audit. Br. J. Surg. 2016, 103, 1855–1863. [Google Scholar] [CrossRef]
- Kuppusamy, M.K.; Low, D.E. Evaluation of International Contemporary Operative Outcomes and Management Trends Associated with Esophagectomy. Ann. Surg. 2022, 275, 515–525. [Google Scholar] [CrossRef]
- Aiolfi, A.; Asti, E.; Rausa, E.; Bonavina, G.; Bonitta, G.; Bonavina, L. Use of C-reactive protein for the early prediction of anastomotic leak after esophagectomy: Systematic review and Bayesian meta-analysis. PLoS ONE 2018, 13, e0209272. [Google Scholar] [CrossRef]
- Watanabe, M.; Miyata, H.; Gotoh, M.; Baba, H.; Kimura, W.; Tomita, N.; Nakagoe, T.; Shimada, M.; Kitagawa, Y.; Sugihara, K.; et al. Total gastrectomy risk model: Data from 20,011 Japanese patients in a nationwide internet-based database. Ann. Surg. 2014, 260, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- van der Werf, L.R.; Busweiler, L.A.D.; van Sandick, J.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Dutch Upper GI Cancer Audit (DUCA) Group. Reporting National Outcomes After Esophagectomy and Gastrectomy According to the Esophageal Complications Consensus Group (ECCG). Ann. Surg. 2020, 271, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Low, D.E.; Kuppusamy, M.K.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.; Davies, A.; D’Journo, X.B.; Gisbertz, S.S.; Griffin, S.M.; et al. Benchmarking Complications Associated with Esophagectomy. Ann. Surg. 2019, 269, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Mandarino, F.V.; Barchi, A.; D’Amico, F.; Fanti, L.; Azzolini, F.; Viale, E.; Esposito, D.; Rosati, R.; Fiorino, G.; Bemelman, W.A.; et al. Endoscopic Vacuum Therapy (EVT) versus Self-Expandable Metal Stent (SEMS) for Anastomotic Leaks after Upper Gastrointestinal Surgery: Systematic Review and Meta-Analysis. Life 2023, 13, 287. [Google Scholar] [CrossRef]
- Donatelli, G.; Cereatti, F.; Dhumane, P.; Vergeau, B.M.; Tuszynski, T.; Marie, C.; Dumont, J.L.; Meduri, B. Closure of gastrointestinal defects with Ovesco clip: Long-term results and clinical implications. Therap. Adv. Gastroenterol. 2016, 9, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Kotzampassi, K.; Eleftheriadis, E. Tissue sealants in endoscopic applications for anastomotic leakage during a 25-year period. Surgery 2015, 157, 79–86. [Google Scholar] [CrossRef]
- Chon, S.H.; Toex, U.; Plum, P.S.; Kleinert, R.; Bruns, C.J.; Goeser, T.; Berlth, F. Efficacy and feasibility of OverStitch suturing of leaks in the upper gastrointestinal tract. Surg. Endosc. 2020, 34, 3861–3869. [Google Scholar] [CrossRef]
- Rosianu, C.G.; Hoara, P.; Achim, F.; Birla, R.; Bolocan, A.; Mohssen, A.; Copca, N.; Constantinoiu, S. The Use of Esophageal Stents in the Management of Postoperative Fistulas—Current Status, Clinical Outcomes and Perspectives—Review. Life 2023, 13, 966. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Jung, C.F.M.; Fabbri, S.; Giuffrida, P.; Sbrancia, M.; Coluccio, C.; Gibiino, G.; Fabbri, C. Endoscopic Management of Postoperative Esophageal and Upper GI Defects—A Narrative Review. Medicina (B Aires) 2023, 59, 136. [Google Scholar]
- Livingstone, I.; Pollock, L.; Sgromo, B.; Mastoridis, S. Current Status of Endoscopic Vacuum Therapy in the Management of Esophageal Perforations and Post-Operative Leaks. Clin. Endosc. 2021, 54, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Mandarino, F.V.; Barchi, A.; Fanti, L.; D’Amico, F.; Azzolini, F.; Esposito, D.; Biamonte, P.; Lauri, G.; Danese, S. Endoscopic vacuum therapy for post-esophagectomy anastomotic dehiscence as rescue treatment: A single center case series. Esophagus 2022, 19, 417–425. [Google Scholar] [CrossRef]
- Lange, J.; Knievel, J.; Wichmann, D.; Kähler, G.; Wiedbrauck, F.; Hellmich, T.; Kandler, M.; Bernhardt, J.; Scholz, D.; Beyna, T.; et al. Clinical implantation of 92 VACStents in the upper gastrointestinal tract of 50 patients—Applicability and safety analysis of an innovative endoscopic concept. Front. Surg. 2023, 10, 1182094. [Google Scholar] [CrossRef]
- Pattynama, L.M.D.; Eshuis, W.J.; van Berge Henegouwen, M.I.; Bergman, J.J.G.H.M.; Pouw, R.E. Vacuum-stent: A combination of endoscopic vacuum therapy and an intraluminal stent for treatment of esophageal transmural defects. Front. Surg. 2023, 10, 1145984. [Google Scholar] [CrossRef] [PubMed]
- Spaander, M.C.W.; van der Bogt, R.D.; Baron, T.H.; Albers, D.; Blero, D.; de Ceglie, A.; Conio, M.; Czakó, L.; Everett, S.; Garcia-Pagán, J.C.; et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2021. Endoscopy 2021, 53, 751–762. [Google Scholar] [CrossRef]
- Paspatis, G.A.; Arvanitakis, M.; Dumonceau, J.M.; Barthet, M.; Saunders, B.; Turino, S.Y.; Dhillon, A.; Fragaki, M.; Gonzalez, J.M.; Repici, A.; et al. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement—Update 2020. Endoscopy 2020, 52, 792–810. [Google Scholar] [CrossRef]
- Spaander, M.C.; Baron, T.H.; Siersema, P.D.; Fuccio, L.; Schumacher, B.; Escorsell, À.; Garcia-Pagán, J.C.; Dumonceau, J.M.; Conio, M.; de Ceglie, A.; et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2016, 48, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Moyes, L.H.; Mackay, C.K.; Forshaw, M.J. The use of self-expanding plastic stents in the management of oesophageal leaks and spontaneous oesophageal perforations. Diagn. Ther. Endosc. 2011, 2011, 418103. [Google Scholar] [CrossRef][Green Version]
- Kamarajah, S.K.; Bundred, J.; Spence, G.; Kennedy, A.; Dasari, B.V.M.; Griffiths, E.A. Critical Appraisal of the Impact of Oesophageal Stents in the Management of Oesophageal Anastomotic Leaks and Benign Oesophageal Perforations: An Updated Systematic Review. World J. Surg. 2020, 44, 1173–1189. [Google Scholar] [CrossRef]
- Dasari, B.V.; Neely, D.; Kennedy, A.; Spence, G.; Rice, P.; Mackle, E.; Epanomeritakis, E. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann. Surg. 2014, 259, 852–860. [Google Scholar] [CrossRef]
- Aryaie, A.H.; Singer, J.L.; Fayezizadeh, M.; Lash, J.; Marks, J.M. Efficacy of endoscopic management of leak after foregut surgery with endoscopic covered self-expanding metal stents (SEMS). Surg. Endosc. 2017, 31, 612–617. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Esposito, D.; Spelta, G.N.E.; Cavestro, G.M.; Rosati, R.; Parise, P.; Gemma, M.F.; Fanti, L. Double layer stent for the treatment of leaks and fistula after upper gastrointestinal oncologic surgery: A retrospective study. Updates Surg. 2022, 74, 1055–1062. [Google Scholar]
- Chan, S.M.; Auyeung, K.K.Y.; Lam, S.F.; Chiu, P.W.Y.; Teoh, A.Y.B. Current status in endoscopic management of upper gastrointestinal perforations, leaks and fistulas. Dig. Endosc. 2022, 34, 43–62. [Google Scholar] [CrossRef]
- Feith, M.; Gillen, S.; Schuster, T.; Theisen, J.; Friess, H.; Gertler, R. Healing occurs in most patients that receive endoscopic stents for anastomotic leakage; dislocation remains a problem. Clin. Gastroenterol. Hepatol. 2011, 9, 202–210. [Google Scholar] [CrossRef]
- Kucukay, F.; Okten, R.S.; Parlak, E.; Disibeyaz, S.; Ozogul, Y.; Bostanci, E.B.; Olcer, T. Self-expanding covered metallic stent treatment of esophagojejunostomy fistulas. Abdom. Imaging. 2013, 38, 244–248. [Google Scholar] [CrossRef]
- Bohle, W.; Louris, I.; Schaudt, A.; Koeninger, J.; Zoller, W.G. Predictors for Treatment Failure of Self-Expandable Metal Stents for Anastomotic Leak after Gastro-Esophageal Resection. J. Gastrointestin Liver Dis. 2020, 29, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Anderloni, A.; Genco, C.; Massidda, M.; Di Leo, M.; Fumagalli, U.R.; Rosati, R.; Correale, L.; Maselli, R.; Ferrara, E.C.; Jovani, M.; et al. Self-Expanding Metal Stents for the Treatment of Post-Surgical Esophageal Leaks: A Tertiary Referral Center Experience. Dig. Surg. 2019, 36, 309–316. [Google Scholar] [CrossRef]
- Plum, P.S.; Herbold, T.; Berlth, F.; Christ, H.; Alakus, H.; Bludau, M.; Chang, D.H.; Bruns, C.J.; Hölscher, A.H.; Chon, S.H. Outcome of Self-Expanding Metal Stents in the Treatment of Anastomotic Leaks After Ivor Lewis Esophagectomy. World J. Surg. 2019, 43, 862–869. [Google Scholar] [CrossRef]
- Sanz Segura, P.; Gotor Delso, J.; García Cámara, P.; Sierra Moros, E.; Val Pérez, J.; Soria Santeodoro, M.T.; Uribarrena Amezaga, R. Use of double-layered covered esophageal stents in post-surgical esophageal leaks and esophageal perforation: Our experience. Gastroenterol. Hepatol. 2022, 45, 198–203. [Google Scholar] [CrossRef]
- Papaefthymiou, A.; Gkolfakis, P.; Basiliya, K.; Ramai, D.; Tziatzios, G.; Sehgal, V.; Telese, A.; Norton, B.; Aslam, N.; Johnson, G.; et al. Success rates of fixation techniques on prevention of esophageal stent migration: A systematic review and meta-analysis. Endoscopy 2024, 56, 22–30. [Google Scholar] [CrossRef]
- Schiemer, M.; Bettinger, D.; Mueller, J.; Schultheiss, M.; Schwacha, H.; Hasselblatt, P.; Thimme, R.; Schmidt, A.; Kuellmer, A. Reduction of esophageal stent migration rate with a novel over-the-scope fixation device (with video). Gastrointest. Endosc. 2022, 96, 1–8. [Google Scholar] [CrossRef]
- Bemelman, W.A.; Baron, T.H. Endoscopic Management of Transmural Defects, Including Leaks, Perforations, and Fistulae. Gastroenterology 2018, 154, 1938–1946.e1. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Fanti, L.; Barchi, A.; Vespa, E.; Danese, S. Endoscopic vacuum therapy for esophageal perforations: Is it risk effective for every size of defect? Endoscopy 2023, 55, 971. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Barchi, A.; Fanti, L.; Azzolini, F.; Rosati, R.; Danese, S. Endoscopic vacuum therapy in the treatment of postesophagectomy leaks: Is intracavitary the way? Gastrointest. Endosc. 2022, 96, 873. [Google Scholar] [CrossRef]
- Kuehn, F.; Loske, G.; Schiffmann, L.; Gock, M.; Klar, E. Endoscopic vacuum therapy for various defects of the upper gastrointestinal tract. Surg. Endosc. 2017, 31, 3449–3458. [Google Scholar] [CrossRef]
- Heits, N.; Stapel, L.; Reichert, B.; Schafmayer, C.; Schniewind, B.; Becker, T.; Hampe, J.; Egberts, J.H. Endoscopic endoluminal vacuum therapy in esophageal perforation. Ann. Thorac. Surg. 2014, 97, 1029–1035. [Google Scholar] [CrossRef]
- Stathopoulos, P.; Zumblick, M.; Wächter, S.; Schiffmann, L.; Gress, T.M.; Bartsch, D.; Seitz, G.; Denzer, U.W. Endoscopic vacuum therapy (EVT) for acute esophageal perforation: Could it replace surgery? Endosc. Int. Open 2022, 10, E686–E693. [Google Scholar] [CrossRef]
- Smallwood, N.R.; Fleshman, J.W.; Leeds, S.G.; Burdick, J.S. The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surg. Endosc. 2016, 30, 2473–2480. [Google Scholar] [CrossRef]
- Jung, D.H.; Huh, C.W.; Min, Y.W.; Park, J.C. Endoscopic vacuum therapy for the management of upper GI leaks and perforations: A multicenter retrospective study of factors associated with treatment failure (with video). Gastrointest. Endosc. 2022, 95, 281–290. [Google Scholar] [CrossRef]
- Momblan, D.; Gimeno Garcia, A.Z.; Busquets, D.; Juzgado, D.; García Lledó, J.; Ferrero, E.; Tejedor-Tejada, J.; Junquera, F.; Díaz-Tasende, J.; Moris, M.; et al. Endoscopic Vacuum Therapy for Upper Gastrointestinal Leaks and Perforations: Analysis from a Multicenter Spanish Registry. Am. J. Gastroenterol. 2023, 118, 1797–1806. [Google Scholar] [CrossRef]
- Pattynama, L.M.D.; Pouw, R.E.; van Berge Henegouwen, M.I.; Daams, F.; Gisbertz, S.S.; Bergman, J.J.G.H.M.; Eshuis, W.J. Endoscopic vacuum therapy for anastomotic leakage after upper gastrointestinal surgery. Endoscopy 2023, 55, 1019–1025. [Google Scholar]
- Luttikhold, J.; Pattynama, L.M.D.; Seewald, S.; Groth, S.; Morell, B.K.; Gutschow, C.A.; Ida, S.; Nilsson, M.; Eshuis, W.J.; Pouw, R.E. Endoscopic vacuum therapy for esophageal perforation: A multicenter retrospective cohort study. Endoscopy 2023, 55, 859–864. [Google Scholar] [CrossRef]
- Müller, P.C.; Morell, B.; Vetter, D.; Raptis, D.A.; Kapp, J.R.; Gubler, C.; Gutschow, C.A. Preemptive Endoluminal Vacuum Therapy to Reduce Morbidity After Minimally Invasive Ivor Lewis Esophagectomy: Including a Novel Grading System for Postoperative Endoscopic Assessment of GI-Anastomoses. Ann. Surg. 2021, 274, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.C.; Vetter, D.; Kapp, J.R.; Gubler, C.; Morell, B.; Raptis, D.A.; Gutschow, C.A. Pre-Emptive Endoluminal Negative Pressure Therapy at the Anastomotic Site in Minimally Invasive Transthoracic Esophagectomy (the preSPONGE Trial): Study Protocol for a Multicenter Randomized Controlled Trial. Int. J. Surg. Protoc. 2021, 25, 7–15. [Google Scholar] [CrossRef]
- Berlth, F.; Bludau, M.; Plum, P.S.; Herbold, T.; Christ, H.; Alakus, H.; Kleinert, R.; Bruns, C.J.; Hölscher, A.H.; Chon, S.H. Self-Expanding Metal Stents Versus Endoscopic Vacuum Therapy in Anastomotic Leak Treatment After Oncologic Gastroesophageal Surgery. J. Gastrointestinal Surg. 2019, 23, 67–75. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Barchi, A.; Leone, L.; Fanti, L.; Azzolini, F.; Viale, E.; Esposito, D.; Salmeri, N.; Puccetti, F.; Barbieri, L.; et al. Endoscopic vacuum therapy versus self-expandable metal stent for treatment of anastomotic leaks < 30 mm following oncologic Ivor-Lewis esophagectomy: A matched case-control study. Surg Endosc. 2023, 37, 7039–7050. [Google Scholar]
- Scognamiglio, P.; Reeh, M.; Karstens, K.; Bellon, E.; Kantowski, M.; Schön, G.; Zapf, A.; Chon, S.H.; Izbicki, J.R.; Tachezy, M. Endoscopic vacuum therapy versus stenting for postoperative esophago-enteric anastomotic leakage: Systematic review and meta-analysis. Endoscopy 2020, 52, 632–642. [Google Scholar] [CrossRef]
- Tachezy, M.; Chon, S.H.; Rieck, I.; Kantowski, M.; Christ, H.; Karstens, K.; Gebauer, F.; Goeser, T.; Rösch, T.; Izbicki, J.R.; et al. Endoscopic vacuum therapy versus stent treatment of esophageal anastomotic leaks (ESOLEAK): Study protocol for a prospective randomized phase 2 trial. Trials 2021, 22, 377. [Google Scholar] [CrossRef]
- Lange, J.; Kähler, G.; Bernhardt, J.; Knievel, J.; Dormann, A.; Hügle, U.; Eisenberger, C.F.; Heiss, M.M. The VACStent trial: Combined treatment of esophageal leaks by covered stent and endoscopic vacuum therapy. Surg. Endosc. 2023, 37, 3657–3668. [Google Scholar] [CrossRef]
- Lange, J.; Dormann, A.; Bulian, D.R.; Hügle, U.; Eisenberger, C.F.; Heiss, M.M. VACStent: Combining the benefits of endoscopic vacuum therapy and covered stents for upper gastrointestinal tract leakage. Endosc. Int. Open 2021, 9, E971–E976. [Google Scholar] [CrossRef]
- Lange, J.; Eisenberger, C.F.; Knievel, J.; Linderer, A.; Heiss, M.M. Preemptive endoluminal vacuum therapy with the VACStent—A pilot study to reduce anastomotic leakage after Ivor Lewis hybrid esophagectomy. Front. Surg. 2023, 28, 1133083. [Google Scholar] [CrossRef] [PubMed]
- Pattynama, L.M.D.; Eshuis, W.J.; Wielenga, M.C.B.; Pouw, R.E. Successful endoscopic management of a large esophageal defect due to Boerhaave syndrome with endoscopic vacuum therapy using vacuum sponge and vacuum stent. VideoGIE 2023, 8, 144–147. [Google Scholar] [CrossRef]
- Chon, S.H.; Scherdel, J.; Rieck, I.; Lorenz, F.; Dratsch, T.; Kleinert, R.; Gebauer, F.; Fuchs, H.F.; Goeser, T.; Bruns, C.J. A new hybrid stent using endoscopic vacuum therapy in treating esophageal leaks: A prospective single-center experience of its safety and feasibility with mid-term follow-up. Dis. Esophagus. 2022, 35, doab067. [Google Scholar] [CrossRef]
- Chon, S.H.; Töx, U.; Lorenz, F.; Rieck, I.; Wagner, B.J.; Kleinert, R.; Fuchs, H.F.; Goeser, T.; Quaas, A.; Bruns, C.J. A Novel Hybrid Stent with Endoscopic Vacuum Therapy for Treating Leaks of the Upper Gastrointestinal Tract. Visc. Med. 2021, 37, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kedia, P.; Stavropoulos, S.N.; Carr-Locke, D. AGA Clinical Practice Update on Endoscopic Management of Perforations in Gastrointestinal Tract: Expert Review. Clin. Gastroenterol. Hepatol. 2021, 19, 2252–2261.e2. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Carpentier, D.; Lemmers, A. Refractory eso-pleural fistula following Roux-en-Y gastric bypass: VacStent to the rescue!! Dig. Endosc. 2023, 36, 221. [Google Scholar] [CrossRef]
- Basiliya, K.; van Helden, E.J.; de Jong, M.; Planting, I.; Holman, F.; Inderson, A. Stent with vacuum therapy for treatment of colonic anastomotic leakage. Endoscopy 2024, 56, E102. [Google Scholar] [CrossRef] [PubMed]
- Chon, S.H.; Bartella, I.; Bürger, M.; Rieck, I.; Goeser, T.; Schröder, W.; Bruns, C.J. VACStent: A new option for endoscopic vacuum therapy in patients with esophageal anastomotic leaks after upper gastrointestinal surgery. Endoscopy 2020, 52, E166–E167. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, C. Don’t be afraid of black holes: Vacuum sponge and vacuum stent treatment of leaks in the upper GI tract—A case series and mini-review. Front. Surg. 2023, 3, 1168541. [Google Scholar] [CrossRef] [PubMed]
- Schweigert, M.; Dubecz, A.; Stadlhuber, R.J.; Muschweck, H.; Stein, H.J. Risk of stent-related aortic erosion after endoscopic stent insertion for intrathoracic anastomotic leaks after esophagectomy. Ann. Thorac. Surg. 2011, 92, 513–518. [Google Scholar] [CrossRef]
- Speer, E.; Dunst, C.M.; Shada, A.; Reavis, K.M.; Swanström, L.L. Covered stents in cervical anastomoses following esophagectomy. Surg. Endosc. 2016, 30, 3297–3303. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Bonura, G.F.; Esposito, D.; Rosati, R.; Parise, P.; Fanti, L. A large anastomotic leakage after esophageal surgery treated with endoluminal vacuum-assisted closure: A case report. J. Surg. Case Rep. 2020, rjaa071. [Google Scholar] [CrossRef]

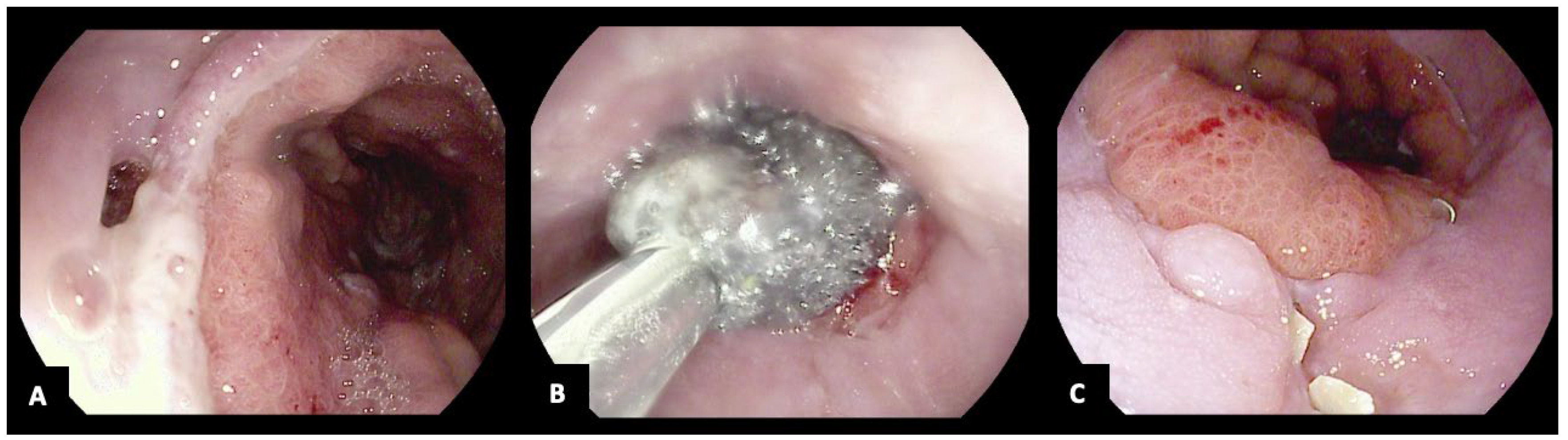

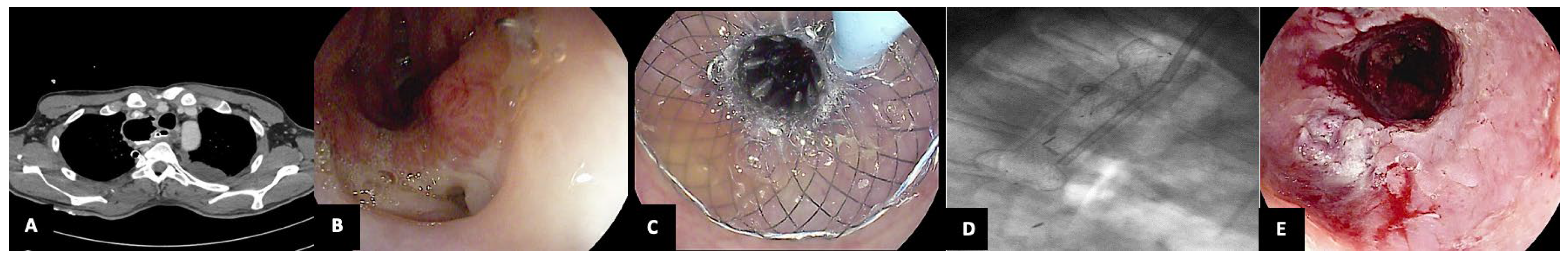
| First Author (Publication Year) | Study Design | N° Patients N° of VAC-Stent (Mean per Patient) | Indications | Previous ET | Clinical Success | Rescue Treatments | Adverse Events |
|---|---|---|---|---|---|---|---|
| L. M. D. Pattynama et al. (2023) [21] | Prospective | 10 patients 15 VAC-Stents | Post-esophagectomy AL (8 cases) Boerhaave syndrome (1 case) Iatrogenic perforation (1 case) | EVT (6 cases—60%) | 10 patients (100%) | None | Anastomotic stricture (1 case undergone previous EVT) |
| J. Lange et al. (2023) [56] | Prospective | 15 patients 41 VAC-Stents (2.7 per patient) | Post-esophagectomy ALs (11 cases) Iatrogenic perforation (3 cases) LINX band explantation (1 case) | EVT (7 cases—47%) | 12 patients (80%) | Surgery (2 cases) | Dislocation (3 cases—7%) Mucosal erosion (9 cases—22%) Local bleeding (5 cases, 12%) Anastomotic stricture (1 case, 6.7%) |
| J. Lange et al. (2021) [57] | Retrospective | 3 patients 4 VAC-Stents (1.3 per patient) | Post-esophagectomy AL (1 case) Boerhaave syndrome (1 case) Iatrogenic perforation (1 case) | SEMS (1 case) EVT (1 case) | 3 patients (100%) | None | None |
| J. Lange et al. (2023) [58] | Prospective | 9 patients 11 VAC-Stents (1.2 per patient) | Pre-emptive (9 cases) | None | 8 cases did not develop AL | None | None |
| L. M. D. Pattynama et al. (2023) [59] | Case report | 1 patient 1 VAC-Stent | Boerhaave syndrome (1 case) | Surgery, EVT | 1 patient (100%) | None | None |
| S.H. Chon et al. (2022) [60] | Prospective | 20 patients 24 VAC-Stents (1.2 per patient) | Post-esophagectomy AL (18 cases) Iatrogenic perforation (2 cases) | EVT (3 cases—15%) | 12 out of 20 (60%) Primary treatment: 12 out of 17 (71%), rescue treatment: 0 out of 3 (0%) | EVT (7 cases) Surgery (1 case) | None |
| S.H. Chon, et al., (2021) [61] | Retrospective | 10 patients 15 VAC-Stents (1.5 per patient) | Post-esophagectomy AL (5 cases) Iatrogenic perforation (1 case) Boerhaave syndrome (2 cases) Esophageal fistula (2 cases) | SEMS (1 case—10%), EVT (2 cases—20%) OTSC (2 cases—20%) | 7 out of 10 (70%). Primary treatment: 4 out of 5 (80%), rescue treatment: 3 out of 5 (60%) | EVT (3 cases) Surgery (1 case) | Adherence to the oesophageal wall during stent removal (3 cases—30%) |
| J. Shah et al. (2023) [63] | Case report | 1 patient 1 VAC-Stent | Esopleural fistula with empyema following a Roux-en-Y gastric bypass | SEMS and double pigtail stents | 1 patient (100%) | None | None |
| K. Basiliya et al. (2024) [64] | Case report | 1 patient/1 VAC-Stent | Anastomotic leak (1; colo-colonic anastomosis) | None | 1 patient (100%) | None | None |
| S. H. Chon et al. (2020) [65] | Case report | 1 patient 2 VAC-Stents | Post-gastrectomy anastomotic leak | OTSC | 1 patient (100%) | None | None |
| VAC-Stent versus SEMSs | VAC-Stent versus EVT | |
|---|---|---|
| Advantages | Vacuum therapy (drainage and aspiration of fluid collection) Greater suitability for the esophageal lumen (less mucosal/vessel trauma) Lower risk of migration Lower stent leakage Rescue drainage strategy Sequential vacuum therapy after intracavitary EVT | Drainage capability associated with stent radial force (less risk of strictures) Resumption of oral feeding Slightly more spaced-out endoscopic procedures (every 5–7 days) Lower risk of AEs related to negative pressure directly in the mediastinum |
| Disadvantages | Dedicated training for endoscopists/nurses Need for hospitalization and monitoring Need for device replacement (every 5–7 days) Discomfort due to nasal tube Only one size Higher costs | Intracavitary placement for ALs associated with cavities not allowed Slightly delayed endoscopic re-evaluation for ALs No possibility for custom-made device Higher costs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Anna, G.; Fanti, L.; Fanizza, J.; Barà, R.; Barchi, A.; Fasulo, E.; Elmore, U.; Rosati, R.; Annese, V.; Laterza, L.; et al. VAC-Stent in the Treatment of Post-Esophagectomy Anastomotic Leaks: A New “Kid on the Block” Who Marries the Best of Old Techniques—A Review. J. Clin. Med. 2024, 13, 3805. https://doi.org/10.3390/jcm13133805
Dell’Anna G, Fanti L, Fanizza J, Barà R, Barchi A, Fasulo E, Elmore U, Rosati R, Annese V, Laterza L, et al. VAC-Stent in the Treatment of Post-Esophagectomy Anastomotic Leaks: A New “Kid on the Block” Who Marries the Best of Old Techniques—A Review. Journal of Clinical Medicine. 2024; 13(13):3805. https://doi.org/10.3390/jcm13133805
Chicago/Turabian StyleDell’Anna, Giuseppe, Lorella Fanti, Jacopo Fanizza, Rukaia Barà, Alberto Barchi, Ernesto Fasulo, Ugo Elmore, Riccardo Rosati, Vito Annese, Liboria Laterza, and et al. 2024. "VAC-Stent in the Treatment of Post-Esophagectomy Anastomotic Leaks: A New “Kid on the Block” Who Marries the Best of Old Techniques—A Review" Journal of Clinical Medicine 13, no. 13: 3805. https://doi.org/10.3390/jcm13133805
APA StyleDell’Anna, G., Fanti, L., Fanizza, J., Barà, R., Barchi, A., Fasulo, E., Elmore, U., Rosati, R., Annese, V., Laterza, L., Fuccio, L., Azzolini, F., Danese, S., & Mandarino, F. V. (2024). VAC-Stent in the Treatment of Post-Esophagectomy Anastomotic Leaks: A New “Kid on the Block” Who Marries the Best of Old Techniques—A Review. Journal of Clinical Medicine, 13(13), 3805. https://doi.org/10.3390/jcm13133805






