Mechanical Valves: Past, Present, and Future—A Review
Abstract
1. Introduction
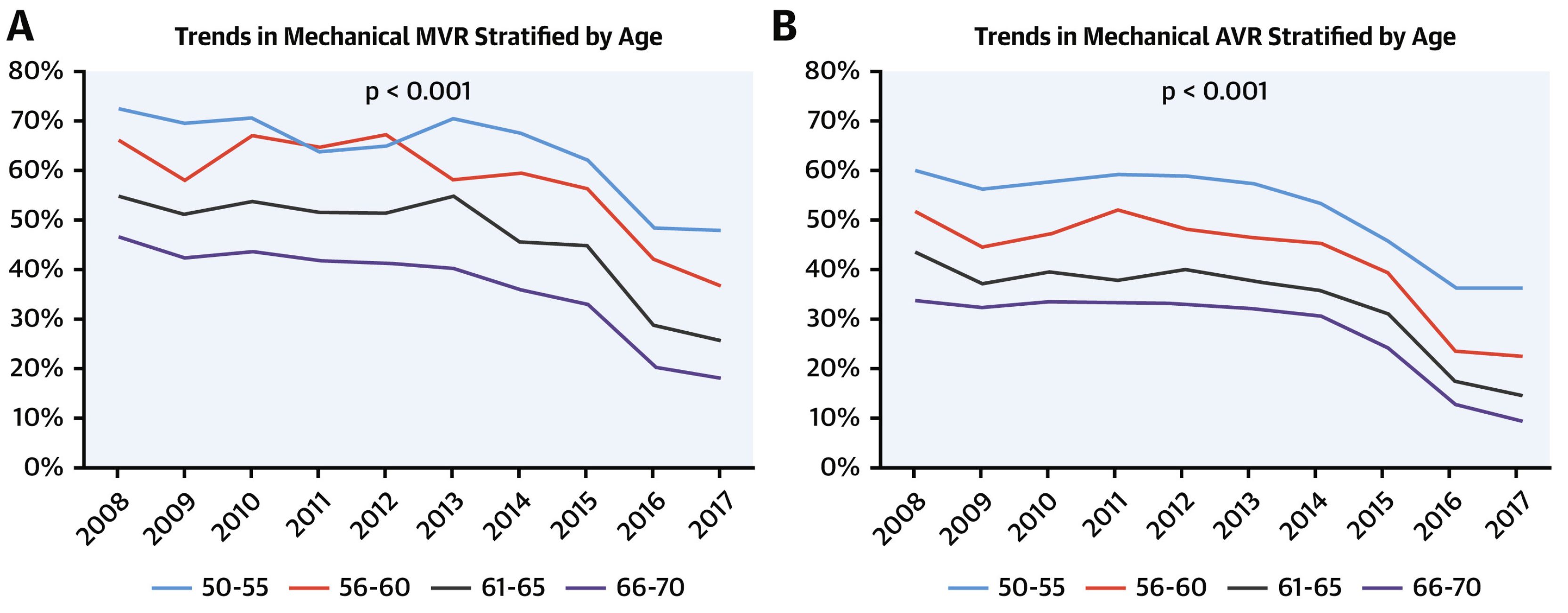
2. History and Trends
3. Current Guidelines
4. Clinical Outcomes of Mechanical vs. Bioprosthetic Valve
4.1. Survival
4.2. Reoperation
4.3. Bleeding
4.4. Stroke
5. Anticoagulants
6. Alternatives
6.1. Aortic Valve
6.2. Mitral Valve
7. Future Directions
7.1. DOACs
7.2. Trileaflet Valve
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wyler von Ballmoos, M.C.; Kaneko, T.; Iribarne, A.; Kim, K.M.; Arghami, A.; Fiedler, A.; Habib, R.; Parsons, N.; Elhalabi, Z.; Krohn, C.; et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2023 Update on Procedure Data and Research. Ann. Thorac. Surg. 2024, 117, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: Executive summary: A report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, C.A.; Harvey, W.P. The surgical correction of aortic regurgitation preliminary report. Bull. Georget. Univ. Med. Cent. 1953, 6, 60–61. [Google Scholar]
- Shiono, M.; Sezai, Y.; Sezai, A.; Hata, M.; Iida, M.; Negishi, N. Long-term results of the cloth-covered Starr-Edwards ball valve. Ann. Thorac. Surg. 2005, 80, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Wu, Y.; Grunkemeier, G.L.; Furnary, A.P.; Starr, A. Forty-year survival with the Starr-Edwards heart valve prosthesis. J. Heart Valve Dis. 2004, 13, 91–96; discussion 96. [Google Scholar] [PubMed]
- Shapira, Y.; Vaturi, M.; Sagie, A. Anticoagulant management of patients with mechanical prosthetic valves undergoing non-cardiac surgery: Indications and unresolved issues. J. Heart Valve Dis. 2001, 10, 380–387. [Google Scholar] [PubMed]
- Blot, W.J.; Ibrahim, M.A.; Ivey, T.D.; Acheson, D.E.; Brookmeyer, R.; Weyman, A.; Defauw, J.; Smith, J.K.; Harrison, D. Twenty-five-year experience with the Björk-Shiley convexoconcave heart valve: A continuing clinical concern. Circulation 2005, 111, 2850–2857. [Google Scholar] [CrossRef] [PubMed]
- Nicoloff, D.M.; Emery, R.W.; Arom, K.V.; Northrup, W.F.; Jorgensen, C.R.; Wang, Y.; Lindsay, W.G. Clinical and hemodynamic results with the St. Jude Medical cardiac valve prosthesis. A three-year experience. J. Thorac. Cardiovasc. Surg. 1981, 82, 674–683. [Google Scholar] [CrossRef]
- Emery, R.W.; Emery, A.M.; Raikar, G.V.; Shake, J.G. Anticoagulation for mechanical heart valves: A role for patient based therapy. J. Thromb. Thrombolysis 2008, 25, 18–25. [Google Scholar] [CrossRef]
- Ikonomidis, J.S.; Kratz, J.M.; Crumbley, A.J.; Stroud, M.R.; Bradley, S.M.; Sade, R.M.; Crawford, F.A. Twenty-year experience with the St Jude Medical mechanical valve prosthesis. J. Thorac. Cardiovasc. Surg. 2003, 126, 2022–2031. [Google Scholar] [CrossRef]
- Antunes, M.J. Requiem for a good mechanical heart valve: Farewell to the Medtronic Hall valve. J. Thorac. Cardiovasc. Surg. 2015, 149, 1492–1494. [Google Scholar] [CrossRef] [PubMed]
- Hiltner, E.; Erinne, I.; Singh, A.; Chen, C.; Kassotis, J.; Russo, M.; Sethi, A. Contemporary trends and in-hospital outcomes of mechanical and bioprosthetic surgical aortic valve replacement in the United States. J. Card. Surg. 2022, 37, 1980–1988. [Google Scholar] [CrossRef]
- Alkhouli, M.; Alqahtani, F.; Kawsara, A.; Pislaru, S.; Schaff, H.V.; Nishimura, R.A. National trends in mechanical valve replacement in patients aged 50 to 70 years. J. Am. Coll. Cardiol. 2020, 76, 2687–2688. [Google Scholar] [CrossRef]
- Alkhouli, M.; Alqahtani, F.; Simard, T.; Pislaru, S.; Schaff, H.V.; Nishimura, R.A. Predictors of Use and Outcomes of Mechanical Valve Replacement in the United States (2008–2017). J. Am. Heart Assoc. 2021, 10, e019929. [Google Scholar] [CrossRef]
- Goldstone, A.B.; Chiu, P.; Baiocchi, M.; Lingala, B.; Patrick, W.L.; Fischbein, M.P.; Woo, Y.J. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N. Engl. J. Med. 2017, 377, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Caus, T.; Chabry, Y.; Nader, J.; Fusellier, J.F.; De Brux, J.L. EpiCard investigators Trends in SAVR with biological vs. mechanical valves in middle-aged patients: Results from a French large multi-centric survey. Front. Cardiovasc. Med. 2023, 10, 1205770. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Dimagli, A.; Fudulu, D.P.; Sinha, S.; Narayan, P.; Dong, T.; Angelini, G.D. Trend and early outcomes in isolated surgical aortic valve replacement in the United Kingdom. Front. Cardiovasc. Med. 2022, 9, 1077279. [Google Scholar] [CrossRef]
- Squiers, J.J.; Robinson, N.B.; Audisio, K.; Ryan, W.H.; Mack, M.J.; Rahouma, M.; Cancelli, G.; Kirov, H.; Doenst, T.; Gaudino, M.; et al. Structural valve degeneration of bioprosthetic aortic valves: A network meta-analysis. J. Thorac. Cardiovasc. Surg. 2023, 166, 52–59. [Google Scholar] [CrossRef]
- Raghav, V.; Okafor, I.; Quach, M.; Dang, L.; Marquez, S.; Yoganathan, A.P. Long-Term Durability of Carpentier-Edwards Magna Ease Valve: A One Billion Cycle In Vitro Study. Ann. Thorac. Surg. 2016, 101, 1759–1765. [Google Scholar] [CrossRef]
- Francica, A.; Benvegnù, L.; San Biagio, L.; Tropea, I.; Luciani, G.B.; Faggian, G.; Onorati, F. Ten-year clinical and echocardiographic follow-up of third-generation biological prostheses in the aortic position. J. Thorac. Cardiovasc. Surg. 2022, 167, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Piperata, A.; Fiocco, A.; Cavicchiolo, A.; Ponzoni, M.; Pesce, R.; Gemelli, M.; Evangelista, G.; Gastino, E.; Michelotti, S.; Mazzaro, E.; et al. Carpentier-Edwards Magna Ease bioprosthesis: A multicentre clinical experience and 12-year durability. Eur. J. Cardiothorac. Surg. 2022, 61, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Alqahtani, F.; Ziada, K.M.; Aljohani, S.; Holmes, D.R.; Mathew, V. Contemporary trends in the management of aortic stenosis in the USA. Eur. Heart J. 2020, 41, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Levy, K.H. Valve-in-valve transcatheter aortic valve replacement versus redo surgical aortic valve replacement: A systematic review and meta-analysis. J. Card. Surg. 2021, 36, 2486–2495. [Google Scholar] [CrossRef] [PubMed]
- Tasoudis, P.T.; Varvoglis, D.N.; Vitkos, E.; Mylonas, K.S.; Sá, M.P.; Ikonomidis, J.S.; Caranasos, T.G.; Athanasiou, T. Mechanical versus bioprosthetic valve for aortic valve replacement: Systematic review and meta-analysis of reconstructed individual participant data. Eur. J. Cardiothorac. Surg. 2022, 62, ezac268. [Google Scholar] [CrossRef] [PubMed]
- Kytö, V.; Sipilä, J.; Ahtela, E.; Rautava, P.; Gunn, J. Mechanical versus biologic prostheses for surgical aortic valve replacement in patients aged 50 to 70. Ann. Thorac. Surg. 2020, 110, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Traxler, D.; Krotka, P.; Laggner, M.; Mildner, M.; Graf, A.; Reichardt, B.; Wendt, R.; Auer, J.; Moser, B.; Mascherbauer, J.; et al. Mechanical aortic valve prostheses offer a survival benefit in 50–65 year olds: AUTHEARTVISIT study. Eur. J. Clin. Investig. 2022, 52, e13736. [Google Scholar] [CrossRef] [PubMed]
- Leviner, D.B.; Witberg, G.; Levi, A.; Landes, U.; Schwartz, N.; Shiran, A.; Kornowski, R.; Sharoni, E. Mechanical vs. Bioprosthetic Aortic Valve Replacement in Patients Younger Than 70 Years of Age: A Hazard Ratio Meta-analysis. Can. J. Cardiol. 2022, 38, 355–364. [Google Scholar] [CrossRef]
- Glaser, N.; Jackson, V.; Holzmann, M.J.; Franco-Cereceda, A.; Sartipy, U. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50–69 years. Eur. Heart J. 2016, 37, 2658–2667. [Google Scholar] [CrossRef]
- Leviner, D.B.; Zafrir, B.; Saliba, W.; Stein, N.; Shiran, A.; Sharoni, E. Biological or mechanical mitral valve replacement in patients 50–70 years of age-a propensity-adjusted analysis. Eur. J. Cardiothorac. Surg. 2022, 62, ezac073. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chan, Y.-H.; Wu, V.C.-C.; Liu, K.-S.; Cheng, Y.-T.; Chu, P.-H.; Chen, S.-W. Bioprosthetic versus mechanical mitral valve replacements in patients with rheumatic heart disease. J. Thorac. Cardiovasc. Surg. 2023, 165, 1050–1060.e8. [Google Scholar] [CrossRef] [PubMed]
- Hammermeister, K.; Sethi, G.K.; Henderson, W.G.; Grover, F.L.; Oprian, C.; Rahimtoola, S.H. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: Final report of the Veterans Affairs randomized trial. J. Am. Coll. Cardiol. 2000, 36, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Oxenham, H.; Bloomfield, P.; Wheatley, D.J.; Lee, R.J.; Cunningham, J.; Prescott, R.J.; Miller, H.C. Twenty year comparison of a Bjork-Shiley mechanical heart valve with porcine bioprostheses. Heart 2003, 89, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Stassano, P.; Di Tommaso, L.; Monaco, M.; Iorio, F.; Pepino, P.; Spampinato, N.; Vosa, C. Aortic valve replacement: A prospective randomized evaluation of mechanical versus biological valves in patients ages 55 to 70 years. J. Am. Coll. Cardiol. 2009, 54, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.P.; Chikwe, J.; Moskowitz, A.J.; Itagaki, S.; Adams, D.H.; Egorova, N.N. Survival and long-term outcomes following bioprosthetic vs. mechanical aortic valve replacement in patients aged 50 to 69 years. JAMA 2014, 312, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.F.; Seco, M.; Wu, J.J.; Edelman, J.B.; Wilson, M.K.; Vallely, M.P.; Byrom, M.J.; Bannon, P.G. Mechanical Versus Bioprosthetic Aortic Valve Replacement in Middle-Aged Adults: A Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2016, 102, 315–327. [Google Scholar] [CrossRef] [PubMed]
- McClure, R.S.; McGurk, S.; Cevasco, M.; Maloney, A.; Gosev, I.; Wiegerinck, E.M.; Salvio, G.; Tokmaji, G.; Borstlap, W.; Nauta, F.; et al. Late outcomes comparison of nonelderly patients with stented bioprosthetic and mechanical valves in the aortic position: A propensity-matched analysis. J. Thorac. Cardiovasc. Surg. 2014, 148, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Attia, T.; Yang, Y.; Svensson, L.G.; Toth, A.J.; Rajeswaran, J.; Blackstone, E.H.; Johnston, D.R. Members of the Cleveland Clinic Aortic Valve Center Similar long-term survival after isolated bioprosthetic versus mechanical aortic valve replacement: A propensity-matched analysis. J. Thorac. Cardiovasc. Surg. 2022, 164, 1444–1455.e4. [Google Scholar] [CrossRef]
- Rodríguez-Caulo, E.A.; Macías, D.; Adsuar, A.; Ferreiro, A.; Arias-Dachary, J.; Parody, G.; Fernández, F.; Daroca, T.; Rodríguez-Mora, F.; Garrido, J.M.; et al. Biological or mechanical prostheses for isolated aortic valve replacement in patients aged 50–65 years: The ANDALVALVE study. Eur. J. Cardiothorac. Surg. 2019, 55, 1160–1167. [Google Scholar] [CrossRef]
- Rodríguez-Caulo, E.A.; Blanco-Herrera, O.R.; Berastegui, E.; Arias-Dachary, J.; Souaf-Khalafi, S.; Parody-Cuerda, G.; Laguna, G. SPAVALVE Study Group Biological versus mechanical prostheses for aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2023, 165, 609–617.e7. [Google Scholar] [CrossRef] [PubMed]
- Chikwe, J.; Chiang, Y.P.; Egorova, N.N.; Itagaki, S.; Adams, D.H. Survival and outcomes following bioprosthetic vs. mechanical mitral valve replacement in patients aged 50 to 69 years. JAMA 2015, 313, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Sonoda, H.; Oishi, Y.; Tanoue, Y.; Nakashima, A.; Shiokawa, Y.; Tominaga, R. Mechanical prosthesis is reasonable for mitral valve replacement in patients approximately 65 years of age. Ann. Thorac. Surg. 2013, 96, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Schnittman, S.R.; Itagaki, S.; Toyoda, N.; Adams, D.H.; Egorova, N.N.; Chikwe, J. Survival and long-term outcomes after mitral valve replacement in patients aged 18 to 50 years. J. Thorac. Cardiovasc. Surg. 2018, 155, 96–102.e11. [Google Scholar] [CrossRef] [PubMed]
- Traxler, D.; Krotka, P.; Reichardt, B.; Copic, D.; Veraar, C.; Mildner, M.; Wendt, R.; Auer, J.; Mascherbauer, J.; Ankersmit, H.J.; et al. Revisiting aortic valve prosthesis choice in patients younger than 50 years: 10 years results of the AUTHEARTVISIT study. Eur. J. Cardiothorac. Surg. 2024, 65, ezad308. [Google Scholar] [CrossRef] [PubMed]
- Mehaffey, H.J.; Hawkins, R.B.; Schubert, S.; Fonner, C.; Yarboro, L.T.; Quader, M.; Speir, A.; Rich, J.; Kron, I.L.; Ailawadi, G. Contemporary outcomes in reoperative mitral valve surgery. Heart 2018, 104, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Ejiofor, J.I.; Hirji, S.A.; Ramirez-Del Val, F.; Norman, A.V.; McGurk, S.; Aranki, S.F.; Shekar, P.S.; Kaneko, T. Outcomes of repeat mitral valve replacement in patients with prior mitral surgery: A benchmark for transcatheter approaches. J. Thorac. Cardiovasc. Surg. 2018, 156, 619–627.e1. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Vassileva, C.M.; Englum, B.; Kim, S.; Yammine, M.; Brennan, M.; Suri, R.M.; Thourani, V.H.; Jacobs, J.P.; Aranki, S. Contemporary Outcomes of Repeat Aortic Valve Replacement: A Benchmark for Transcatheter Valve-in-Valve Procedures. Ann. Thorac. Surg. 2015, 100, 1298–1304; discussion 1304. [Google Scholar] [CrossRef] [PubMed]
- Narayan, P.; Dimagli, A.; Fudulu, D.P.; Sinha, S.; Dong, T.; Chan, J.; Angelini, G.D. Risk factors and outcomes of reoperative surgical aortic valve replacement in the United Kingdom. Ann. Thorac. Surg. 2023, 116, 759–766. [Google Scholar] [CrossRef]
- Mahboubi, R.; Kakavand, M.; Soltesz, E.G.; Rajeswaran, J.; Blackstone, E.H.; Svensson, L.G.; Johnston, D.R. The decreasing risk of reoperative aortic valve replacement: Implications for valve choice and transcatheter therapy. J. Thorac. Cardiovasc. Surg. 2023, 166, 1043–1053.e7. [Google Scholar] [CrossRef]
- Cannegieter, S.C.; Rosendaal, F.R.; Briët, E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994, 89, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Deviri, E.; Sareli, P.; Wisenbaugh, T.; Cronje, S.L. Obstruction of mechanical heart valve prostheses: Clinical aspects and surgical management. J. Am. Coll. Cardiol. 1991, 17, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Dürrleman, N.; Pellerin, M.; Bouchard, D.; Hébert, Y.; Cartier, R.; Perrault, L.P.; Basmadjian, A.; Carrier, M. Prosthetic valve thrombosis: Twenty-year experience at the Montreal Heart Institute. J. Thorac. Cardiovasc. Surg. 2004, 127, 1388–1392. [Google Scholar] [CrossRef] [PubMed]
- Cannegieter, S.C.; Rosendaal, F.R.; Wintzen, A.R.; van der Meer, F.J.; Vandenbroucke, J.P.; Briët, E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N. Engl. J. Med. 1995, 333, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, T.; Lim, V.; Inamo, J.; Miller, F.A.; Mahoney, D.W.; Schaff, H.V.; Enriquez-Sarano, M. Achieved anticoagulation vs. prosthesis selection for mitral mechanical valve replacement: A population-based outcome study. Chest 2009, 136, 1503–1513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- MacIsaac, S.; Jaffer, I.H.; Belley-Côté, E.P.; McClure, G.R.; Eikelboom, J.W.; Whitlock, R.P. How did we get here?: A historical review and critical analysis of anticoagulation therapy following mechanical valve replacement. Circulation 2019, 140, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Saour, J.N.; Sieck, J.O.; Mamo, L.A.; Gallus, A.S. Trial of different intensities of anticoagulation in patients with prosthetic heart valves. N. Engl. J. Med. 1990, 322, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Rouvier, J.; Gurfinkel, E.; D’Ortencio, O.; Manzanel, R.; de La Fuente, L.; Favaloro, R.G. Comparison of two levels of anticoagulant therapy in patients with substitute heart valves. J. Thorac. Cardiovasc. Surg. 1991, 101, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Acar, J.; Iung, B.; Boissel, J.P.; Samama, M.M.; Michel, P.L.; Teppe, J.P.; Pony, J.C.; Breton, H.L.; Thomas, D.; Isnard, R.; et al. AREVA: Multicenter randomized comparison of low-dose versus standard-dose anticoagulation in patients with mechanical prosthetic heart valves. Circulation 1996, 94, 2107–2112. [Google Scholar] [CrossRef]
- Torella, M.; Torella, D.; Chiodini, P.; Franciulli, M.; Romano, G.; De Santo, L.; De Feo, M.; Amarelli, C.; Sasso, F.C.; Salvatore, T.; et al. LOWERing the INtensity of oral anticoaGulant Therapy in patients with bileaflet mechanical aortic valve replacement: Results from the “LOWERING-IT” Trial. Am. Heart J. 2010, 160, 171–178. [Google Scholar] [CrossRef]
- Puskas, J.; Gerdisch, M.; Nichols, D.; Quinn, R.; Anderson, C.; Rhenman, B.; Fermin, L.; McGrath, M.; Kong, B.; Hughes, C.; et al. Reduced anticoagulation after mechanical aortic valve replacement: Interim results from the prospective randomized on-X valve anticoagulation clinical trial randomized Food and Drug Administration investigational device exemption trial. J. Thorac. Cardiovasc. Surg. 2014, 147, 1202–1210; discussion 1210. [Google Scholar] [CrossRef]
- Mok, C.K.; Boey, J.; Wang, R.; Chan, T.K.; Cheung, K.L.; Lee, P.K.; Chow, J.; Ng, R.P.; Tse, T.F. Warfarin versus dipyridamole-aspirin and pentoxifylline-aspirin for the prevention of prosthetic heart valve thromboembolism: A prospective randomized clinical trial. Circulation 1985, 72, 1059–1063. [Google Scholar] [CrossRef]
- Little, S.H.; Massel, D.R. Antiplatelet and anticoagulation for patients with prosthetic heart valves. Cochrane Database Syst. Rev. 2003, CD003464. [Google Scholar] [CrossRef]
- Puskas, J.D.; Gerdisch, M.; Nichols, D.; Fermin, L.; Rhenman, B.; Kapoor, D.; Copeland, J.; Quinn, R.; Hughes, G.C.; Azar, H.; et al. Anticoagulation and Antiplatelet Strategies After On-X Mechanical Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 2717–2726. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Connolly, S.J.; Brueckmann, M.; Granger, C.B.; Kappetein, A.P.; Mack, M.J.; Blatchford, J.; Devenny, K.; Friedman, J.; Guiver, K.; et al. Dabigatran versus warfarin in patients with mechanical heart valves. N. Engl. J. Med. 2013, 369, 1206–1214. [Google Scholar] [CrossRef]
- Wang, T.Y.; Svensson, L.G.; Wen, J.; Vekstein, A.; Gerdisch, M.; Rao, V.U.; Moront, M.; Johnston, D.; Lopes, R.D.; Chavez, A.; et al. Apixaban or Warfarin in Patients with an On-X Mechanical Aortic Valve. NEJM Evid. 2023, 2, EVIDoa2300067. [Google Scholar] [CrossRef]
- Duraes, A.R.; de Souza Lima Bitar, Y.; Schonhofen, I.S.; Travassos, K.S.O.; Pereira, L.V.; Filho, J.A.L.; Neto, M.G.; Junior, R.A.; Roever, L. Rivaroxaban Versus Warfarin in Patients with Mechanical Heart Valves: Open-Label, Proof-of-Concept trial-The RIWA study. Am. J. Cardiovasc. Drugs 2021, 21, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.A.; van Riet, S. The On-X heart valve: Mid-term results in a poorly anticoagulated population. J. Heart Valve Dis. 2006, 15, 80–86. [Google Scholar] [PubMed]
- Chu, M.W.A.; Ruel, M.; Graeve, A.; Gerdisch, M.W.; Damiano, R.J.; Smith, R.L.; Keeling, W.B.; Wait, M.A.; Hagberg, R.C.; Quinn, R.D.; et al. Low-Dose vs. Standard Warfarin After Mechanical Mitral Valve Replacement: A Randomized Trial. Ann. Thorac. Surg. 2023, 115, 929–938. [Google Scholar] [CrossRef]
- Tamer, S.; Mastrobuoni, S.; Vancraeynest, D.; Lemaire, G.; Navarra, E.; Khoury, G.E.; de Kerchove, L. Late results of aortic valve repair for isolated severe aortic regurgitation. J. Thorac. Cardiovasc. Surg. 2023, 165, 995–1006.e3. [Google Scholar] [CrossRef]
- Ashikhmina, E.; Sundt, T.M.; Dearani, J.A.; Connolly, H.M.; Li, Z.; Schaff, H.V. Repair of the bicuspid aortic valve: A viable alternative to replacement with a bioprosthesis. J. Thorac. Cardiovasc. Surg. 2010, 139, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, M.J.; Kosiorowska, K.; Gocol, R.; Jasinski, J.; Nowicki, R.; Bielicki, G.; Berezowski, M.; Przybylski, R.; Obremska, M.; Lukaszewski, M.; et al. Bicuspid aortic valve repair: Outcomes after 17 years of experience. Eur. J. Cardiothorac. Surg. 2021, 60, 1053–1061. [Google Scholar] [CrossRef]
- de Meester, C.; Pasquet, A.; Gerber, B.L.; Vancraeynest, D.; Noirhomme, P.; El Khoury, G.; Vanoverschelde, J.-L.J. Valve repair improves the outcome of surgery for chronic severe aortic regurgitation: A propensity score analysis. J. Thorac. Cardiovasc. Surg. 2014, 148, 1913–1920. [Google Scholar] [CrossRef]
- Girdauskas, E.; Balaban, Ü.; Herrmann, E.; Bauer, T.; Beckmann, A.; Bekeredjian, R.; Ensminger, S.; Frerker, C.; Möllmann, H.; Petersen, J.; et al. Aortic Valve Repair Results in Better 1-Year Survival Than Replacement: Results from German Aortic Valve Registry. Ann. Thorac. Surg. 2023, 117, 517–525. [Google Scholar] [CrossRef]
- Jabagi, H.; Chan, V.; Ruel, M.; Mesana, T.G.; Boodhwani, M. Aortic Valve Repair Decreases Risks of VRE in AI at 10 Years: A Propensity Score-Matched Analysis. Ann. Thorac. Surg. 2022, 113, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Malaisrie, S.C.; Kislitsina, O.N.; Wilsbacher, L.; Mendelson, M.; Puthumana, J.J.; Vassallo, P.; Kruse, J.; Andrei, A.-C.; McCarthy, P.M. Valve-sparing versus valve-replacing aortic root replacement in patients with aortic root aneurysm. J. Card. Surg. 2022, 37, 1947–1956. [Google Scholar] [CrossRef]
- Price, J.; Magruder, J.T.; Young, A.; Grimm, J.C.; Patel, N.D.; Alejo, D.; Dietz, H.C.; Vricella, L.A.; Cameron, D.E. Long-term outcomes of aortic root operations for Marfan syndrome: A comparison of Bentall versus aortic valve-sparing procedures. J. Thorac. Cardiovasc. Surg. 2016, 151, 330–336. [Google Scholar] [CrossRef]
- Lansac, E.; Di Centa, I.; Danial, P.; Bouchot, O.; Arnaud-Crozat, E.; Hacini, R.; Doguet, F.; Demaria, R.; Verhoye, J.P.; Jouan, J.; et al. Aortic valve repair versus mechanical valve replacement for root aneurysm: The CAVIAAR multicentric study. Eur. J. Cardiothorac. Surg. 2022, 62, ezac283. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, T.; Abeln, K.B.; Froede, L.; Burgard, C.; Giebels, C.; Schäfers, H.-J. Valve-sparing aortic root replacement-for all patients? J. Thorac. Cardiovasc. Surg. 2023; in press. [Google Scholar] [CrossRef]
- Sharma, V.J.; Kangarajah, A.; Yang, A.; Kim, M.; Seevayanagam, S.; Matalanis, G. Valve-sparing aortic root replacement: Long-term variables significantly associated with mortality and morbidity. J. Thorac. Cardiovasc. Surg. 2023; in press. [Google Scholar] [CrossRef]
- Flynn, C.D.; Tian, D.H.; Wilson-Smith, A.; David, T.; Matalanis, G.; Misfeld, M.; Mastrobuoni, S.; El Khoury, G.; Yan, T.D. Systematic review and meta-analysis of surgical outcomes in Marfan patients undergoing aortic root surgery by composite-valve graft or valve sparing root replacement. Ann. Cardiothorac. Surg. 2017, 6, 570–581. [Google Scholar] [CrossRef]
- Ouzounian, M.; Rao, V.; Manlhiot, C.; Abraham, N.; David, C.; Feindel, C.M.; David, T.E. Valve-Sparing Root Replacement Compared with Composite Valve Graft Procedures in Patients with Aortic Root Dilation. J. Am. Coll. Cardiol. 2016, 68, 1838–1847. [Google Scholar] [CrossRef]
- Rosenblum, J.M.; Leshnower, B.G.; Moon, R.C.; Lasanajak, Y.; Binongo, J.; McPherson, L.; Chen, E.P. Durability and safety of David V valve-sparing root replacement in acute type A aortic dissection. J. Thorac. Cardiovasc. Surg. 2019, 157, 14–23.e1. [Google Scholar] [CrossRef]
- Buratto, E.; Shi, W.Y.; Wynne, R.; Poh, C.L.; Larobina, M.; O’Keefe, M.; Goldblatt, J.; Tatoulis, J.; Skillington, P.D. Improved survival after the ross procedure compared with mechanical aortic valve replacement. J. Am. Coll. Cardiol. 2018, 71, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Sá, M.P.; Van den Eynde, J.; Jacquemyn, X.; Tasoudis, P.; Erten, O.; McDonald, C.; Weymann, A.; Ruhparwar, A.; Clavel, M.-A.; Pibarot, P.; et al. Long-Term Outcomes of Ross Procedure versus Mechanical Aortic Valve Replacement: Meta-Analysis of Reconstructed Time-To-Event Data. Trends Cardiovasc. Med. 2024, 34, 29–36. [Google Scholar] [CrossRef]
- El-Hamamsy, I.; Toyoda, N.; Itagaki, S.; Stelzer, P.; Varghese, R.; Williams, E.E.; Erogova, N.; Adams, D.H. Propensity-Matched Comparison of the Ross Procedure and Prosthetic Aortic Valve Replacement in Adults. J. Am. Coll. Cardiol. 2022, 79, 805–815. [Google Scholar] [CrossRef]
- Gofus, J.; Fila, P.; Drabkova, S.; Zacek, P.; Ondrasek, J.; Nemec, P.; Sterba, J.; Tuna, M.; Jarkovsky, J.; Vojacek, J. Ross procedure provides survival benefit over mechanical valve in adults: A propensity-matched nationwide analysis. Eur. J. Cardiothorac. Surg. 2022, 61, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Mazine, A.; El-Hamamsy, I.; Verma, S.; Peterson, M.D.; Bonow, R.O.; Yacoub, M.H.; David, T.E.; Bhatt, D.L. Ross Procedure in Adults for Cardiologists and Cardiac Surgeons: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2761–2777. [Google Scholar] [CrossRef] [PubMed]
- Mazine, A.; David, T.E.; Rao, V.; Hickey, E.J.; Christie, S.; Manlhiot, C.; Ouzounian, M. Long-Term Outcomes of the Ross Procedure Versus Mechanical Aortic Valve Replacement: Propensity-Matched Cohort Study. Circulation 2016, 134, 576–585. [Google Scholar] [CrossRef]
- Mazine, A.; Rocha, R.V.; El-Hamamsy, I.; Ouzounian, M.; Yanagawa, B.; Bhatt, D.L.; Verma, S.; Friedrich, J.O. Ross Procedure vs. Mechanical Aortic Valve Replacement in Adults: A Systematic Review and Meta-analysis. JAMA Cardiol. 2018, 3, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Stolte, T.; Boeddinghaus, J.; Allegra, G.; Leibundgut, G.; Reuthebuch, O.; Kaiser, C.; Müller, C.; Nestelberger, T. Incidence and Outcomes of Valve-in-Valve Transcatheter Aortic Valve Implantation in Failed Bioprosthetic Valves. J. Clin. Med. 2023, 12, 5868. [Google Scholar] [CrossRef]
- Demal, T.J.; Gordon, C.; Bhadra, O.D.; Linder, M.; Ludwig, S.; Grundmann, D.; Voigtländer, L.; Waldschmidt, L.; Schirmer, J.; Schofer, N.; et al. Contemporary Outcome Trends in Transcatheter Aortic Valve-in-Valve Implantation Versus Redo Aortic Valve Replacement. Am. J. Cardiol. 2022, 171, 115–121. [Google Scholar] [CrossRef]
- Tam, D.Y.; Dharma, C.; Rocha, R.V.; Ouzounian, M.; Wijeysundera, H.C.; Austin, P.C.; Chikwe, J.; Gaudino, M.; Fremes, S.E. Transcatheter ViV Versus Redo Surgical AVR for the Management of Failed Biological Prosthesis: Early and Late Outcomes in a Propensity-Matched Cohort. JACC Cardiovasc. Interv. 2020, 13, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Bleiziffer, S.; Simonato, M.; Webb, J.G.; Rodés-Cabau, J.; Pibarot, P.; Kornowski, R.; Windecker, S.; Erlebach, M.; Duncan, A.; Seiffert, M.; et al. Long-term outcomes after transcatheter aortic valve implantation in failed bioprosthetic valves. Eur. Heart J. 2020, 41, 2731–2742. [Google Scholar] [CrossRef] [PubMed]
- Lazam, S.; Vanoverschelde, J.L.; Tribouilloy, C.; Grigioni, F.; Suri, R.M.; Avierinos, J.F.; de Meester, C.; Barbieri, A.; Rusinaru, D.; Russo, A.; et al. Twenty-Year Outcome After Mitral Repair Versus Replacement for Severe Degenerative Mitral Regurgitation: Analysis of a Large, Prospective, Multicenter, International Registry. Circulation 2017, 135, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.C.; Jang, M.-J.; Hwang, H.Y. Meta-Analysis Comparing Mitral Valve Repair Versus Replacement for Degenerative Mitral Regurgitation Across All Ages. Am. J. Cardiol. 2019, 123, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Shuhaiber, J.; Anderson, R.J. Meta-analysis of clinical outcomes following surgical mitral valve repair or replacement. Eur. J. Cardiothorac. Surg. 2007, 31, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Whisenant, B.K.; Bleiziffer, S.; Delgado, V.; Dhoble, A.; Schofer, N.; Eschenbach, L.; Bansal, E.; Murdoch, D.J.; Ancona, M.; et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur. Heart J. 2019, 40, 441–451. [Google Scholar] [CrossRef]
- Simonato, M.; Whisenant, B.; Ribeiro, H.B.; Webb, J.G.; Kornowski, R.; Guerrero, M.; Wijeysundera, H.; Søndergaard, L.; De Backer, O.; Villablanca, P.; et al. Transcatheter Mitral Valve Replacement after Surgical Repair or Replacement: Comprehensive Midterm Evaluation of Valve-in-Valve and Valve-in-Ring Implantation from the VIVID Registry. Circulation 2021, 143, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Ismayl, M.; Abbasi, M.A.; Mostafa, M.R.; Aboeata, A.; Vora, A.N.; Ben-Dor, I.; Anavekar, N.S.; Goldsweig, A.M. Meta-Analysis Comparing Valve-in-Valve Transcatheter Mitral Valve Replacement Versus Redo Surgical Mitral Valve Replacement in Degenerated Bioprosthetic Mitral Valve. Am. J. Cardiol. 2023, 189, 98–107. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Silverio, A.; Di Maio, M.; Prota, C.; De Angelis, E.; Radano, I.; Citro, R.; Carrizzo, A.; Ciccarelli, M.; Vecchione, C.; Capodanno, D.; et al. Safety and efficacy of non-vitamin K antagonist oral anticoagulants in elderly patients with atrial fibrillation: Systematic review and meta-analysis of 22 studies and 440 281 patients. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, f20–f29. [Google Scholar] [CrossRef]
- Schaller, T.; Scharfschwerdt, M.; Schubert, K.; Prinz, C.; Lembke, U.; Sievers, H.-H. Aortic valve replacement in sheep with a novel trileaflet mechanical heart valve prosthesis without anticoagulation. JTCVS Open 2021, 7, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Schubert, K.; Schaller, T.; Stojenthin, E.; Stephan, C.; Sievers, H.-H.; Scharfschwerdt, M. A novel trileaflet mechanical heart valve: First in vitro results. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Carrel, T.; Vogt, P.R.; Obrist, D.; Schaff, H. Evolving technology: The TRIFLO tri-leaflet mechanical valve without oral anticoagulation: A potential major innovation in valve surgery. Front. Cardiovasc. Med. 2023, 10, 1220633. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, R.P.; Rivard, A.L.; Suwan, P.T.; Black, S.; Bertog, S.; Steinseifer, U.; Armien, A.; Lahti, M.; Bianco, R.W. In-vivo experience with the Triflo trileaflet mechanical heart valve. J. Heart Valve Dis. 2006, 15, 791–799. [Google Scholar] [PubMed]

| ACC/AHA | ESC/EACTS | ||
|---|---|---|---|
| AVR | Mechanical | <50 years | <60 years |
| Bioprosthetic | >65 years | ||
| Shared decision-making | 50–65 years | 60–65 years | |
| MVR | Mechanical | <65 years | |
| Bioprosthetic | >65 years | >70 years | |
| Shared decision-making | N/I | 65–70 years | |
| Age | AVR/MVR | |
|---|---|---|
| Survival | <50 years | Favors mechanical |
| 50–65 years | Inconclusive | |
| >65 years | Favors bioprosthetic | |
| Reoperation | <65 years | Favors mechanical |
| Bleeding | All ages | Favors bioprosthetic |
| Stroke | All ages | No difference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leviner, D.B.; Abraham, D.; Ronai, T.; Sharoni, E. Mechanical Valves: Past, Present, and Future—A Review. J. Clin. Med. 2024, 13, 3768. https://doi.org/10.3390/jcm13133768
Leviner DB, Abraham D, Ronai T, Sharoni E. Mechanical Valves: Past, Present, and Future—A Review. Journal of Clinical Medicine. 2024; 13(13):3768. https://doi.org/10.3390/jcm13133768
Chicago/Turabian StyleLeviner, Dror B., Dana Abraham, Tom Ronai, and Erez Sharoni. 2024. "Mechanical Valves: Past, Present, and Future—A Review" Journal of Clinical Medicine 13, no. 13: 3768. https://doi.org/10.3390/jcm13133768
APA StyleLeviner, D. B., Abraham, D., Ronai, T., & Sharoni, E. (2024). Mechanical Valves: Past, Present, and Future—A Review. Journal of Clinical Medicine, 13(13), 3768. https://doi.org/10.3390/jcm13133768






