Prospective Study to Evaluate Rectus Femoris Muscle Ultrasound for Body Composition Analysis in Patients Undergoing Bariatric Surgery
Abstract
1. Introduction
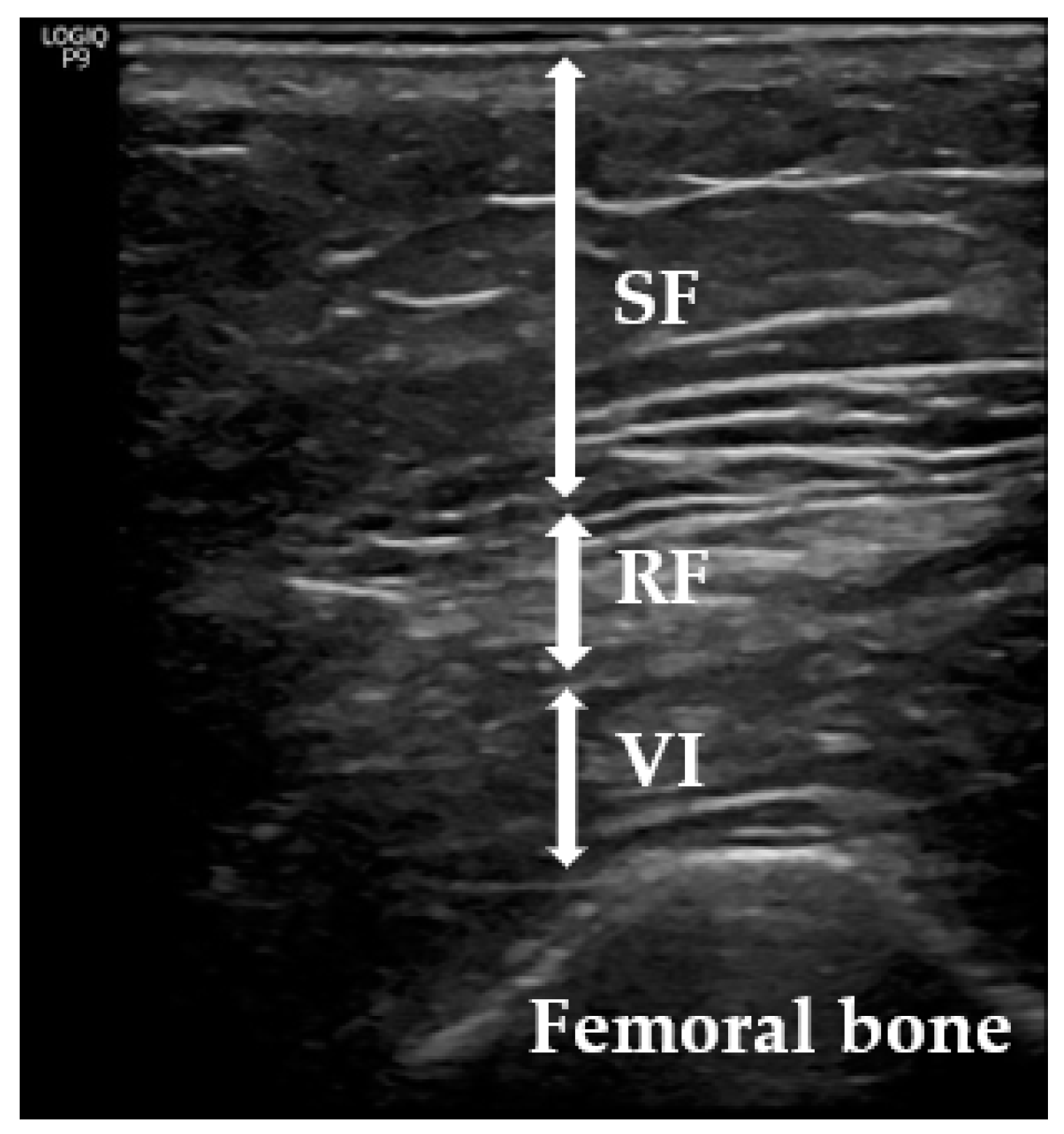
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayedi, A.; Soltani, S.; Motlagh, S.Z.T.; Emadi, A.; Shahinfar, H.; Moosavi, H.; Shab-Bidar, S. Anthropometric and adiposity indicators and risk of type 2 diabetes: Systematic review and dose-response meta-analysis of cohort studies. BMJ 2022, 376, e067516. [Google Scholar] [CrossRef] [PubMed]
- Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; de Gonzalez, A.B.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; Joshy, G.; et al. Body-mass index and all-cause mortality: Individual-participant-data metaanalysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ambrosi, J.; Silva, C.; Galofré, J.C.; Escalada, J.; Santos, S.; Millán, D.; Vila, N.; Ibañez, P.; Gil, M.J.; Valentí, V.; et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int. J. Obes. 2012, 36, 286–294. [Google Scholar] [CrossRef]
- Palmas, F.; Ciudin, A.; Guerra, R.; Eiroa, D.; Espinet, C.; Roson, N.; Burgos, R.; Simo, R. Comparison of computed tomography and dual-energy X-ray absorptiometry in the evaluation of body composition in patients with obesity. Front. Endocrinol. 2023, 14, 1161116. [Google Scholar] [CrossRef]
- Kim, G.; Tan, C.S.; Tan, K.W.; Lim, S.P.Y.; So, J.B.Y.; Shabbir, A. Sleeve gastrectomy and roux-en-Y gastric bypass lead to comparable changes in body composition in a multiethnic Asian population. J. Gastrointest. Surg. 2019, 23, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Matos, O.; Ruthes, E.M.P.; Malinowski, A.K.C.; Lima, A.L.; Veiga, M.S.; Krause, M.P.; Farah, L.; Souza, C.J.F.; Lass, A.D.; Castelo-Branco, C. Changes in bone mass and body composition after bariatric surgery. Gynecol. Endocrinol. 2020, 36, 578–581. [Google Scholar] [CrossRef]
- Baad, V.M.A.; Bezerra, L.R.; de Holanda, N.C.P.; dos Santos, A.C.O.; da Silva, A.A.M.; Bandeira, F.; Cavalcante, T.C.F. Body Composition, Sarcopenia and Physical Performance After Bariatric Surgery: Differences Between Sleeve Gastrectomy and Roux-En-Y Gastric Bypass. Obes. Surg. 2022, 32, 3830–3838. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shin, S.Y.; Shin, M.J. Sarcopenic obesity is associated with lower indicators of psychological health and quality of life in Koreans. Nutr. Res. 2015, 35, 384–392. [Google Scholar] [CrossRef]
- Tian, S.; Xu, Y. Association of sarcopenic obesity with the risk of all-cause mortality: A meta-analysis of prospective cohort studies. Geriatr. Gerontol. Int. 2016, 16, 155–166. [Google Scholar] [CrossRef]
- Sizoo, D.; de Heide, L.J.; Emous, M.; van Zutphen, T.; Navis, G.; van Beek, A.P. Measuring Muscle Mass and Strength in Obesity: A Review of Various Methods. Obes. Surg. 2021, 31, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Balasekaran, G.; Victor Govindaswamy, V.; Yong Hwa, C.; Meng Shun, L. Comparison of body composition with bioelectric impedance (BIA) and dual energy Xray absorptiometry (DEXA) among Singapore Chinese. J. Sci. Med. Sport 2011, 14, 33–35. [Google Scholar] [CrossRef]
- Toombs, R.J.; Ducher, G.; Shepherd, J.A.; De Souza, M.J. The impact of recent technological advances on the trueness and precision of DXA to assess body composition. Obesity 2012, 20, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Pineau, J.C.; Guihard-Costa, A.M.; Bocquet, M. Validation of ultrasound techniques applied to body fat measurement. A comparison between ultrasound techniques, air displacement plethysmography and bioelectrical impedance vs. dual-energy X-ray absorptiometry. Ann. Nutr. Metab. 2007, 51, 421–427. [Google Scholar] [CrossRef]
- Ticinesi, A.; Narici, M.V.; Lauretani, F.; Nouvenne, A.; Colizzi, E.; Mantovani, M.; Corsonello, A.; Landi, F.; Meschi, T.; Maggio, M. Assessing sarcopenia with vastus lateralis muscle ultrasound: An operative protocol. Aging Clin. Exp. Res. 2018, 30, 1437–1443. [Google Scholar] [CrossRef]
- Simó-Servat, A.; Ibarra, M.; Libran, M.; Quirós, C.; Puértolas, N.; Alonso, N.; Perea, V.; Simó, R.; Barahona, M. Usefulness of Ultrasound in Assessing the Impact of Bariatric Surgery on Body Composition: A Pilot Study. Obes. Surg. 2023, 33, 1211–1217. [Google Scholar] [CrossRef]
- Deniz, O.; Cruz-Jentoft, A.; Sengul Aycicek, G.; Unsal, P.; Esme, M.; Ucar, Y.; Burkuk, S.; Sendur, A.; Yavuz, B.B.; Cankurtaran, M.; et al. Role of Ultrasonography in Estimating Muscle Mass in Sarcopenic Obesity. J. Parenter. Enter. Nutr. 2020, 44, 1398–1406. [Google Scholar] [CrossRef]
- Ido, A.; Nakayama, Y.; Ishii, K.; Iemitsu, M.; Sato, K.; Fujimoto, M.; Kurihara, T.; Hamaoka, T.; Satoh-Asahara, N.; Sanada, K. Ultrasound-Derived Abdominal Muscle Thickness Better Detects Metabolic Syndrome Risk in Obese Patients tan Skeletal Muscle Index Measured by Dual-Energy X-ray Absorptiometry. PLoS ONE 2015, 10, e0143858. [Google Scholar] [CrossRef] [PubMed]
- Perkisas, S.; Bastijns, S.; Baudry, S.; Bauer, J.; Beaudart, C.; Beckwée, D.; Cruz-Jentoft, A.; Gasowski, J.; Hobbelen, H.; Jager-Wittenaar, H.; et al. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update. Eur. Geriatr. Med. 2021, 12, 45–59. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Simó-Servat, A.; Ibarra, M.; Libran, M.; Rodríguez, S.; Perea, V.; Quirós, C.; Orois, A.; Pérez, N.; Simó, R.; Barahona, M.J. Usefulness of Muscle Ultrasound to Study Sarcopenic Obesity: A Pilot Case-Control Study. J. Clin. Med. 2022, 11, 2886. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Ando, K.; Kobayashi, K.; Ito, K.; Tsushima, M.; Kobayakawa, T.; Morozumi, M.; Tanaka, S.; Machino, M.; Ota, K.; et al. Ultrasound measurement of thigh muscle thickness for assessment of sarcopenia. Nagoya J. Med. Sci. 2018, 80, 519–527. [Google Scholar] [PubMed]
- Kawai, H.; Kera, T.; Hirayama, R.; Hirano, H.; Fujiwara, Y.; Ihara, K.; Kojima, M.; Obuchi, S. Morphological and qualitative characteristics of the quadriceps muscle of community-dwelling older adults based on ultrasound imaging: Classification using latent class analysis. Aging Clin. Exp. Res. 2018, 30, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.V.; Moorey, H.; Stringer, H.; Sahbudin, I.; Filer, A.; Lord, J.M.; Sapey, E. Bilateral Anterior Thigh Thickness: A New Diagnostic Tool for the Identification of Low Muscle Mass? J. Am. Med. Dir. Assoc. 2019, 20, 1247–1253. [Google Scholar] [CrossRef]
- Souza, V.A.; Oliveira, D.; Cupolilo, E.N.; Miranda, C.S.; Colugnati, F.A.B.; Mansur, H.N.; Fernandes, N.M.D.S.; Bastos, M.G. Rectus femoris muscle mass evaluation by ultrasound: Facilitating sarcopenia diagnosis in pre-dialysis chronic kidney disease stages. Clinics 2018, 73, e392. [Google Scholar] [CrossRef]
- Welch, D.; Ndanyo, L.S.; Brown, S.; Agyapong-Badu, S.; Warner, M.; Stokes, M.; Samuel, D. Thigh muscle and subcutaneous tissue thickness measured using ultrasound imaging in older females living in extended care: A preliminary study. Aging Clin. Exp. Res. 2018, 30, 463–469. [Google Scholar] [CrossRef]
- Minetto, M.A.; Caresio, C.; Menapace, T.; Hajdareviv, A.; Marchini, A.; Molinari, F.; Maffiuletti, N.A. Ultrasound-based detection of low muscle mass for diagnosis of sarcopenia in older adults. PM&R 2016, 8, 453–462. [Google Scholar]
- Benaiges, D.; Goday, A.; Pedro-Botet, J.; Más, A.; Chillarón, J.J.; Flores-Le Roux, J.A. Bariatric surgery: To whom and when? Minerva Endocrinol. 2015, 40, 119–128. [Google Scholar] [PubMed]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric surgery: A systematic review and meta-analysis. JAMA 2004, 292, 1724–1737. [Google Scholar] [CrossRef]
- de Luis Román, D.; Garrachón Vallo, F.; Carretero Gómez, J.; Santabalbina, F.J.T.; Rolo, G.G.; Almeida, J.M.G.; Paris, A.S. La masa muscular disminuida en la diabetes de tipo 2. Una comorbilidad oculta que debemos tener en cuenta [Decreased muscle mass in type-2 diabetes. A hidden comorbidity to consider]. Nutr. Hosp. 2023, 40, 59–66. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Bozan, A.; Erhan, B. The relationship between quadriceps femoris thickness measured by US and femoral cartilage thickness in knee osteoarthritis, its effect on radiographic stage and clinical parameters: Comparison with healthy young population. J. Frailty Sarcopenia Falls 2023, 8, 155–162. [Google Scholar] [CrossRef]
- Jung, S.Y.; Kim, H.J.; Oh, K.T. Comparative Analysis of Preoperative and Postoperative Muscle Mass around Hip Joint by Computed Tomography in Patients with Hip Fracture. Hip Pelvis 2022, 34, 10–17. [Google Scholar] [CrossRef]
- Martínez, M.C.; Meli, E.F.; Candia, F.P.; Filippi, F.; Vilallonga, R.; Cordero, E.; Hernández, I.; Eguinoa, A.Z.; Burgos, R.; Vila, A.; et al. The Impact of Bariatric Surgery on the Muscle Mass in Patients with Obesity: 2-Year Follow-up. Obes. Surg. 2021, 32, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, N.; Kazemi, A.; Asbaghi, O.; Jafarian, F.; Moeinvaziri, N.; Hosseini, B.; Amini, M. Long-term effect of bariatric surgery on body composition in patients with morbid obesity: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Giraudo, C.; Cavaliere, A.; Lupi, A.; Guglielmi, G.; Quaia, E. Established paths and new avenues: A review of the main radiological techniques for investigating sarcopenia. Quant. Imaging Med. Surg. 2020, 10, 1602–1613. [Google Scholar] [CrossRef]
- Tosato, M.; Marzetti, E.; Cesari, M.; Savera, G.; Miller, R.R.; Bernabei, R.; Landi, F.; Calvani, R. Measurement of muscle mass in sarcopenia: From imaging to biochemical markers. Aging Clin. Exp. Res. 2017, 29, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ikai, M.; Fukunaga, T. The size and strength per unit cross-sectional area of human muscle by means of ultrasonic measurement. Int. Z. Angew. Physiol. 1968, 26, 26–32. [Google Scholar]
- Young, A.; Stokes, M.; Crowe, M. The size and strength of the quadriceps muscles of old and young woman. Eur. J. Clin. Investig. 1984, 14, 282–287. [Google Scholar] [CrossRef]
- Ochi, M.; Tabara, Y.; Kido, T.; Uetani, E.; Ochi, N.; Igase, M.; Miki, T.; Kohara, K. Quadriceps sarcopenia and visceral obesity are risk factors for postural instability in the middle-aged to elderly population. Geriatr. Gerontol. Int. 2010, 10, 233–243. [Google Scholar] [CrossRef] [PubMed]


| Total sample size (n) | 77 |
| Female (%) | 50 (64.9) |
| Age (years) mean ± SD | 53.2 ± 8.67 |
| BMI * (kg/m2) mean ± SD | 43.82 ± 5.08 |
| Type 2 DM (%) | 40 (51.9) |
| Type 1 DM (%) | 2 (2.59) |
| Prediabetes (%) | 9 (11.68) |
| Hypertension (%) | 50 (64.9) |
| Dyslipidaemia (%) | 42 (54.54) |
| Pre-Surgery (Mean ± SD) | Post-Surgery (Mean ± SD) | p * | |
|---|---|---|---|
| RFT (cm) | 1.05 ± 0.067 | 0.77 ± 0.03 | 0.0002 |
| iFFM (%) | 23.79 ± 0.38 | 21.07 ± 0.59 | 0.001 |
| AMI | 7.99 ± 0.18 | 7.16 ± 0.14 | 0.001 |
| Lower-Extremity AMI | 6.02 ± 0.12 | 5.39 ± 0.11 | 0.001 |
| Patient | RFT * Pre-Surgery (cm) | RFT Post-Surgery (cm) | Increase in RFT (cm) | QoL * Pre-Surgery | QoL Post-Surgery | Increase in QoL |
|---|---|---|---|---|---|---|
| 7 | 0.46 | 0.71 | +0.25 | −2 | 1.5 | +3.5 |
| 19 | 0.63 | 0.7 | +0.07 | −1.25 | 1.75 | +3 |
| 28 | 0.64 | 0.96 | +0.32 | −0.25 | 2.75 | +3 |
| 41 | 0.86 | 0.89 | +0.03 | −1 | 0.75 | +1.75 |
| 42 | 1.6 | 1.73 | +0.13 | −0.25 | 2.25 | +2.5 |
| 60 | 1.02 | 1.13 | +0.11 | −2.5 | 2.25 | +4.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simó-Servat, A.; Ibarra, M.; Libran, M.; Escobar, L.; Perea, V.; Quirós, C.; Puig-Jové, C.; Barahona, M.-J. Prospective Study to Evaluate Rectus Femoris Muscle Ultrasound for Body Composition Analysis in Patients Undergoing Bariatric Surgery. J. Clin. Med. 2024, 13, 3763. https://doi.org/10.3390/jcm13133763
Simó-Servat A, Ibarra M, Libran M, Escobar L, Perea V, Quirós C, Puig-Jové C, Barahona M-J. Prospective Study to Evaluate Rectus Femoris Muscle Ultrasound for Body Composition Analysis in Patients Undergoing Bariatric Surgery. Journal of Clinical Medicine. 2024; 13(13):3763. https://doi.org/10.3390/jcm13133763
Chicago/Turabian StyleSimó-Servat, Andreu, Montse Ibarra, Mireia Libran, Lilian Escobar, Verónica Perea, Carmen Quirós, Carlos Puig-Jové, and Maria-José Barahona. 2024. "Prospective Study to Evaluate Rectus Femoris Muscle Ultrasound for Body Composition Analysis in Patients Undergoing Bariatric Surgery" Journal of Clinical Medicine 13, no. 13: 3763. https://doi.org/10.3390/jcm13133763
APA StyleSimó-Servat, A., Ibarra, M., Libran, M., Escobar, L., Perea, V., Quirós, C., Puig-Jové, C., & Barahona, M.-J. (2024). Prospective Study to Evaluate Rectus Femoris Muscle Ultrasound for Body Composition Analysis in Patients Undergoing Bariatric Surgery. Journal of Clinical Medicine, 13(13), 3763. https://doi.org/10.3390/jcm13133763








