Efficacy and Safety of Direct Oral Anticoagulants versus Warfarin in Obese Patients (BMI ≥ 30 kg/m2) with Atrial Fibrillation or Venous Thromboembolism: An Updated Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Information Sources and Search Strategy
2.2. Eligibility Criteria
2.2.1. Population and Interventions
2.2.2. Outcomes
2.2.3. Study Design
2.2.4. Exclusion Criteria
- Case reports/case series, narrative reviews;
- Expert opinions, dissertations, protocols;
- Including animals and/or in vitro studies.
2.3. Selection Process
2.4. Data Extraction
2.5. Quality Assessments
2.6. Data Analysis
3. Results
3.1. Selection Process and Study Characteristics
3.2. Efficacy Outcomes
3.3. Safety Outcomes
3.4. Subgroup Analysis, Sensitivity Analysis, and Meta-Regression
4. Discussion
4.1. Strengths and Limitations
4.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Obesity Atlas 2023|World Obesity Federation. Available online: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2023 (accessed on 26 December 2023).
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke Off. J. Int. Stroke Soc. 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A.; Global Burden of Cardiovascular Diseases and Risks Collaborators. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Parise, H.; Levy, D.; D’Agostino, R.B.S.; Wolf, P.A.; Vasan, R.S.; Benjamin, E.J. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004, 292, 2471–2477. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhi, H.; Yang, S.; Yu, E.Y.-W.; Wang, L. Body Mass Index and the Risk of Atrial Fibrillation: A Mendelian Randomization Study. Nutrients 2022, 14, 1878. [Google Scholar] [CrossRef] [PubMed]
- Ntinopoulou, P.; Ntinopoulou, E.; Papathanasiou, I.V.; Fradelos, E.C.; Kotsiou, O.; Roussas, N.; Raptis, D.G.; Gourgoulianis, K.I.; Malli, F. Obesity as a Risk Factor for Venous Thromboembolism Recurrence: A Systematic Review. Medicina 2022, 58, 1290. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I.; Farjat, A.E.; Ageno, W.; Turpie, A.G.G.; Haas, S.; Goto, S.; Goldhaber, S.Z.; Angchaisuksiri, P.; Gibbs, H.; GARFIELD-VTE Investigators; et al. Influence of body mass index on clinical outcomes in venous thromboembolism: Insights from GARFIELD-VTE. J. Thromb. Haemost. 2021, 19, 3031–3043. [Google Scholar] [CrossRef] [PubMed]
- Aronis, K.N.; Hylek, E.M. Evidence Gaps in the Era of Non-Vitamin K Oral Anticoagulants. J. Am. Heart Assoc. 2018, 7, e007338. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland JCJr Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; Heidenreich, P.A.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart. J. Am. Coll. Cardiol. 2019, 74, 104–132. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022). Cochrane. 2022. Available online: www.training.cochrane.org/handbook (accessed on 22 May 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Ottawa Hospital Research Institute. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 17 January 2023).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Weber, F.; Knapp, G.; Ickstadt, K.; Kundt, G.; Glass, Ä. Zero-cell corrections in random-effects meta-analyses. Res. Synth. Methods 2020, 11, 913–919. [Google Scholar] [CrossRef]
- Chapter 10: Analysing Data and Undertaking Meta-Analyses | Cochrane Training. Available online: https://training.cochrane.org/handbook/current/chapter-10#section-10-10 (accessed on 8 January 2024).
- Guarascio, M.; Bertù, L.; Dona dini, M.P.; Antonucci, E.; Palareti, G.; Ageno, W. DOACs use in extreme body-weighted patients: Results from the prospective START-register. Intern. Emerg. Med. 2023, 18, 1681–1687. [Google Scholar] [CrossRef]
- Pilkerton, C.S.; Adelman, M.; Crocetti, E.; Xiang, J.; Strick, V.; Ponte, C.D.; Peckens, S.; Jackson, B.P.; Whipp, K.; Ashcraft, A.M. Direct Oral Anticoagulants: Probability of Recurrent Venous Thromboembolism and Bleeding Risk in an Obese Population. Ann. Pharmacother. 2023, 10600280231212186. [Google Scholar] [CrossRef]
- Costa, O.S.; Beyer-Westendorf, J.; Ashton, V.; Milentijevic, D.; Moore, K.T.; Bunz, T.J.; Coleman, C.I. Rivaroxaban Versus Warfarin for Management of Obese African Americans with Non-Valvular Atrial Fibrillation or Venous Thromboembolism: A Retrospective Cohort Analysis. Clin. Appl. Thromb. Off. J. Int. Acad. Clin. Appl. Thromb. 2020, 26, 1076029620954910. [Google Scholar] [CrossRef]
- Alberts, M.J.; He, J.; Kharat, A.; Ashton, V. Effectiveness and Safety of Rivaroxaban versus Warfarin Among Nonvalvular Atrial Fibrillation Patients with Obesity and Polypharmacy. Am. J. Cardiovasc. Drugs 2022, 22, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Crouch, A.; Ng, T.H.; Kelley, D.; Knight, T.; Edwin, S.; Giuliano, C. Multi-center retrospective study evaluating the efficacy and safety of apixaban versus warfarin for treatment of venous thromboembolism in patients with severe obesity. Pharmacotherapy 2022, 42, 119–133. [Google Scholar] [CrossRef]
- Deitelzweig, S.; Luo, X.; Nguyen, J.L.; Malhotra, D.; Emir, B.; Russ, C.; Li, X.; Lee, T.C.; Ferri, M.; Wiederkehr, D.; et al. Thrombotic and bleeding events, mortality, and anticoagulant use among 546,656 hospitalized patients with COVID-19 in the United States: A retrospective cohort study. J. Thromb. Thrombolysis 2022, 53, 766–776. [Google Scholar] [CrossRef]
- Perino, A.C.; Fan, J.; Schmitt, S.; Guo, J.D.; Hlavacek, P.; Din, N.; Kothari, M.; Pundi, K.; Russ, C.; Emir, B.; et al. Anticoagulation Treatment and Outcomes of Venous Thromboembolism by Weight and Body Mass Index: Insights from the Veterans Health Administration. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e008005. [Google Scholar] [CrossRef]
- Berger, J.S.; Laliberté, F.; Kharat, A.; Lejeune, D.; Moore, K.T.; Jung, Y.; Lefebvre, P.; Ashton, V. Real-world effectiveness and safety of rivaroxaban versus warfarin among non-valvular atrial fibrillation patients with obesity in a US population. Curr. Med. Res. Opin. 2021, 37, 881–890. [Google Scholar] [CrossRef]
- Berger, J.S.; Laliberté, F.; Kharat, A.; Lejeune, D.; Moore, K.T.; Jung, Y.; Lefebvre, P.; Ashton, V. Comparative Effectiveness and Safety of Rivaroxaban and Warfarin Among Nonvalvular Atrial Fibrillation (NVAF) Patients with Obesity and Polypharmacy in the United States (US). Adv. Ther. 2021, 38, 3771–3788. [Google Scholar] [CrossRef]
- Samaranayake, C.B.; Keir, G.; Slader, S.A.A.; Tseng, T.; Tran, K.; Anderson, J.; McCann, A.; McCabe, C.; Upham, J.W. Use of direct oral anticoagulants for acute pulmonary embolisms in obesity: A propensity-matched, multicentre case-control study. ERJ Open Res. 2021, 7, 00379-2021. [Google Scholar] [CrossRef]
- Costa, O.S.; Beyer-Westendorf, J.; Ashton, V.; Milentijevic, D.; Moore, K.T.; Bunz, T.J.; Coleman, C.I. Effectiveness and safety of rivaroxaban versus warfarin in obese patients with acute venous thromboembolism: Analysis of electronic health record data. J. Thromb. Thrombolysis 2021, 51, 349–358. [Google Scholar] [CrossRef]
- Cohen, A.T.; Pan, S.; Byon, W.; Ilyas, B.S.; Taylor, T.; Lee, T.C. Correction to: Efficacy, Safety, and Exposure of Apixaban in Patients with High Body Weight or Obesity and Venous Thromboembolism: Insights from AMPLIFY. Adv. Ther. 2021, 38, 4596–4597. [Google Scholar] [CrossRef]
- Martin, K.A.; Lancki, N.; Li, C.; Eyster, M.E.; Sanfilippo, K.; Woller, I.A.; Woller, S.C.; Kreuziger, L.B.; Rosovsky, R.P. DOAC compared with warfarin for VTE in patients with obesity: A retrospective cohort study conducted through the VENUS network. J. Thromb. Thrombolysis 2023, 55, 685–690. [Google Scholar] [CrossRef]
- Weir, M.R.; Chen, Y.-W.; He, J.; Bookhart, B.; Campbell, A.; Ashton, V. Effectiveness and safety of rivaroxaban versus warfarin among nonvalvular atrial fibrillation patients with obesity and diabetes. J. Diabetes Complicat. 2021, 35, 108029. [Google Scholar] [CrossRef]
- Barakat, A.F.; Jain, S.; Masri, A.; Alkukhun, L.; Senussi, M.; Sezer, A.; Wang, Y.; Thoma, F.; Bhonsale, A.; Saba, S.; et al. Outcomes of Direct Oral Anticoagulants in Atrial Fibrillation Patients Across Different Body Mass Index Categories. JACC Clin. Electrophysiol. 2021, 7, 649–658. [Google Scholar] [CrossRef]
- Cohen, A.; Sah, J.; Lee, T.; Rosenblatt, L.; Hlavacek, P.; Emir, B.; Keshishian, A.; Yuce, H.; Luo, X. Effectiveness and Safety of Apixaban vs. Warfarin in Venous Thromboembolism Patients with Obesity and Morbid Obesity. J. Clin. Med. 2021, 10, 200. [Google Scholar] [CrossRef]
- Perales, I.J.; San Agustin, K.; DeAngelo, J.; Campbell, A.M. Rivaroxaban Versus Warfarin for Stroke Prevention and Venous Thromboembolism Treatment in Extreme Obesity and High Body Weight. Ann. Pharmacother. 2020, 54, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Falk, K.; McComb, M.N.; Shapiro, N.L.; Uppuluri, E.M. Prescribing Pattern of Oral Anticoagulants in Patients with Obesity. J. Pharm. Pract. 2022, 35, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Deitelzweig, S.; Keshishian, A.; Kang, A.; Dhamane, A.D.; Luo, X.; Li, X.; Balachander, N.; Rosenblatt, L.; Mardekian, J.; Pan, X.; et al. Effectiveness and Safety of Oral Anticoagulants among NVAF Patients with Obesity: Insights from the ARISTOPHANES Study. J. Clin. Med. 2020, 9, 1633. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Merino, J.L.; Banach, M.; de Groot, J.R.; Maier, L.S.; Themistoclakis, S.; Boriani, G.; Jin, J.; Melino, M.; Winters, S.M.; et al. Impact of Body Mass Index on Outcomes in the Edoxaban Versus Warfarin Therapy Groups in Patients Underwent Cardioversion of Atrial Fibrillation (from ENSURE-AF). Am. J. Cardiol. 2019, 123, 592–597. [Google Scholar] [CrossRef]
- Kido, K.; Ngorsuraches, S. Comparing the Efficacy and Safety of Direct Oral Anticoagulants with Warfarin in the Morbidly Obese Population with Atrial Fibrillation. Ann. Pharmacother. 2019, 53, 165–170. [Google Scholar] [CrossRef]
- Kalani, C.; Awudi, E.; Alexander, T.; Udeani, G.; Surani, S. Evaluation of the efficacy of direct oral anticoagulants (DOACs) in comparison to warfarin in morbidly obese patients. Hosp. Pract. (1995) 2019, 47, 181–185. [Google Scholar] [CrossRef]
- Briasoulis, A.; Mentias, A.; Mazur, A.; Alvarez, P.; Leira, E.C.; Vaughan Sarrazin, M.S. Comparative Effectiveness and Safety of Direct Oral Anticoagulants in Obese Patients with Atrial Fibrillation. Cardiovasc. Drugs Ther. 2021, 35, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Hattaway, Q.; Starr, J.A.; Pinner, N.A. Direct Oral Anticoagulants for the Treatment of Venous Thromboembolism in Obesity. J. Pharm. Technol. JPT Off. Publ. Assoc. Pharm. Technol. 2023, 39, 269–273. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Ashton, V.; Chen, Y.-W.; Wu, B.; Peterson, E.D. Rivaroxaban versus warfarin treatment among morbidly obese patients with venous thromboembolism: Comparative effectiveness, safety, and costs. Thromb. Res. 2019, 182, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, M.; Choi, Y.; Eisenberg, R.; Rao, D.; Tolu, S.; Gao, J.; Mowrey, W.; Billett, H.H. Efficacy and safety of direct oral factor Xa inhibitors compared with warfarin in patients with morbid obesity: A single-centre, retrospective analysis of chart data. Lancet Haematol. 2019, 6, e359–e365. [Google Scholar] [CrossRef]
- Peterson, E.D.; Ashton, V.; Chen, Y.-W.; Wu, B.; Spyropoulos, A.C. Comparative effectiveness, safety, and costs of rivaroxaban and warfarin among morbidly obese patients with atrial fibrillation. Am. Heart J. 2019, 212, 113–119. [Google Scholar] [CrossRef]
- Coons, J.C.; Albert, L.; Bejjani, A.; Iasella, C.J. Effectiveness and Safety of Direct Oral Anticoagulants versus Warfarin in Obese Patients with Acute Venous Thromboembolism. Pharmacotherapy 2020, 40, 204–210. [Google Scholar] [CrossRef]
- Bianco, C.; Wen, S.; Cai, Y.; Finch, C.; Kimble, W.; Caccamo, M.; Sokos, G.G. Novel oral anticoagulants are safe and effective for thromboembolic prophylaxis in obese patients with atrial fibrillation. J. Am. Coll. Cardiol. 2020, 75, 505. [Google Scholar] [CrossRef]
- Nakao, Y.M.; Nakao, K.; Wu, J.; Nadarajah, R.; Camm, A.J.; Gale, C.P. Risks and benefits of oral anticoagulants for stroke prophylaxis in atrial fibrillation according to body mass index: Nationwide cohort study of primary care records in England. EClinicalMedicine 2022, 54, 101709. [Google Scholar] [CrossRef] [PubMed]
- Patil, T.; Lebrecht, M. A single center retrospective cohort study evaluating use of direct oral anticoagulants (DOACs) in morbidly obese veteran population. Thromb. Res. 2020, 192, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.A.; Linneman, T.W. Comparing Safety and Efficacy of Direct Oral Anticoagulants Versus Warfarin in Extreme Obesity. J. Pharm. Pract. 2023, 36, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, S.N.; Hasan, H.; Metwali, H.; Aseeri, M. Comparing the Efficacy and Safety of Apixaban Versus Warfarin in Morbidly Obese Patients. Cureus 2022, 14, e30303. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.M.; Smith, C.B.; Crannage, E.F.; Challen, L.M. Examination of the Effectiveness of Direct Oral Anticoagulants in Comparison to Warfarin in an Obese Population. J. Pharm. Technol. JPT Off. Publ. Assoc. Pharm. Technol. 2022, 38, 26–30. [Google Scholar] [CrossRef]
- Weaver, P.; Ng, T.H.; Breeden, T.; Edwin, S.B.; Haan, B.; Giuliano, C. Management of Venous Thromboembolism in Morbid Obesity with Rivaroxaban or Warfarin. Ann. Pharmacother. 2022, 56, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.A.; Beyer-Westendorf, J.; Davidson, B.L.; Huisman, M.V.; Sandset, P.M.; Moll, S. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: Updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J. Thromb. Haemost. 2021, 19, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef]
- Alalawneh, M.; Rachid, O.; Abdallah, I.; Mahfouz, A.; Elewa, H.; Danjuma, M.I.; Mohamed, A.E.; Awaisu, A. Trends in prescribing and outcomes in obese versus non-obese patients receiving rivaroxaban therapy: An observational study using real-world data. Eur. J. Clin. Pharmacol. 2023, 79, 1675–1685. [Google Scholar] [CrossRef]
- Lavalle, C.; Pierucci, N.; Mariani, M.V.; Piro, A.; Borrelli, A.; Grimaldi, M.; Rossillo, A.; Notarstefano, P.; Compagnucci, P.; Dello Russo, A.; et al. Italian Registry in the Setting of Atrial Fibrillation Ablation with Rivaroxaban -IRIS. Minerva Cardiol. Angiol. 2024. preprint. [Google Scholar] [CrossRef] [PubMed]
- Riaz, I.B.; Fuentes, H.; Deng, Y.; Naqvi, S.A.A.; Yao, X.; Sangaralingham, L.R.; Houghton, D.E.; Padrnos, L.J.; Shamoun, F.E.; Wysokinski, W.E.; et al. Comparative Effectiveness of Anticoagulants in Patients with Cancer-Associated Thrombosis. JAMA Netw. Open 2023, 6, e2325283. [Google Scholar] [CrossRef]
- Harrington, J.; Carnicelli, A.P.; Hua, K.; Wallentin, L.; Patel, M.R.; Hohnloser, S.H.; Giugliano, R.P.; Fox, K.A.A.; Hijazi, Z.; Lopes, R.D.; et al. Direct Oral Anticoagulants Versus Warfarin Across the Spectrum of Kidney Function: Patient-Level Network Meta-Analyses From COMBINE AF. Circulation 2023, 147, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- A Multicenter, RandomiZed, Active-ControLled Study to Evaluate the Safety and Tolerability of Two Blinded Doses of Abelacimab Compared with Open-Label Rivaroxaban in Patients with Atrial Fibrillation-American College of Cardiology. Available online: https://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2023/11/10/22/46/azalea-timi-71 (accessed on 27 December 2023).
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; RE-LY Steering Committee and Investigators; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Balla, S.R.; Cyr, D.D.; Lokhnygina, Y.; Becker, R.C.; Berkowitz, S.D.; Breithardt, G.; Fox, K.A.A.; Hacke, W.; Halperin, J.L.; Hankey, G.J.; et al. Relation of Risk of Stroke in Patients with Atrial Fibrillation to Body Mass Index (from Patients Treated with Rivaroxaban and Warfarin in the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of St. Am. J. Cardiol. 2017, 119, 1989–1996. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; ARISTOTLE Committees and Investigators; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Hokusai-VTE Investigators; Büller, H.R.; Décousus, H.; Grosso, M.A.; Mercuri, M.; Middeldorp, S.; Prins, M.H.; Raskob, G.E.; Schellong, S.M.; Schwocho, L.; et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N. Engl. J. Med. 2013, 369, 1406–1415. [Google Scholar] [CrossRef]
- Agnelli, G.; Buller, H.R.; Cohen, A.; Curto, M.; Gallus, A.S.; Johnson, M.; Masiukiewicz, U.; Pak, R.; Thompson, J.; et al.; AMPLIFY Investigators Oral apixaban for the treatment of acute venous thromboembolism. N. Engl. J. Med. 2013, 369, 799–808. [Google Scholar] [CrossRef]
- EINSTEIN Investigators; Bauersachs, R.; Berkowitz, S.D.; Brenner, B.; Buller, H.R.; Decousus, H.; Gallus, A.S.; Lensing, A.W.; Misselwitz, F.; Prins, M.H.; et al. Oral rivaroxaban for symptomatic venous thromboembolism. N. Engl. J. Med. 2010, 363, 2499–2510. [Google Scholar] [CrossRef]
- Malik, A.H.; Yandrapalli, S.; Shetty, S.; Aronow, W.S.; Jain, D.; Frishman, W.H.; Cooper, H.A.; Panza, J.A.; MAGIC (Meta-analysis And oriGinal Investigations in Cardiology) Investigators. Impact of weight on the efficacy and safety of direct-acting oral anticoagulants in patients with non-valvular atrial fibrillation: A meta-analysis. EP Eur. 2020, 22, 361–367. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, H.; Wang, X.; Zhu, Z.; Duan, F. Effectiveness and safety of non-vitamin K antagonist oral anticoagulant in the treatment of patients with morbid obesity or high body weight with venous thromboembolism: A meta-analysis. Medicine 2023, 102, e35015. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Fudim, M.; Alexander, J.H.; Wojdyla, D.M.; Ezekowitz, J.A.; Hanna, M.; Atar, D.; Hijazi, Z.; Bahit, M.C.; Al-Khatib, S.M.; et al. Efficacy and Safety of Apixaban Versus Warfarin in Patients with Atrial Fibrillation and Extremes in Body Weight. Circulation 2019, 139, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.M.; Braunwald, E.; Steffel, J.; Boriani, G.; Palazzolo, M.G.; Antman, E.M.; Bohula, E.A.; Carnicelli, A.P.; Connolly, S.J.; COMBINE AF (A Collaboration Between Multiple Institutions to Better Investigate Non-Vitamin K Antagonist Oral Anticoagulant Use in Atrial Fibrillation) Investigators; et al. Efficacy and Safety of Non-Vitamin-K Antagonist Oral Anticoagulants Versus Warfarin Across the Spectrum of Body Mass Index and Body Weight: An Individual Patient Data Meta-Analysis of 4 Randomized Clinical Trials of Patients with Atrial Fibrillation. Circulation 2024, 149, 932–943. [Google Scholar] [CrossRef]
- Reilly, P.A.; Lehr, T.; Haertter, S.; Connolly, S.J.; Yusuf, S.; Eikelboom, J.W.; Ezekowitz, M.D.; Nehmiz, G.; Wang, S.; Wallentin, L.; et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: The RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J. Am. Coll. Cardiol. 2014, 63, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Piran, S.; Traquair, H.; Chan, N.; Bhagirath, V.; Schulman, S. Peak plasma concentration of direct oral anticoagulants in obese patients weighing over 120 kilograms: A retrospective study. Res. Pract. Thromb. Haemost. 2018, 2, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Mueck, W.; Lensing, A.W.A.; Agnelli, G.; Decousus, H.; Prandoni, P.; Misselwitz, F. Rivaroxaban: Population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin. Pharmacokinet. 2011, 50, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Willmann, S.; Zhang, L.; Frede, M.; Kubitza, D.; Mueck, W.; Schmidt, S.; Solms, A.; Yan, X.; Garmann, D. Integrated Population Pharmacokinetic Analysis of Rivaroxaban Across Multiple Patient Populations. Integrated Population Pharmacokinetic Analysis of Rivaroxaban Across Multiple Patient Populations. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 309–320. [Google Scholar] [CrossRef]
- Elad, B.; Maman, N.; Ayalon, S.; Goldstein, L.H. Effectiveness and Safety of Direct Oral Anticoagulants for Stroke Prevention in Atrial Fibrillation Patients with Extreme Obesity. Am. J. Cardiol. 2023, 202, 223–228. [Google Scholar] [CrossRef]
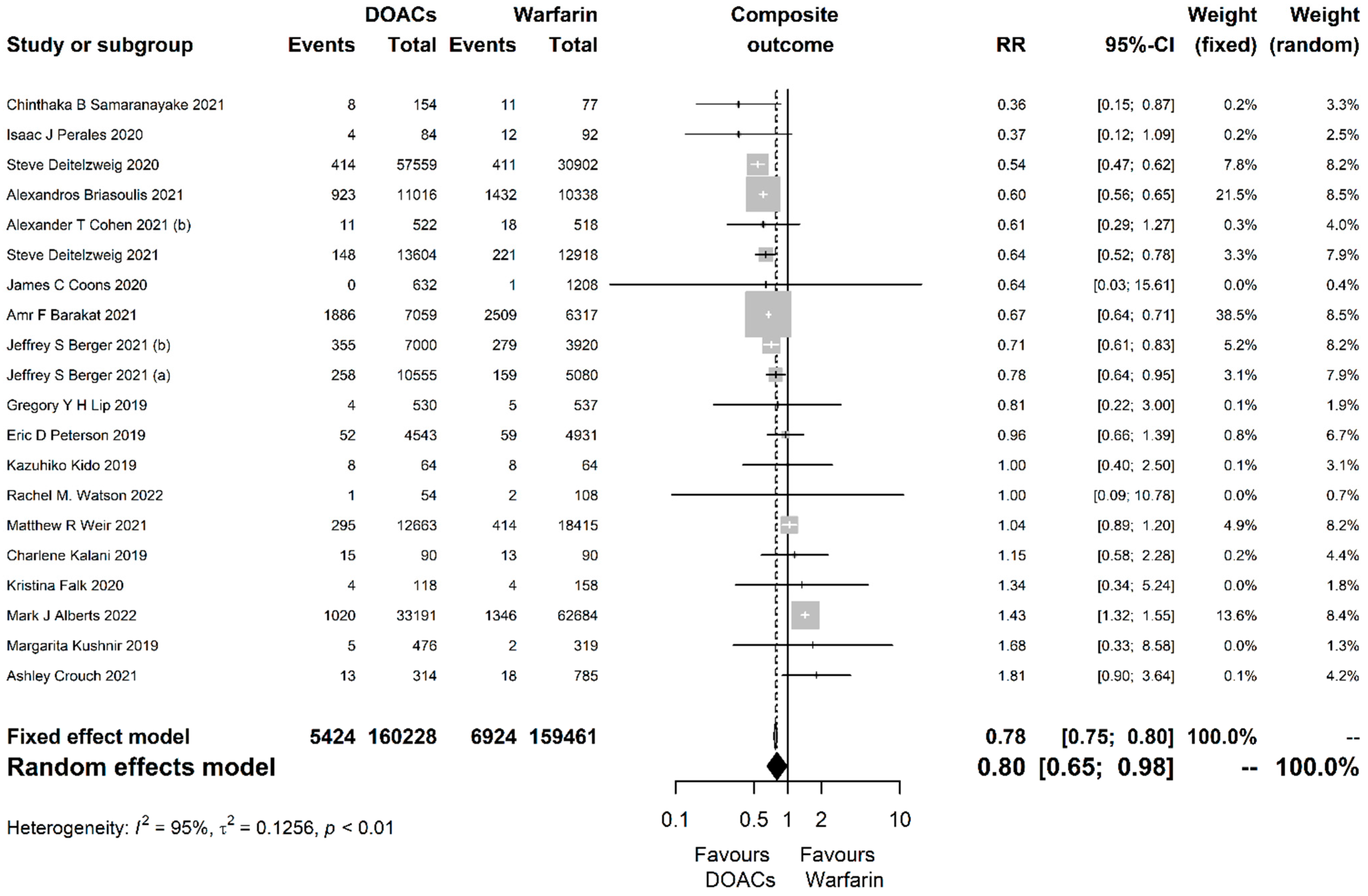
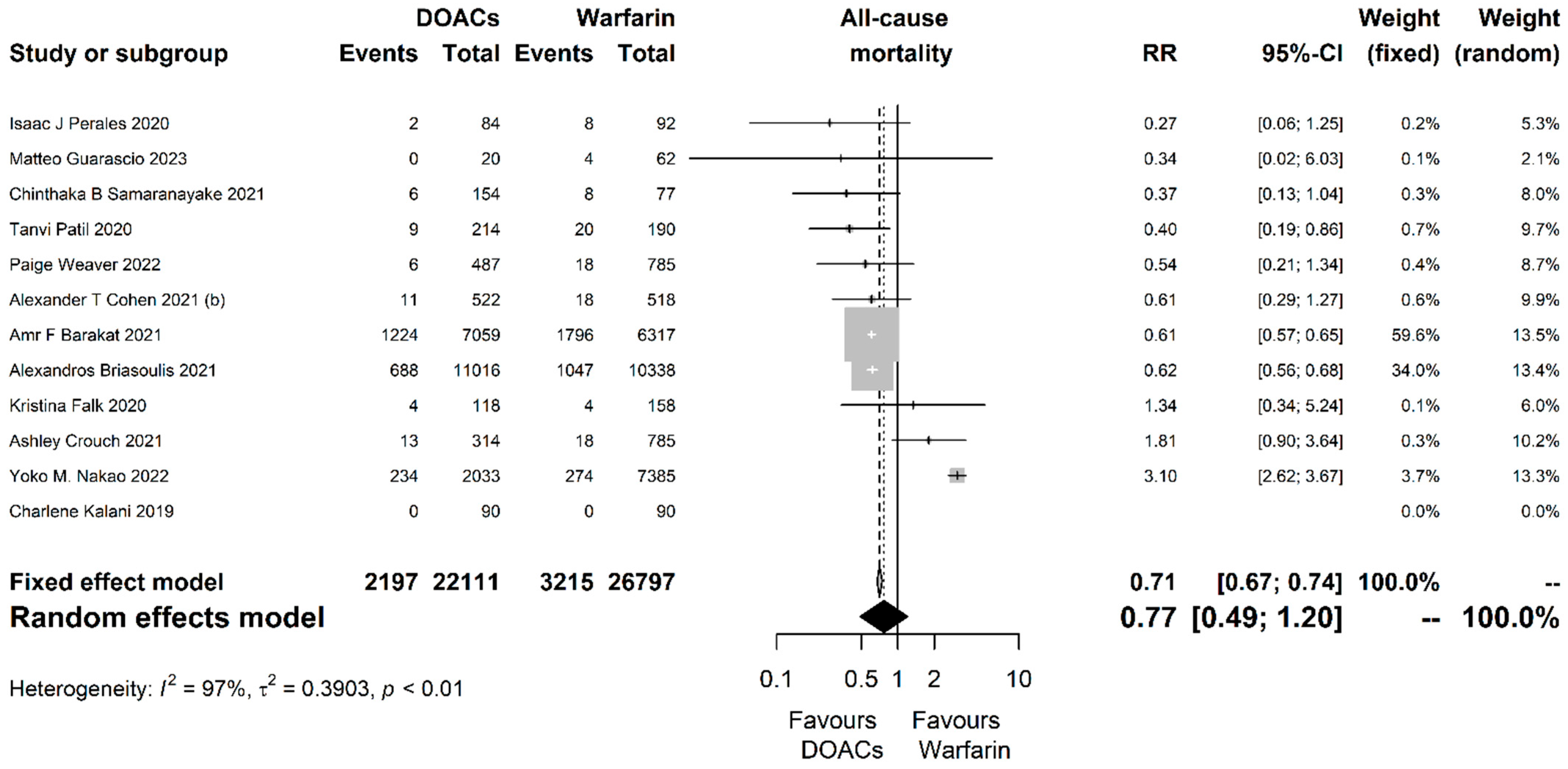
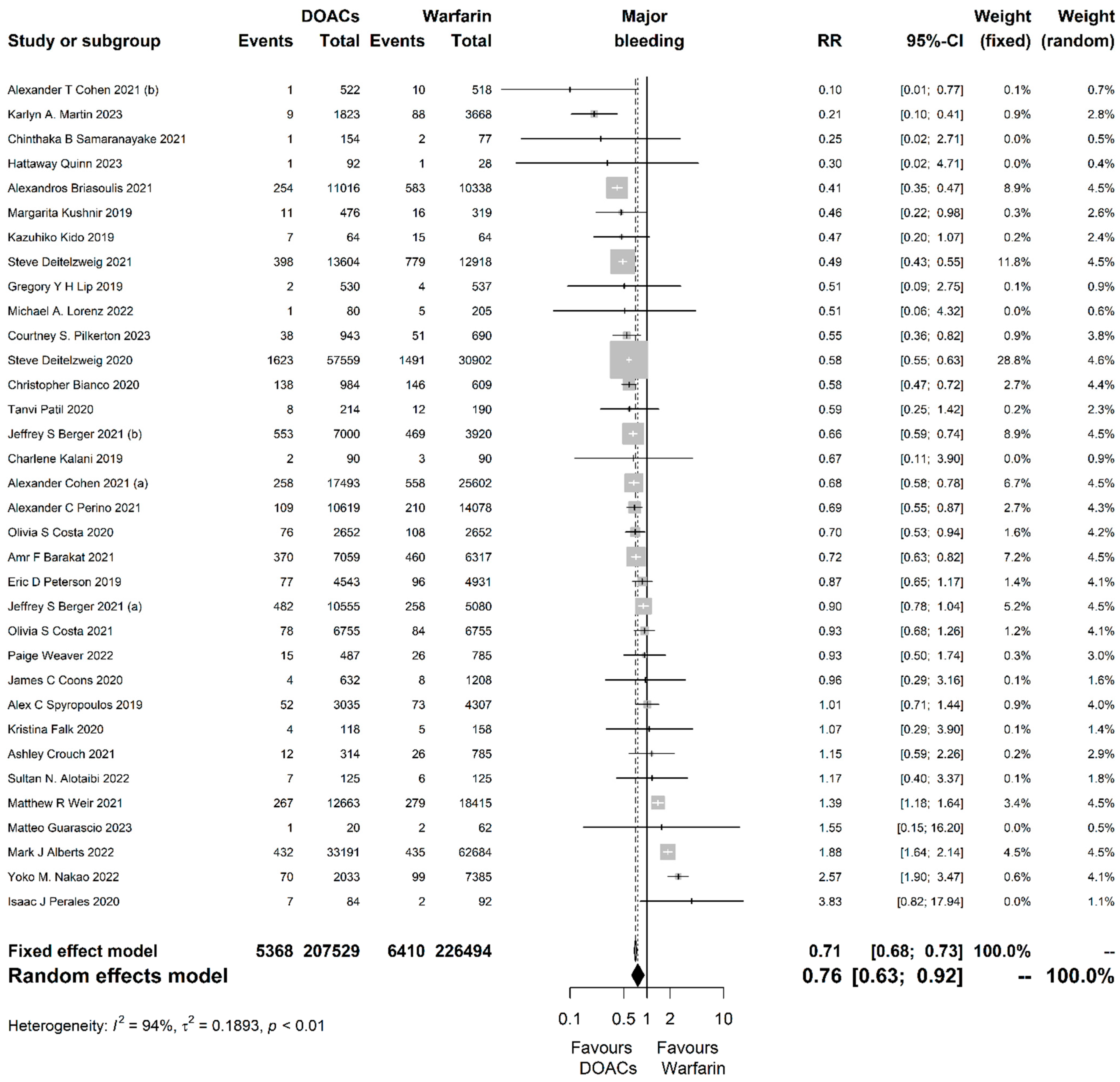
| Outcome | Studies (N) | Participants (N) | RR with 95% CI | p-Value | Prediction Interval | I2 with 95% CI | Cochran’s Q p-Value | Egger’s Test p-Value |
|---|---|---|---|---|---|---|---|---|
| A. Efficacy outcomes | ||||||||
| Composite | 20 | 319,689 | 0.8 (0.65, 0.98) | 0.033 | (0.37, 1.74) | 0.95 (0.93, 0.96) | <0.001 | 0.742 |
| All-cause mortality | 12 | 48,908 | 0.77 (0.49, 1.2) | 0.247 | (0.17, 3.46) | 0.97 (0.96, 0.98) | <0.001 | 0.633 |
| Stroke or systemic embolism | 18 | 331,210 | 0.9 (0.71, 1.15) | 0.409 | (0.35, 2.35) | 0.94 (0.92, 0.95) | <0.001 | 0.562 |
| Myocardial infarction | 3 | 34,910 | 0.82 (0.51, 1.32) | 0.413 | (0, 185.92) | 0.88 (0.65, 0.96) | <0.001 | 0.897 |
| Ischemic stroke | 13 | 317,292 | 0.94 (0.72, 1.22) | 0.649 | (0.36, 2.48) | 0.93 (0.9, 0.95) | <0.001 | 0.454 |
| Hemorrhagic stroke | 9 | 305,061 | 0.58 (0.38, 0.88) | 0.011 | (0.14, 2.49) | 0.92 (0.86, 0.95) | <0.001 | 0.136 |
| Systemic embolism | 7 | 268,722 | 0.81 (0.63, 1.05) | 0.113 | (0.47, 1.42) | 0.26 (0, 0.68) | 0.23 | 0.074 |
| Venous thromboembolism | 20 | 110,160 | 0.8 (0.63, 1) | 0.052 | (0.33, 1.89) | 0.87 (0.81, 0.91) | <0.001 | 0.502 |
| B. Safety outcomes | ||||||||
| Major bleeding | 34 | 434,023 | 0.76 (0.63, 0.92) | 0.004 | (0.31, 1.89) | 0.94 (0.92, 0.95) | <0.001 | 0.524 |
| Minor bleeding | 14 | 76,479 | 0.88 (0.71, 1.09) | 0.257 | (0.48, 1.62) | 0.77 (0.62, 0.86) | <0.001 | 0.437 |
| Gastrointestinal bleeding | 5 | 181,272 | 0.59 (0.49, 0.72) | <0.001 | (0.3, 1.16) | 0.88 (0.74, 0.94) | <0.001 | 0.491 |
| Intracranial bleeding | 6 | 165,384 | 0.45 (0.33, 0.6) | <0.001 | (0.19, 1.05) | 0.52 (0, 0.82) | 0.08 | 0.202 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakasis, P.; Ktenopoulos, N.; Pamporis, K.; Sagris, M.; Soulaidopoulos, S.; Gerogianni, M.; Leontsinis, I.; Giannakoulas, G.; Tousoulis, D.; Fragakis, N.; et al. Efficacy and Safety of Direct Oral Anticoagulants versus Warfarin in Obese Patients (BMI ≥ 30 kg/m2) with Atrial Fibrillation or Venous Thromboembolism: An Updated Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 3784. https://doi.org/10.3390/jcm13133784
Karakasis P, Ktenopoulos N, Pamporis K, Sagris M, Soulaidopoulos S, Gerogianni M, Leontsinis I, Giannakoulas G, Tousoulis D, Fragakis N, et al. Efficacy and Safety of Direct Oral Anticoagulants versus Warfarin in Obese Patients (BMI ≥ 30 kg/m2) with Atrial Fibrillation or Venous Thromboembolism: An Updated Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(13):3784. https://doi.org/10.3390/jcm13133784
Chicago/Turabian StyleKarakasis, Paschalis, Nikolaos Ktenopoulos, Konstantinos Pamporis, Marios Sagris, Stergios Soulaidopoulos, Maria Gerogianni, Ioannis Leontsinis, George Giannakoulas, Dimitris Tousoulis, Nikolaos Fragakis, and et al. 2024. "Efficacy and Safety of Direct Oral Anticoagulants versus Warfarin in Obese Patients (BMI ≥ 30 kg/m2) with Atrial Fibrillation or Venous Thromboembolism: An Updated Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 13: 3784. https://doi.org/10.3390/jcm13133784
APA StyleKarakasis, P., Ktenopoulos, N., Pamporis, K., Sagris, M., Soulaidopoulos, S., Gerogianni, M., Leontsinis, I., Giannakoulas, G., Tousoulis, D., Fragakis, N., & Tsioufis, K. (2024). Efficacy and Safety of Direct Oral Anticoagulants versus Warfarin in Obese Patients (BMI ≥ 30 kg/m2) with Atrial Fibrillation or Venous Thromboembolism: An Updated Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(13), 3784. https://doi.org/10.3390/jcm13133784












