Unexpected Genetic Twists in Patients with Cardiac Devices
Abstract
1. Introduction
2. Materials and Methods
- Study Design: Retrospective observational study.
- Inclusion criteria: (I) Patients diagnosed with arrhythmias as a first cardiac event (e.g., ventricular tachycardia, ventricular fibrillation, and 3rd-degree atrioventricular block) who are being treated with cardiac devices, such as pacemakers (PMKs), internal cardioverter-defibrillators (T-ICDs), subcutaneous internal cardioverter-defibrillators (S-ICDs), and undergoing genetic screening; (II) Patients with syncope of unknown cause, who are being treated with loop recorders and undergoing genetic screening; (III) Patients with cardiac resynchronization therapy (CRT) indications, heart failure (HF) belonging to New York Heart Association (NYHA) class II–IV, left ventricular ejection fraction (LVEF) ≤ 35%, QRS complex ≥ 130 ms, left bundle branch block (LBBB) pattern, and optimal pharmacological treatment 3 months prior to CRT, who are undergoing genetic testing.
- Exclusion criteria: patients with incomplete medical records or missing genetic data.
- Data collection:
- 4.1
- Patient demographics: age, gender, and family history of SCD.
- 4.2
- Clinical characteristics: symptoms, type of arrhythmias, and history of cardiac arrest.
- 4.3
- Cardiac imaging: (1) Echocardiographic measurements in all patients (valvular regurgitation and ejection fraction (EF)) and (2) Cardiac Magnetic Resonance Imaging (MRI) if available: ejection fraction, fibrosis, or scar.
- 4.4
- Interventions: type of cardiac device (PMK, ICD, S-ICD, CRT, or loop recorder) and type of ablation if it was performed.
- 4.5
- Genetic testing used next-generation sequencing panels. The testing focused on channelopathies and cardiomyopathies, used commercially available panels, ranged from 106–174 genes, and were chosen at the discretion of the attending physician.
Statistical Analysis
3. Results
- -
- Clinical response to CRT, defined as improvement in NYHA functional class.
- -
- Echocardiographic response (defined as >5% increase in LVEF and decreased mitral regurgitation degree).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A2ML1 | Alpha 2 Macroglobulin Like 1 |
| ACM | Arrhythmogenic Cardiomyopathy |
| ACMG | American College of Medical Genetics and Genomics |
| ACTC1 | Actin Alpha Cardiac Muscle 1 |
| ACTN2 | Actinin Alpha 2 |
| ACEI | Angiotensin-Coverting Enzyme Inhibitor |
| AGL | Amylo-Alpha-1, 6-Glucosidase, 4-Alpha-Glucanotransferase |
| ARNI | Angiotensin Receptor Neprylisin Inhibitor |
| ATP | Anti-Tachycardia Pacing |
| AV | Atrioventricular |
| BrS | Brugada |
| CACNB2 | Calcium Voltage-Gated Channel Auxiliary Subunit Beta 2 |
| CALM 1 | Calmodulin 1 |
| CALM 2 | Calmodulin 2 |
| CALM 3 | Calmodulin 3 |
| CCD | Cardiac Conduction Defects |
| COPD | Chronic Obstructive Pulmonary Disease |
| CPVT | Catecholaminergic Polymorphic Ventricular Tachycardia |
| CRT | Cardiac Resynchronization Therapy |
| CRT-D | Cardiac Resynchronization Therapy Defibrillator |
| CRT-P | Cardiac Resynchronization Therapy Pacemaker |
| CTNNA3 | Catenin Alpha 3 |
| DCM | Dilated Cardiomyopathy |
| DES | Desmin |
| DSG2 | Desmoglein 2 |
| DMD | Dystrophin |
| DSP | Desmoplakin |
| DSC2 | Desmocollin 2 |
| EF | Ejection Fraction |
| EMD | Emerin |
| FBN1 | Fibrillin 1 |
| FLNC | Filamin C |
| FO | Follow-Up |
| HCM | Hypertrophic Cardiomyopathy |
| HF | Heart Failure |
| ICD | Implantable Cardioverter Defibrillator |
| JUP | Junction Plakoglobin |
| KCNQ1 | Potassium Voltage Gated Channel Subfamily Q Member 1 |
| KCNH2 | Potassium Voltage Gated Channel Subfamily H Member 2 |
| LBBB | Left Bundle Branch Block |
| LP | Likely Pathogenic |
| LMNA | Lamin A/C |
| LQTs | Long QT Syndrome |
| LV | Left Ventricle |
| LVEF | Left Ventricular Ejection Fraction |
| MRI | Magnetic Resonance Imaging |
| MR | Mitral Regurgitation |
| MYBPC3 | Myosin Binding Protein C, Cardiac |
| MYH7 | Myosin Heavy Chain 7 |
| MYLK | Myosin Light Chain Kinase |
| MYL2 | Myosin Light Chain 2 |
| MYL3 | Myosin Light Chain 3 |
| NDUFB3 | NADH: Ubiquinone Oxidoreductase Subunit B3 |
| NEXN | Nexilin F-actin binding protein |
| NOAC | Non-Vitamin K Antagonist Oral Anticoagulant |
| NYHA | New York Heart Association |
| P | Pathogenic |
| PLN | Phospholamban |
| PKP2 | Plakophilin 2 |
| PMK | Pacemaker |
| RBM20 | RNA Binding Motif Protein 20 |
| RYR2 | Ryanodine Receptor 2 |
| SCD | Sudden Cardiac Death |
| SCN5A | Sodium Voltage Gated Channel Alpha Subunit 5 |
| SGCD | Sarcoglycan Delta |
| SGLT2 | Sodium-Glucose Co-transporter 2 Inhibitors |
| S-ICD | Subcutaneous Implantable Cardioverter Defibrillator |
| SOS1 | SOS Ras/Rac Guanine Nucleotide Exchange Factor 1 |
| SR | Superresponder |
| SD | Standard Deviation |
| T-ICD | Transvenous Implantable Cardioverter Defibrillator |
| TMEM43 | Transmembrane Protein 43 |
| TNNI3K | TNNI3 Interacting Kinase |
| TNNI3 | Troponin I3, Cardiac Type |
| TNNT2 | Troponin T2, Cardiac Type |
| TPM1 | Tropomyosin 1 |
| TR | Tricuspid Regurgitation |
| TRMP4 | Transient Receptor Potential Cation Channel Subfamily M Member 4 |
| TTN | Titin |
| TTN-DCM | Titin Related Dilated Cardiomyopathy |
| VUS | Variant of Uncertain Significance |
References
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabe, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA). Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; A Blom, N.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, J.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Lenarczyk, R.; Zeppenfeld, K.; Tfelt-Hansen, J.; Heinzel, F.R.; Deneke, T.; Ene, E.; Meyer, C.; Wilde, A.; Arbelo, E.; Jędrzejczyk-Patej, E.; et al. Management of patients with an electrical storm or clustered ventricular arrhythmias: A clinical consensus statement of the European Heart Rhythm Association of the ESC—Endorsed by the Asia-Pacific Heart Rhythm Society, Heart Rhythm Society, and Latin-American Heart Rhythm Society. EP Eur. 2024, 26, euae049. [Google Scholar] [CrossRef]
- Tzeis, S.; Gerstenfeld, E.P.; Kalman, J.; Saad, E.B.; Shamloo, A.S.; Andrade, J.G.; Barbhaiya, C.R.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. EP Eur. 2024, 26, euae043. [Google Scholar] [CrossRef]
- Wilde, A.A.M.; Semsarian, C.; Marquez, M.F.; Shamloo, A.S.; Ackerman, M.J.; Ashley, E.A.; Sternick, E.B.; Barajas-Martinez, H.; Behr, E.R.; Bezzina, C.R.; et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) Expert Consensus Statement on the state of genetic testing for cardiac diseases. EP Eur. 2022, 24, 1367. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; A Blom, N.; A de Boer, R.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Novelli, V.; Amin, A.S.; Abiusi, E.; Care, M.; Nannenberg, E.A.; Feilotter, H.; Amenta, S.; Mazza, D.; Bikker, H.; et al. An international, multicentered, evidence-based reappraisal of genes reported to cause congenital long QT syndrome. Circulation 2020, 141, 418–428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shimizu, W.; Horie, M. Phenotypic manifestations of mutations in genes encoding subunits of cardiac potassium channels. Circ. Res. 2011, 109, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Crotti, L.; Odening, K.E.; Sanguinetti, M.C. Heritable arrhythmias associated with abnormal function of cardiac potassium channels. Cardiovasc. Res. 2020, 116, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Ackerman, M.J.; Antzelevitch, C.; Bezzina, C.R.; Borggrefe, M.; Cuneo, B.F.; Wilde, A.A.M. Inherited cardiac arrhythmias. Nat. Rev. Dis. Primers 2020, 6, 58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Probst, V.; Wilde, A.A.; Barc, J.; Sacher, F.; Babuty, D.; Mabo, P.; Mansourati, J.; Le Scouarnec, S.; Kyndt, F.; Le Caignec, C.; et al. SCN5A mutations and the role of genetic background in the pathophysiology of Brugada syndrome. Circ. Cardiovasc. Genet. 2009, 2, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Bezzina, C.R.; Barc, J.; Mizusawa, Y.; Remme, C.A.; Gourraud, J.-B.; Simonet, F.; Verkerk, A.O.; Schwartz, P.J.; Crotti, L.; Dagradi, F.; et al. Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat. Genet. 2013, 45, 1044–1049, Erratum in Nat. Genet. 2013, 45, 1409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neu, A.; Eiselt, M.; Paul, M.; Sauter, K.; Stallmeyer, B.; Isbrandt, D.; Schulze-Bahr, E. A homozygous SCN5A mutation in a severe, recessive type of cardiac conduction disease. Hum. Mutat. 2010, 31, E1609-21. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.B.; Gando, I.; Bu, L.; Cecchin, F.; Coetzee, W. A homozygous SCN5A mutation associated with atrial standstill and sudden death. Pacing Clin. Electrophysiol. 2018, 41, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Daumy, X.; Amarouch, M.-Y.; Lindenbaum, P.; Bonnaud, S.; Charpentier, E.; Bianchi, B.; Nafzger, S.; Baron, E.; Fouchard, S.; Thollet, A.; et al. Targeted resequencing identifies TRPM4 as a major gene predisposing to progressive familial heart block type I. Int. J. Cardiol. 2016, 207, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Alfares, A.A.; Kelly, M.A.; McDermott, G.; Funke, B.H.; Lebo, M.S.; Baxter, S.B.; Shen, J.; McLaughlin, H.M.; Clark, E.H.; Babb, L.J.; et al. Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: Expanded panels offer limited additional sensitivity. Genet. Med. 2015, 17, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Day, S.M.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Jacoby, D.; Cirino, A.L.; Fox, J.C.; Lakdawala, N.K.; Ware, J.; et al. For the SHaRe Investigators. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: Insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 2018, 138, 1387–1398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazzarotto, F.; Tayal, U.; Buchan, R.J.; Midwinter, W.; Wilk, A.; Whiffin, N.; Govind, R.; Mazaika, E.; de Marvao, A.; Dawes, T.J.; et al. Reevaluating the genetic contribution of monogenic dilated cardiomyopathy. Circulation 2020, 141, 387–398. [Google Scholar] [CrossRef]
- Haas, J.; Frese, K.S.; Peil, B.; Kloos, W.; Keller, A.; Nietsch, R.; Feng, Z.; Müller, S.; Kayvanpour, E.; Vogel, B.; et al. Atlas of the clinical genetics of human dilated cardiomyopathy. Eur. Heart J. 2015, 36, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Gerull, B.; Brodehl, A. Insights into Genetics and Pathophysiology of Arrhythmogenic Cardiomyopathy. Curr. Heart Fail Rep. 2021, 18, 378–390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ackerman, M.J.; Priori, S.G.; Willems, S.; Berul, C.; Brugada, R.; Calkins, H.; Camm, A.J.; Ellinor, P.T.; Gollob, M.; Hamilton, R.; et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm 2011, 8, 1308–1339. [Google Scholar] [CrossRef]
- Chirita-Emandi, A.; Andreescu, N.; Zimbru, C.G.; Tutac, P.; Arghirescu, S.; Serban, M.; Puiu, M. Challenges in reporting pathogenic/potentially pathogenic variants in 94 cancer predisposing genes—In pediatric patients screened with NGS panels. Sci. Rep. 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lincoln, S.E.; Kobayashi, Y.; Anderson, M.J.; Yang, S.; Desmond, A.J.; Mills, M.A.; Nilsen, G.B.; Jacobs, K.B.; Monzon, F.A.; Kurian, A.W.; et al. A Systematic Comparison of Traditional and Multigene Panel Testing for Hereditary Breast and Ovarian Cancer Genes in More Than 1000 Patients. J. Mol. Diagn. 2015, 17, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, S.E.; Truty, R.; Lin, C.-F.; Zook, J.M.; Paul, J.; Ramey, V.H.; Salit, M.; Rehm, H.L.; Nussbaum, R.L.; Lebo, M.S. A Rigorous Interlaboratory Examination of the Need to Confirm Next-Generation Sequencing–Detected Variants with an Orthogonal Method in Clinical Genetic Testing. J. Mol. Diagn. 2019, 21, 318–329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burri, H.; Prinzen, F.W.; Gasparini, M.; Leclercq, C. Left univentricular pacing for cardiac resynchronization therapy. Europace 2017, 19, 912–919. [Google Scholar] [CrossRef]
- Køber, L.; Thune, J.J.; Nielsen, J.C.; Haarbo, J.; Videbæk, L.; Korup, E.; Jensen, G.; Hildebrandt, P.; Steffensen, F.H.; Bruun, N.E.; et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N. Engl. J. Med. 2016, 375, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Thuillot, M.; Maupain, C.; Gandjbakhch, E.; Waintraub, X.; Hidden-Lucet, F.; Isnard, R.; Ader, F.; Rouanet, S.; Richard, P.; Charron, P. External validation of risk factors for malignant ventricular arrhythmias in lamin A/C mutation carriers. Eur. J. Heart Fail 2019, 21, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Kumar, S.; Elliott, P.; Kalman, J.M.; Fatkin, D. Arrhythmic genotypes in familial dilated cardiomyopathy: Implications for genetic testing and clinical management. Heart Lung Circ. 2019, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Genga, M.F.; Cuenca, S.; Ferro, M.D.; Zorio, E.; Salgado-Aranda, R.; Climent, V.; Padrón-Barthe, L.; Duro-Aguado, I.; Jiménez-Jáimez, J.; Hidalgo-Olivares, V.M.; et al. Truncating FLNC mutations are associated with high-risk dilated and arrhythmogenic cardiomyopathies. J. Am. Coll. Cardiol. 2016, 68, 2440–2451. [Google Scholar] [CrossRef] [PubMed]
- Wahbi, K.; Béhin, A.; Charron, P.; Dunand, M.; Richard, P.; Meune, C.; Vicart, P.; Laforêt, P.; Stojkovic, T.; Bécane, H.M.; et al. High cardiovascular morbidity and mortality in myofibrillar myopathies due to DES gene mutations: A 10-year longitudinal study. Neuromuscul. Disord. 2012, 22, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Gigli, M.; Merlo, M.; Graw, S.L.; Barbati, G.; Rowland, T.J.; Slavov, D.B.; Stolfo, D.; Haywood, M.E.; Ferro, M.D.; Altinier, A.; et al. Genetic risk of arrhythmic phenotypes in patients with dilated cardiomyopathy. J. Am. Coll. Cardiol. 2019, 74, 1480–1490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arbustini, E.; Narula, N.; Dec, G.W.; Reddy, K.S.; Greenberg, B.; Kushwaha, S.; Marwick, T.; Pinney, S.; Bellazzi, R.; Favalli, V.; et al. The MOGE(S) classification for a phenotype-genotype nomenclature of cardiomyopathy: Endorsed by the World Heart Federation. J. Am. Coll. Cardiol. 2013, 62, 2046–2072. [Google Scholar] [CrossRef]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; De Groote, P.; Imazio, M.; et al. MProposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef]
- Bondue, A.; Arbustini, E.; Bianco, A.; Ciccarelli, M.; Dawson, D.; De Rosa, M.; Hamdani, N.; Hilfiker-Kleiner, D.; Meder, B.; Leite-Moreira, A.F.; et al. Complex roads from genotype to phenotype in dilated cardiomyopathy: Scientific update from the Working Group of Myocardial Function of the European Society of Cardiology. Cardiovasc. Res. 2018, 114, 1287–1303. [Google Scholar] [CrossRef]
- Hazebroek, M.; Kemna, M.; Schalla, S.; Wijk, S.S.-V.; Gerretsen, S.; Dennert, R.; Merken, J.; Kuznetsova, T.; Staessen, J.; Rocca, H.B.-L.; et al. Prevalence and prognostic relevance of cardiac involvement in ANCA-associated vasculitis: Eosinophilic granulomatosis with polyangiitis and granulomatosis with polyangiitis. Int. J. Cardiol. 2015, 199, 170–179. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 4901. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2024, 45, 53. [Google Scholar] [CrossRef]
- Beggs, S.A.S.; Jhund, P.S.; Jackson, C.E.; McMurray, J.J.V.; Gardner, R.S. Non-ischaemic cardiomyopathy, sudden death and implantable defibrillators: A review and meta-analysis. Heart 2018, 104, 144–150. [Google Scholar] [CrossRef]
- Stroud, M.J.; Fang, X.; Zhang, J.; Guimarães-Camboa, N.; Veevers, J.; Dalton, N.D.; Gu, Y.; Bradford, W.H.; Peterson, K.L.; Evans, S.M.; et al. Luma is not essential for murine cardiac development and function. Cardiovasc. Res. 2018, 114, 378–388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zink, M.; Seewald, A.; Rohrbach, M.; Brodehl, A.; Liedtke, D.; Williams, T.; Childs, S.J.; Gerull, B. Decreased survival and cardiac performance of mutant TMEM43 in transgenic zebrafish. Circulation 2018, 138 (Suppl. 1), A15878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ratnavadivel, S.; de Toledo, M.S.; Rasmussen, T.B.; Šarić, T.; Gummert, J.; Zenke, M.; Milting, H. Human pluripotent stem cell line (HDZi001-A) derived from a patient carrying the ARVC-5 associated mutation TMEM43-p S358L. Stem Cell Res. 2020, 48, 101957. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.S.; Krahn, A.D.; Bashir, J.; Amit, G.; McIntyre, W.F.; Tsang, B.; Joza, J.; Exner, D.V.; Birnie, D.H.; Sadek, M.; et al. Perioperative Safety andarly Patient and Device Outcomes Among Subcutaneous Versus Transvenous Implantable Cardioverter Defibrillator Implantations: A Randomized, Multicenter Trial. Ann. Intern. Med. 2022, 175, 1658–1665. [Google Scholar] [CrossRef]
- Wang, W.; Gasperetti, A.; Sears, S.F.; Tichnell, C.; Murray, B.; Tandri, H.; James, C.A.; Calkins, H. Subcutaneous and Transvenous Defibrillators in Arrhythmogenic Right Ventricular Cardiomyopathy: A Comparison of Clinical and Quality-of-Life Outcomes. JACC Clin. Electrophysiol. 2023, 9, 394–402. [Google Scholar] [CrossRef]
- Honarbakhsh, S.; Protonotarios, A.; Monkhouse, C.; Hunter, R.J.; Elliott, P.M.; Lambiase, P.D. Right ventricular function is a predictor for sustained ventricular tachycardia requiring anti-tachycardic pacing in arrhythmogenic ventricular cardiomyopathy: Insight into transvenous vs. subcutaneous implantable cardioverter defibrillator insertion. Europace 2023, 25, euad073. [Google Scholar] [CrossRef]
- McNally, E.M.; Mestroni, L. Dilated cardiomyopathy: Genetic determinants and mechanisms. Circ. Res. 2017, 121, 731–748. [Google Scholar] [CrossRef]
- Herman, D.S.; Lam, L.; Taylor, M.R.; Wang, L.; Teekakirikul, P.; Christodoulou, D.; Conner, L.; DePalma, S.R.; McDonough, B.; Sparks, E.; et al. Truncations of titin causing dilated cardiomyopathy. N. Engl. J. Med. 2012, 366, 619–628. [Google Scholar] [CrossRef]
- Chauveau, C.; Rowell, J.; Ferreiro, A. A rising titan: TTN review and mutation update. Hum. Mutat. 2014, 35, 1046–1059. [Google Scholar] [CrossRef]
- Kayvanpour, E.; Sedaghat-Hamedani, F.; Amr, A.; Lai, A.; Haas, J.; Holzer, D.B.; Frese, K.S.; Keller, A.; Jensen, K.; Katus, H.A.; et al. Genotype-phenotype associations in dilated cardiomyopathy: Meta-analysis on more than 8000 individuals. Clin. Res. Cardiol. 2017, 106, 127–139. [Google Scholar] [CrossRef]
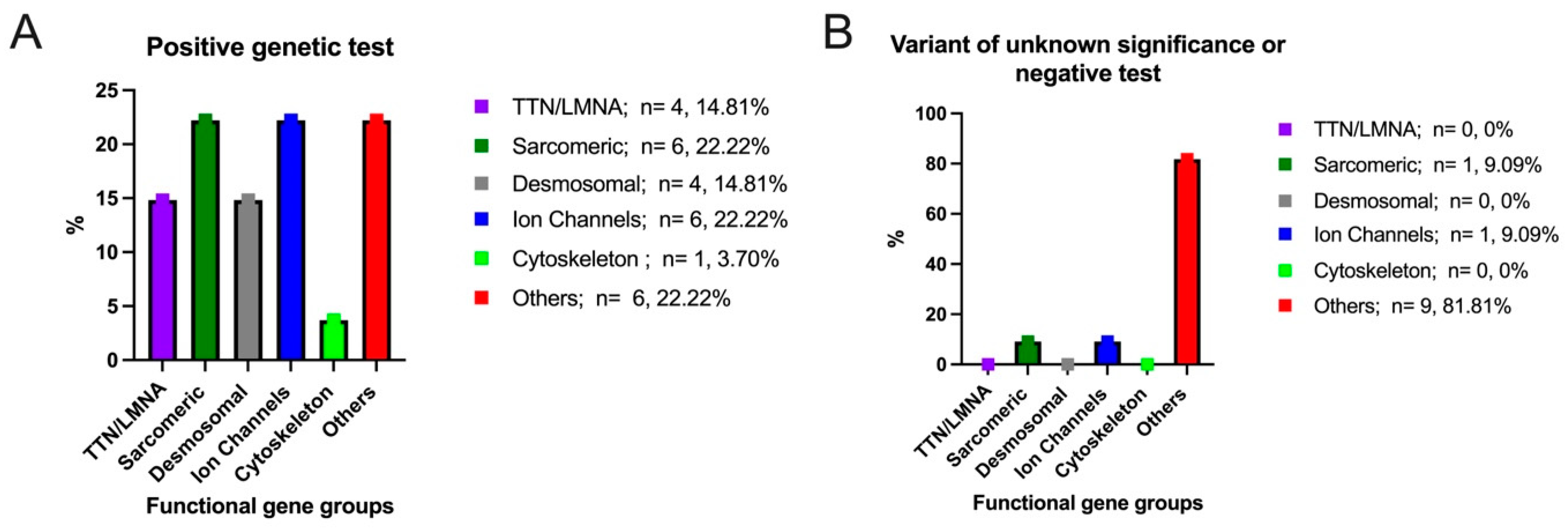
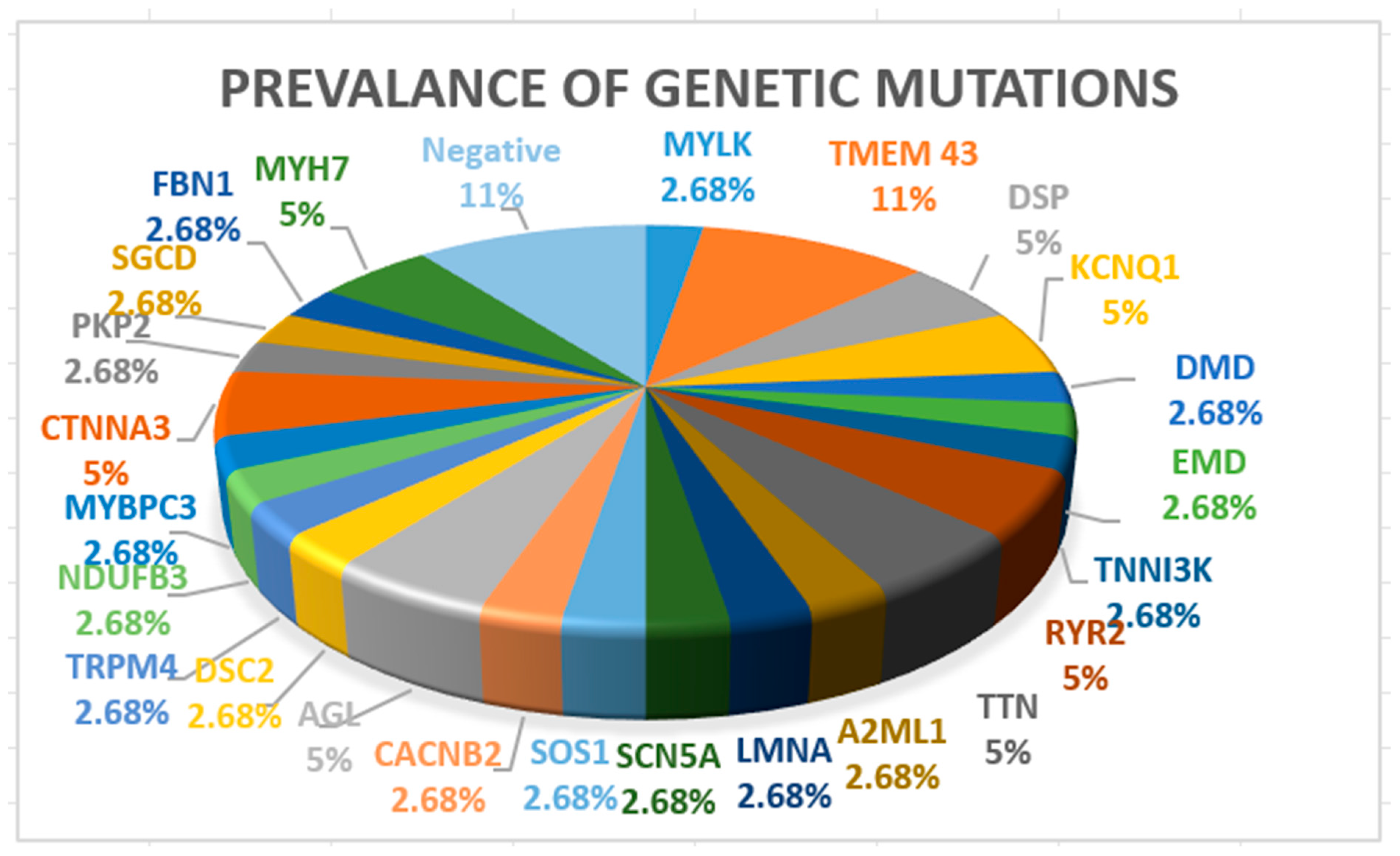
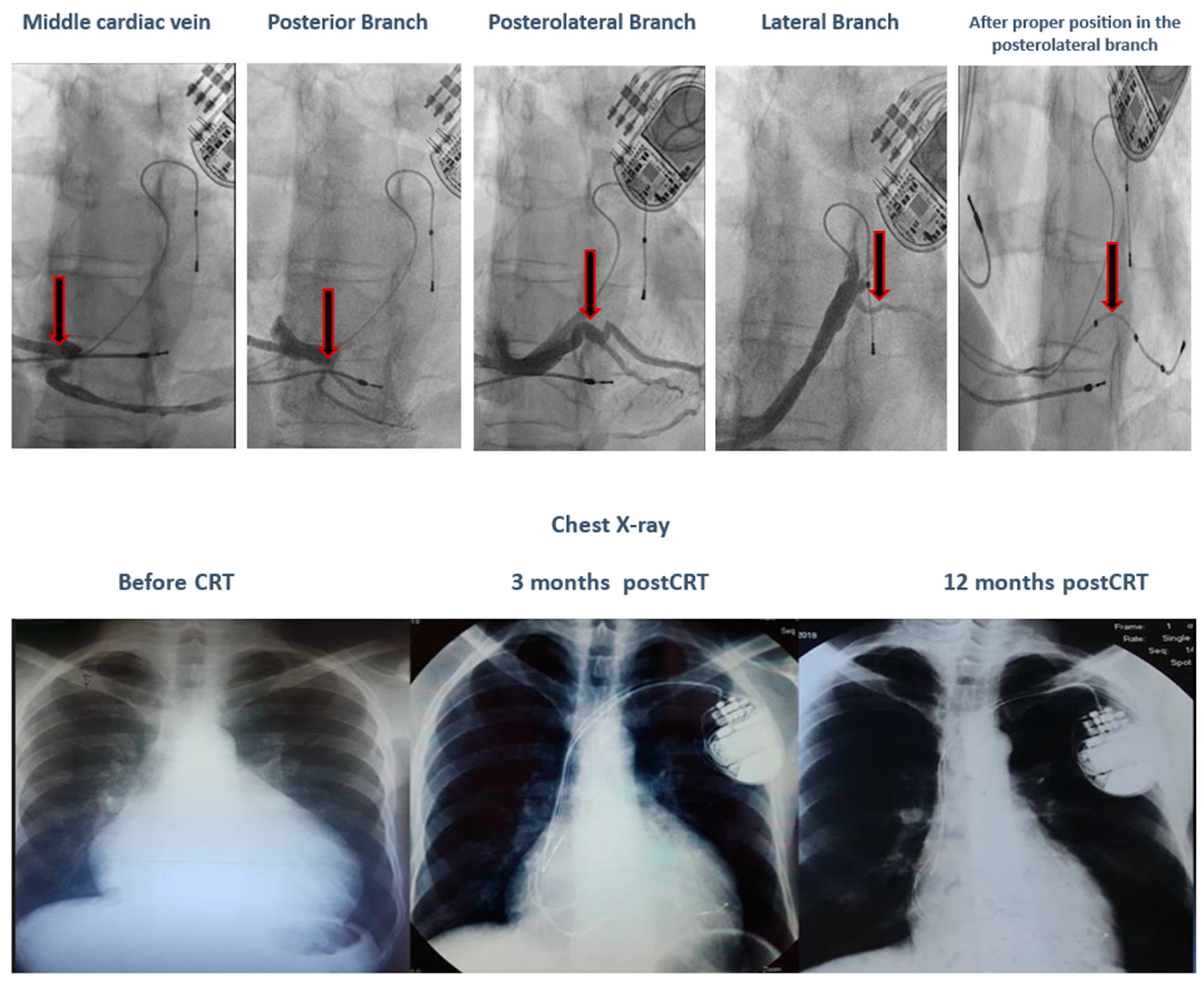
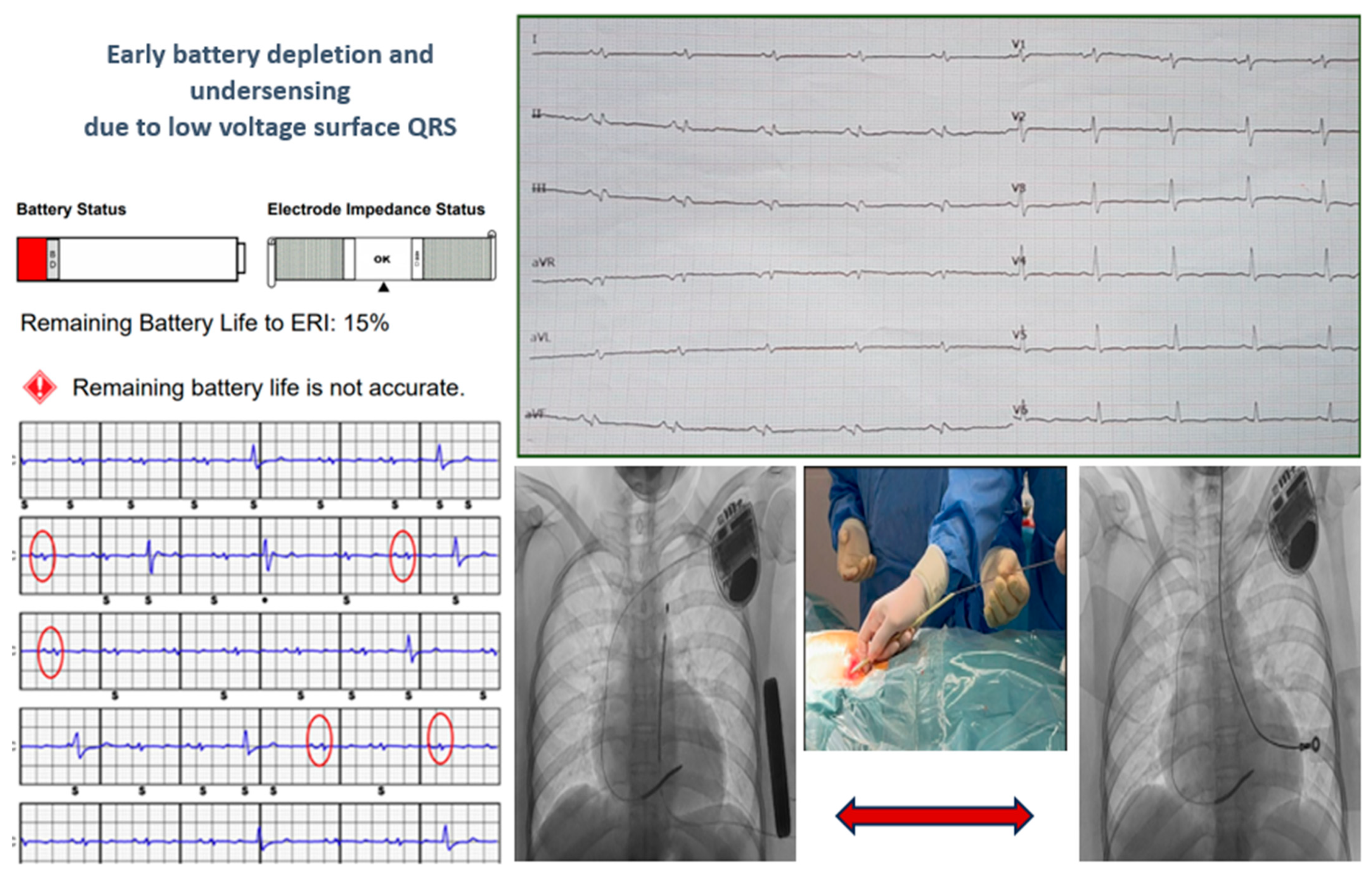
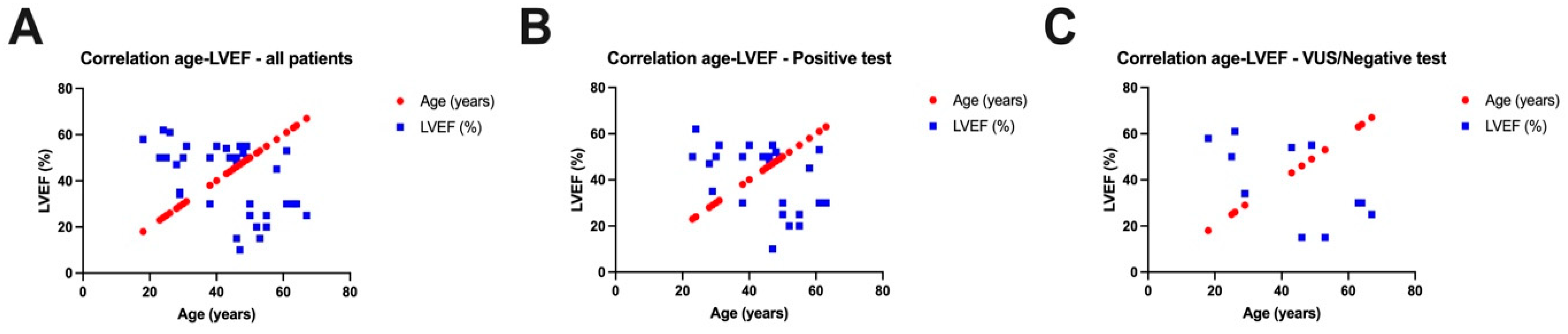
| Male gender, % | 22 (58%) | |
| Age, y.o., mean ± SD | 44.5 ± 13.1 | |
| Main arrhythmia or symptoms leading to cardiac evaluation | Cardiac arrest | 10 (26%) |
| Ventricular tachycardia | 34 (34%) | |
| Atrial fibrillation | 3 (8%) | |
| 3rd degree atrioventricular block | 5 (13%) | |
| Heart failure symptoms | 2 (5%) | |
| Syncope | 5 (13%) | |
| Associated pathology, n, % | Hypertension | 5 (13%) |
| Coronary artery disease | 3 (8%) | |
| Diabetes Mellitus | 3 (8%) | |
| COPD | 1 (3%) | |
| LVEF (%) | 40.3 ± 14.7 | |
| Severe MR, n, % | 5 (13%) | |
| Moderate MR, n, % | 13 (34%) | |
| Mild MR, n, % | 14 (37%) | |
| Severe TR, n, % | 1 (3%) | |
| Moderate TR, n, % | 8 (21%) | |
| Mild TR, n, % | 24 (63%) |
| Medical Treatment | N, % |
|---|---|
| Bblockers | 25 (66%) |
| Ivabradine | 1 (3%) |
| Class Ic antiarrhythmic | 2 (5%) |
| Class III antiarrhythmic | 14 (37%) |
| NOAC | 6 (16%) |
| Antialdosteronics | 16 (42%) |
| SGLT2 inhibitors | 9 (24%) |
| ARB + ARNI | 13 (34%) |
| ACEI | 6 (16%) |
| Sex | Age (Years) | Gene | OMIM Number | Transcript | Zygosity | Classification |
|---|---|---|---|---|---|---|
| M | 49 | TMEM43 | #612048 | c.1073C>T (p.Ser358Leu) | heterozygous | P |
| M | 50 | TMEM43&TTN | #612048𮆨 | c.1073C>T (p.Ser358Leu)&c.107635C>T (p.Gln35879*) | heterozygous | P&P |
| F | 28 | DSP | #125647 | Deletion (Exons 7-10) | heterozygous | LP |
| M | 30 | TMEM43 | #612048 | c.1073C>T (p.Ser358Leu) | heterozygous | P |
| M | 49 | DSP | #125647 | c.939C>T (Silent) | heterozygous | LP |
| M | 48 | KCNQ1 | #607542 | c.691C>T (p.Arg231Cys) | heterozygous | P |
| F | 44 | KCNQ1 | #607542 | c.604G>A (p.Asp202Asn) | heterozygous | P |
| M | 29 | DMD | #300377 | Deletion (Exons 45-47) | hemizygous | P |
| M | 24 | EMD | #300384 | c.187+1G>A (Splice donor) | hemizygous | P |
| F | 50 | TNNI3K | #613932 | c.2302G>A (p.Glu768Lys) | heterozygous | P |
| M | 52 | RYR2 | #180902 | c.10631C>G (p.Pro3544Arg) | heterozygous | LP |
| M | 50 | TTN | #188840 | c.93166C>T (p.Arg31056*) | heterozygous | LP |
| F | 40 | LMNA | #150330 | c.604G>T (p.Glu202*) | heterozygous | P |
| F | 47 | SCN5A | #600163 | c.5971C>T (p.Arg1991Trp) | heterozygous | LP |
| F | 61 | DSC2 | #125645 | c.397G>A (p.Ala133Thr) | heterozygous | LP |
| M | 31 | TRPM4 | #606936 | c.1127T>C (p.Ile376Thr) | heterozygous | P |
| M | 63 | MYBPC3 | #600958 | c.712C>T (p.Arg238Cys) | heterozygous | LP |
| F | 23 | CTNNA3 | #607667 | Deletion (Exon 10) | heterozygous | LP |
| M | 38 | SCN5A | #600163 | c.2989G>A (p.Ala997Thr) | heterozygous | LP |
| M | 46 | RYR2 | #180902 | c.5776G>A (p.Val1926IIe) | heterozygous | LP |
| F | 45 | MYH7 | #160760 | c.1615A>G (p.Met539Val) | heterozygous | LP |
| M | 47 | PKP2 | #602861 | c.1510+1G>T | heterozygous | LP |
| M | 38 | TTN | #188840 | c.69224delA | heterozygous | LP |
| M | 55 | SGCD | #601411 | c.448T>G | heterozygous | VUS |
| F | 61 | TMEM43 | #612048 | c.1073C>T (p.Ser358Leu) | heterozygous | P |
| F | 55 | FBN1 | #134797 | c.718C>T | heterozygous | P |
| F | 58 | MYH7 | #160760 | c.1615A >G | heterozygous | LP |
| M | 46 | MYLK | #600922 | c.3610C>T (p.Arg1204Trp) | heterozygous | VUS |
| M | 53 | A2ML1 | #610627 | c.2464G>A (p.Val822Ile) | heterozygous | VUS |
| M | 64 | SOS1 | #182530 | c.2165G>A (p.Arg722Lys) | heterozygous | VUS |
| F | 26 | CACNB2 | #600003 | c.998C>T (p.Thr333Ile) | heterozygous | VUS |
| F | 63 | AGL | #610860 | c.1333A>G (p.Met445Val) | heterozygous | VUS |
| F | 67 | AGL | #610860 | c.3235C>T (p.Gln1079*) | heterozygous | P |
| F | 25 | NDUFB3 | #603839 | c.208G>T (p.Gly70*) | heterozygous | VUS |
| SRs (N = 5, 50%) | Non-SRs (N = 5, 50%) | ||
|---|---|---|---|
| Age, y.o., mean ± SD | 51 ± 3.3 | 63 ± 6.5 | |
| LV lead position n, % | Posterolateral | 2 (40%) | 0 (0%) |
| Lateral | 3 (60%) | 3 (60%) | |
| Posterior | 0 (0%) | 1 (20%) | |
| Anterolateral | 0 (0%) | 1 (20%) | |
| AV paced interval, mean ± SD | 120 ± 7.5 | 110 ± 10 | |
| AV sensed interval, mean ± SD | 100 ± 8 | 90 ± 6.3 | |
| LBBB | Typical pattern | 5 (100%) | 2 (40%) |
| Atypical pattern | 0 (0%) | 3 (60%) | |
| QRS duration mean ± SD | 180 ± 0 | 140 ± 9.8 | |
LVEF (%)
| |||
| 20 ± 7.1 | 30 ± 2.4 | ||
| 50 ± 3.7 | 35 ± 4.4 | ||
Severe MR, n, %
| 3 (60%) | 0 (0%) | |
| 0 (0%) | 0 (0%) | |
Moderate MR, n, %
| 2 (40%) | 5 (100%) | |
| 0 (0%) | 4 (80%) | |
| Genetic testing (gene mutation) | PKP2 | A2ML1 | |
| SGCD | AGL | ||
| TNNI3K | AGL | ||
| MYLK | MYBCP3 | ||
| RYR2 | TTN&TMEM43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goanta, E.-V.; Vacarescu, C.; Tartea, G.; Ungureanu, A.; Militaru, S.; Muraretu, A.; Faur-Grigori, A.-A.; Petrescu, L.; Vătăsescu, R.; Cozma, D. Unexpected Genetic Twists in Patients with Cardiac Devices. J. Clin. Med. 2024, 13, 3801. https://doi.org/10.3390/jcm13133801
Goanta E-V, Vacarescu C, Tartea G, Ungureanu A, Militaru S, Muraretu A, Faur-Grigori A-A, Petrescu L, Vătăsescu R, Cozma D. Unexpected Genetic Twists in Patients with Cardiac Devices. Journal of Clinical Medicine. 2024; 13(13):3801. https://doi.org/10.3390/jcm13133801
Chicago/Turabian StyleGoanta, Emilia-Violeta, Cristina Vacarescu, Georgica Tartea, Adrian Ungureanu, Sebastian Militaru, Alexandra Muraretu, Adelina-Andreea Faur-Grigori, Lucian Petrescu, Radu Vătăsescu, and Dragos Cozma. 2024. "Unexpected Genetic Twists in Patients with Cardiac Devices" Journal of Clinical Medicine 13, no. 13: 3801. https://doi.org/10.3390/jcm13133801
APA StyleGoanta, E.-V., Vacarescu, C., Tartea, G., Ungureanu, A., Militaru, S., Muraretu, A., Faur-Grigori, A.-A., Petrescu, L., Vătăsescu, R., & Cozma, D. (2024). Unexpected Genetic Twists in Patients with Cardiac Devices. Journal of Clinical Medicine, 13(13), 3801. https://doi.org/10.3390/jcm13133801






