Effects of Combined Low-Dose Spironolactone Plus Vitamin E versus Vitamin E Monotherapy on Lipidomic Profile in Non-Alcoholic Fatty Liver Disease: A Post Hoc Analysis of a Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Serum Lipidomic Analysis
2.3. Liquid Chromatography–Mass Spectrometry Analysis
2.4. Lipids Identification and Quantification
2.5. Data Analysis and Visualization
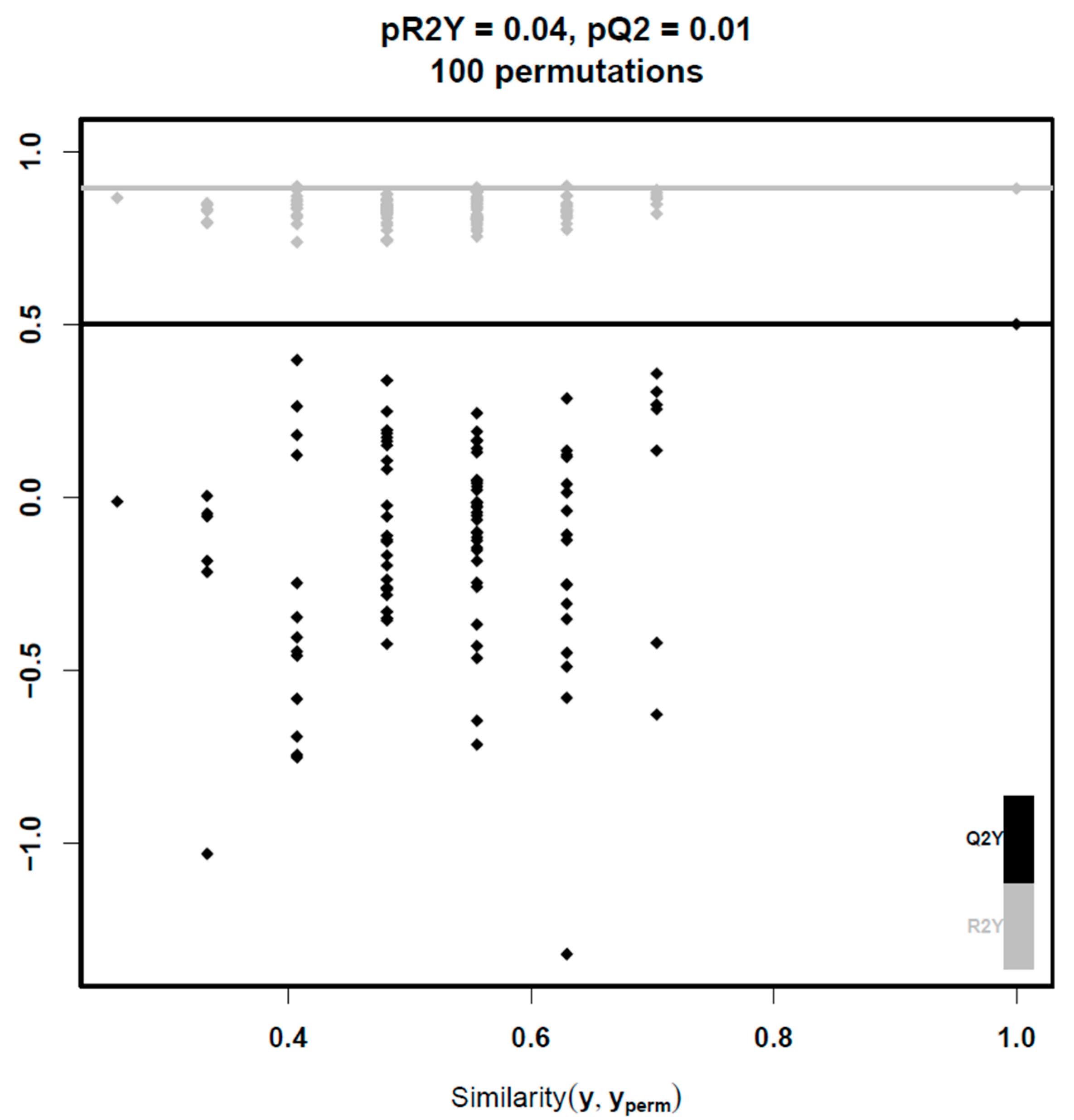
3. Results
3.1. Univariate Analysis
3.2. Multivariate Analysis
3.3. Linear Regression Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perakakis, N.; Polyzos, S.A.; Yazdani, A.; Sala-Vila, A.; Kountouras, J.; Anastasilakis, A.D.; Mantzoros, C.S. Non-invasive diagnosis of non-alcoholic steatohepatitis and fibrosis with the use of omics and supervised learning: A proof of concept study. Metabolism 2019, 101, 154005. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Mantzoros, C.S. Making progress in nonalcoholic fatty liver disease (NAFLD) as we are transitioning from the era of NAFLD to dys-metabolism associated fatty liver disease (DAFLD). Metabolism 2020, 111, 154318. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kang, E.S.; Boutari, C.; Rhee, E.-J.; Mantzoros, C.S. Current and emerging pharmacological options for the treatment of nonalcoholic steatohepatitis. Metabolism 2020, 111S, 154203. [Google Scholar] [CrossRef]
- Kartsoli, S.; Kostara, C.E.; Tsimihodimos, V.; Bairaktari, E.T.; Christodoulou, D.K. Lipidomics in non-alcoholic fatty liver disease. World J Hepatol. 2020, 12, 436–450. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P.; Mantzoros, C.S. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism 2016, 65, 1109–1123. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kechagias, S.; Tsochatzis, E.A. Review article: Non-alcoholic fatty liver disease and cardiovascular diseases: Associations and treatment considerations. Aliment. Pharmacol. Ther. 2021, 54, 1013–1025. [Google Scholar] [CrossRef]
- Perakakis, N.; Stefanakis, K.; Mantzoros, C.S. The role of omics in the pathophysiology, diagnosis and treatment of non-alcoholic fatty liver disease. Metabolism 2020, 111S, 154320. [Google Scholar] [CrossRef]
- Béland-Bonenfant, S.; Rouland, A.; Petit, J.-M.; Vergès, B. Concise review of lipidomics in nonalcoholic fatty liver disease. Diabetes Metab. 2023, 49, 101432. [Google Scholar] [CrossRef]
- Raza, S.; Rajak, S.; Upadhyay, A.; Tewari, A.; Anthony Sinha, R. Current treatment paradigms and emerging therapies for NAFLD/NASH. Front. Biosci. (Landmark Ed.) 2021, 26, 206–237. [Google Scholar] [CrossRef]
- Nagashimada, M.; Ota, T. Role of vitamin E in nonalcoholic fatty liver disease. IUBMB Life 2019, 71, 516–522. [Google Scholar] [CrossRef]
- Leoni, S.; Tovoli, F.; Napoli, L.; Serio, I.; Ferri, S.; Bolondi, L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J. Gastroenterol. 2018, 24, 3361–3373. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef]
- Papaefthymiou, A.; Doulberis, M.; Karafyllidou, K.; Chatzimichael, E.; Deretzi, G.; Exadaktylos, A.K.; Sampsonas, F.; Gelasakis, A.; Papamichos, S.I.; Kotronis, G.; et al. Effect of spironolactone on pharmacological treatment of nonalcoholic fatty liver disease. Minerva Endocrinol. 2023, 48, 346–359. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Cleland, J.G.; Ferreira, J.P.; Mariottoni, B.; Pellicori, P.; Cuthbert, J.; Verdonschot, J.A.; Petutschnigg, J.; Ahmed, F.Z.; Cosmi, F.; Brunner La Rocca, H.P.; et al. The effect of spironolactone on cardiovascular function and markers of fibrosis in people at increased risk of developing heart failure: The heart “OMics” in AGEing (HOMAGE) randomized clinical trial. Eur. Heart J. 2021, 42, 684–696. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S.; Polymerou, V.; Katsinelos, P. Effects of combined low-dose spironolactone plus vitamin E vs. vitamin E monotherapy on insulin resistance, non-invasive indices of steatosis and fibrosis, and adipokine levels in non-alcoholic fatty liver disease: A randomized controlled trial. Diabetes Obes. Metab. 2017, 19, 1805–1809. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Zafeiriadou, E.; Patsiaoura, K.; Katsiki, E.; Deretzi, G.; Zavos, C.; Tsarouchas, G.; Rakitzi, P.; Slavakis, A. Effect of spironolactone and vitamin E on serum metabolic parameters and insulin resistance in patients with nonalcoholic fatty liver disease. J. Renin Angiotensin Aldosterone Syst. 2011, 12, 498–503. [Google Scholar] [CrossRef]
- Castañé, H.; Baiges-Gaya, G.; Hernández-Aguilera, A.; Rodríguez-Tomàs, E.; Fernández-Arroyo, S.; Herrero, P.; Delpino-Rius, A.; Canela, N.; Menendez, J.A.; Camps, J.; et al. Coupling Machine Learning and Lipidomics as a Tool to Investigate Metabolic Dysfunction-Associated Fatty Liver Disease. A General Overview. Biomolecules 2021, 11, 473. [Google Scholar] [CrossRef]
- Checa, A.; Bedia, C.; Jaumot, J. Lipidomic data analysis: Tutorial, practical guidelines and applications. Anal. Chim. Acta 2015, 885, 1–16. [Google Scholar] [CrossRef]
- Krug, A.W.; Stelzner, L.; Rao, A.D.; Lichtman, A.H.; Williams, G.H.; Adler, G.K. Effect of low dose mineralocorticoid receptor antagonist eplerenone on glucose and lipid metabolism in healthy adult males. Metabolism 2013, 62, 386–391. [Google Scholar] [CrossRef]
- Lin, M.; Heizati, M.; Wang, L.; Nurula, M.; Yang, Z.; Wang, Z.; Abudoyreyimu, R.; Wu, Z.; Li, N. A systematic review and meta-analysis of effects of spironolactone on blood pressure, glucose, lipids, renal function, fibrosis and inflammation in patients with hypertension and diabetes. Blood Press. 2021, 30, 145–153. [Google Scholar] [CrossRef]
- Muneyyirci-Delale, O.; Kaplan, J.; Joulak, I.; Yang, L.; Von Gizycki, H.; Nacharaju, V.L. Serum free fatty acid levels in PCOS patients treated with glucophage, magnesium oxide and spironolactone. Gynecol. Endocrinol. 2013, 29, 474–477. [Google Scholar] [CrossRef]
- Šmíd, V.; Dvořák, K.; Šedivý, P.; Kosek, V.; Leníček, M.; Dezortová, M.; Hajšlová, J.; Hájek, M.; Vítek, L.; Bechyňská, K.; et al. Effect of Omega-3 Polyunsaturated Fatty Acids on Lipid Metabolism in Patients With Metabolic Syndrome and NAFLD. Hepatol. Commun. 2022, 6, 1336–1349. [Google Scholar] [CrossRef]
- Nording, M.L.; Yang, J.; Georgi, K.; Karbowski, C.H.; German, J.B.; Weiss, R.H.; Hogg, R.J.; Trygg, J.; Hammock, B.D.; Zivkovic, A.M. Individual variation in lipidomic profiles of healthy subjects in response to omega-3 Fatty acids. PLoS ONE 2013, 8, e76575. [Google Scholar] [CrossRef]
- Pereira, S.; Cline, D.L.; Glavas, M.M.; Covey, S.D.; Kieffer, T.J. Tissue-Specific Effects of Leptin on Glucose and Lipid Metabolism. Endocr. Rev. 2021, 42, 1–28. [Google Scholar] [CrossRef]
- Puri, P.; Baillie, R.A.; Wiest, M.M.; Mirshahi, F.; Choudhury, J.; Cheung, O.; Sargeant, C.; Contos, M.J.; Sanyal, A.J. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007, 46, 1081–1090. [Google Scholar] [CrossRef]
- Kalhan, S.C.; Guo, L.; Edmison, J.; Dasarathy, S.; McCullough, A.J.; Hanson, R.W.; Milburn, M. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 2011, 60, 404–413. [Google Scholar] [CrossRef]
- Chiappini, F.; Coilly, A.; Kadar, H.; Gual, P.; Tran, A.; Desterke, C.; Samuel, D.; Duclos-Vallée, J.-C.; Touboul, D.; Bertrand-Michel, J.; et al. Metabolism dysregulation induces a specific lipid signature of nonalcoholic steatohepatitis in patients. Sci. Rep. 2017, 7, 46658. [Google Scholar] [CrossRef]
- Deng, K.-Q.; Huang, X.; Lei, F.; Zhang, X.-J.; Zhang, P.; She, Z.-G.; Cai, J.; Ji, Y.-X.; Li, H. Role of hepatic lipid species in the progression of nonalcoholic fatty liver disease. Am. J. Physiol. Cell Physiol. 2022, 323, C630–C639. [Google Scholar] [CrossRef]
- López-Vicario, C.; González-Périz, A.; Rius, B.; Morán-Salvador, E.; García-Alonso, V.; Lozano, J.J.; Bataller, R.; Cofán, M.; Kang, J.X.; Arroyo, V.; et al. Molecular interplay between Δ5/Δ6 desaturases and long-chain fatty acids in the pathogenesis of non-alcoholic steatohepatitis. Gut 2014, 63, 344–355. [Google Scholar] [CrossRef]
- Caussy, C.; Chuang, J.-C.; Billin, A.; Hu, T.; Wang, Y.; Subramanian, G.M.; Djedjos, C.S.; Myers, R.P.; Dennis, E.A.; Loomba, R. Plasma eicosanoids as noninvasive biomarkers of liver fibrosis in patients with nonalcoholic steatohepatitis. Ther. Adv. Gastroenterol. 2020, 13, 1756284820923904. [Google Scholar] [CrossRef]
- Nikolova-Karakashian, M. Sphingolipids at the Crossroads of NAFLD and Senescence. In Advances in Cancer Research; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 155–190. [Google Scholar]
- Régnier, M.; Polizzi, A.; Guillou, H.; Loiseau, N. Sphingolipid metabolism in non-alcoholic fatty liver diseases. Biochimie 2019, 159, 9–22. [Google Scholar] [CrossRef]
- Tiwari-Heckler, S.; Gan-Schreier, H.; Stremmel, W.; Chamulitrat, W.; Pathil, A. Circulating Phospholipid Patterns in NAFLD Patients Associated with a Combination of Metabolic Risk Factors. Nutrients 2018, 10, 649. [Google Scholar] [CrossRef]
- Zhou, Y.; Orešič, M.; Leivonen, M.; Gopalacharyulu, P.; Hyysalo, J.; Arola, J.; Verrijken, A.; Francque, S.; Van Gaal, L.; Hyötyläinen, T.; et al. Noninvasive Detection of Nonalcoholic Steatohepatitis Using Clinical Markers and Circulating Levels of Lipids and Metabolites. Clin. Gastroenterol. Hepatol. 2016, 14, 1463–1472.e6. [Google Scholar] [CrossRef]
- Barr, J.; Vázquez-Chantada, M.; Alonso, C.; Pérez-Cormenzana, M.; Mayo, R.; Galán, A.; Caballería, J.; Martín-Duce, A.; Tran, A.; Wagner, C.; et al. Liquid chromatography-mass spectrometry-based parallel metabolic profiling of human and mouse model serum reveals putative biomarkers associated with the progression of nonalcoholic fatty liver disease. J. Proteome Res. 2010, 9, 4501–4512. [Google Scholar] [CrossRef]
- Peng, K.-Y.; Watt, M.J.; Rensen, S.S.; Greve, J.W.; Huynh, K.; Jayawardana, K.S.; Meikle, P.J.; Meex, R.C.R. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J. Lipid Res. 2018, 59, 1977–1986. [Google Scholar] [CrossRef]
- Gorden, D.; Myers, D.S.; Ivanova, P.T.; Fahy, E.; Maurya, M.R.; Gupta, S.; Min, J.; Spann, N.J.; McDonald, J.G.; Kelly, S.L.; et al. Biomarkers of NAFLD progression: A lipidomics approach to an epidemic. J. Lipid Res. 2015, 56, 722–736. [Google Scholar] [CrossRef]

| Characteristic | VitE Group (n = 15) | SPL + VitE Group (n = 12) | p-Value † |
|---|---|---|---|
| Women/Men (n) | 9/6 | 11/1 | 0.09 |
| Omega-3 Supplementation [n (%)] | 3 (20%) | 2 (17%) | 0.99 |
| Age (years) | 54.0 ± 3.7 | 56.8 ± 1.8 | 0.62 |
| BMI (kg/m2) | 34.1 ± 1.8 | 32.3 ± 1.5 | 0.49 |
| Waist circumference (cm) | 110 ± 4 | 105 ± 3 | 0.43 |
| Systolic blood pressure (mmHg) | 130 ± 5 | 132 ± 2 | 0.90 |
| Diastolic blood pressure (mmHg) | 83 ± 4 | 84 ± 2 | 0.71 |
| AST (U/L) | 39 ± 8 | 29 ± 2 | 0.94 |
| ALT (U/L) | 52 ± 11 | 36 ± 4 | 0.51 |
| GGT (U/L) | 61 ± 16 | 37 ± 6 | 0.94 |
| Total cholesterol (mg/dL) | 219 ± 9 | 231 ± 14 | 0.57 |
| Triglycerides (mg/dL) | 219 ± 31 | 205 ± 21 | 0.99 |
| HDL-C (mg/dL) | 49 ± 2 | 51 ± 3 | 0.61 |
| LDL-C (mg/dL) | 127 ± 10 | 139 ± 11 | 0.52 |
| Glucose (mg/dL) | 106 ± 7 | 103 ± 6 | 0.85 |
| Insulin (μU/mL) | 12.0 ± 2.5 | 12.7 ± 3.0 | 0.83 |
| HOMA-IR | 3.2 ± 0.7 | 3.3 ± 0.7 | 0.83 |
| Lipid Molecule † | Q-Value | FC ‡ | RSD% in QC Samples |
|---|---|---|---|
| CE 16:1 | <0.001 | 1.094 | 3.589 |
| CE 20:4 | <0.001 | 0.821 | 10.924 |
| CE 20:5 | <0.001 | 1.742 | 2.988 |
| CE 22:6 | <0.001 | 1.405 | 6.470 |
| DG 34:1 | <0.001 | 1.106 | 4.861 |
| LPC 22:6 | <0.001 | 1.309 | 6.009 |
| PC 32:2 | 0.042 | 1.040 | 5.801 |
| PC 36:5 | <0.001 | 1.072 | 5.044 |
| PC 38:2 | <0.001 | 1.103 | 1.572 |
| PC 38:4 | 0.016 | 0.955 | 4.536 |
| PC 38:5|PC 16:0_22:5 | <0.001 | 1.075 | 3.868 |
| PC 38:5|PC 18:0_20:5 | <0.001 | 1.278 | 1.910 |
| PC 38:6|PC 18:1_20:5 | <0.001 | 1.380 | 8.073 |
| PC 38:6|PC 18:2_20:4 | 0.001 | 0.915 | 9.372 |
| PC 40:5 | <0.001 | 1.186 | 2.177 |
| PC 40:6 | <0.001 | 1.114 | 5.770 |
| PC O-34:2 | <0.001 | 0.787 | 0.909 |
| PC O-34:3 | <0.001 | 0.929 | 1.280 |
| PC O-36:2 | 0.037 | 0.833 | 5.387 |
| PC O-36:5 | <0.001 | 0.903 | 3.099 |
| PC O-38:4 | <0.001 | 0.911 | 6.656 |
| SM 40:2;O2 | <0.001 | 1.121 | 0.851 |
| SM 41:2;O2 | <0.001 | 1.168 | 6.084 |
| TG 42:1 | 0.011 | 1.400 | 10.674 |
| TG 44:1 | <0.001 | 1.132 | 11.708 |
| TG 46:1 | <0.001 | 1.149 | 2.731 |
| TG 46:2 | <0.001 | 1.151 | 11.023 |
| TG 48:1 | 0.001 | 1.040 | 6.055 |
| TG 48:2 | <0.001 | 1.067 | 6.009 |
| TG 48:3 | 0.014 | 1.065 | 10.302 |
| TG 50:0 | <0.001 | 0.906 | 1.938 |
| TG 52:0 | 0.005 | 0.828 | 14.065 |
| TG 52:1 | <0.001 | 1.072 | 6.095 |
| TG 52:5 | <0.001 | 1.079 | 13.191 |
| TG 54:6 | 0.008 | 0.807 | 14.337 |
| TG 56:7 | <0.001 | 1.464 | 11.718 |
| Lipid Molecule † | Q-Value | FC ‡ | RSD% in QC Samples |
|---|---|---|---|
| FA 20:5 | 0.024 | 1.731 | 3.703 |
| FA 22:6 | <0.001 | 1.364 | 2.322 |
| LPC 16:0 | <0.001 | 0.989 | 2.111 |
| LPC 18:0 | <0.001 | 1.017 | 1.837 |
| PC 34:1 | <0.001 | 1.085 | 3.446 |
| SM 32:1;O2 | <0.001 | 1.172 | 3.561 |
| SM 34:2;O2 | <0.001 | 1.141 | 3.137 |
| Lipid Molecule † | Q-Value | VIP | FC ‡ | RSD% in QC Samples |
|---|---|---|---|---|
| FA 16:1 | 0.006 | 1.499 | 0.886 | 3.929 |
| FA 20:5 | <0.001 | 1.836 | 1.731 | 3.703 |
| FA 22:5 | 0.046 | 1.354 | 1.182 | 2.635 |
| FA 22:6 | <0.001 | 1.739 | 1.364 | 2.322 |
| PC 36:5 | <0.001 | 1.323 | 1.454 | 1.390 |
| SM 42:3;O2 | <0.001 | 1.427 | 1.256 | 3.679 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semertzidis, A.; Mouskeftara, T.; Gika, H.; Pousinis, P.; Makedou, K.; Goulas, A.; Kountouras, J.; Polyzos, S.A. Effects of Combined Low-Dose Spironolactone Plus Vitamin E versus Vitamin E Monotherapy on Lipidomic Profile in Non-Alcoholic Fatty Liver Disease: A Post Hoc Analysis of a Randomized Controlled Trial. J. Clin. Med. 2024, 13, 3798. https://doi.org/10.3390/jcm13133798
Semertzidis A, Mouskeftara T, Gika H, Pousinis P, Makedou K, Goulas A, Kountouras J, Polyzos SA. Effects of Combined Low-Dose Spironolactone Plus Vitamin E versus Vitamin E Monotherapy on Lipidomic Profile in Non-Alcoholic Fatty Liver Disease: A Post Hoc Analysis of a Randomized Controlled Trial. Journal of Clinical Medicine. 2024; 13(13):3798. https://doi.org/10.3390/jcm13133798
Chicago/Turabian StyleSemertzidis, Anastasios, Thomai Mouskeftara, Helen Gika, Petros Pousinis, Kali Makedou, Antonis Goulas, Jannis Kountouras, and Stergios A. Polyzos. 2024. "Effects of Combined Low-Dose Spironolactone Plus Vitamin E versus Vitamin E Monotherapy on Lipidomic Profile in Non-Alcoholic Fatty Liver Disease: A Post Hoc Analysis of a Randomized Controlled Trial" Journal of Clinical Medicine 13, no. 13: 3798. https://doi.org/10.3390/jcm13133798
APA StyleSemertzidis, A., Mouskeftara, T., Gika, H., Pousinis, P., Makedou, K., Goulas, A., Kountouras, J., & Polyzos, S. A. (2024). Effects of Combined Low-Dose Spironolactone Plus Vitamin E versus Vitamin E Monotherapy on Lipidomic Profile in Non-Alcoholic Fatty Liver Disease: A Post Hoc Analysis of a Randomized Controlled Trial. Journal of Clinical Medicine, 13(13), 3798. https://doi.org/10.3390/jcm13133798








