Postoperative Atrial Fibrillation Prediction by Left Atrial Size in Coronary Artery Bypass Grafting and Five-Year Survival Outcome
Abstract
1. Introduction
2. Patients and Methods
2.1. Ethical Statement
2.2. Patient Recruitment
2.3. Echocardiography
2.4. Primary Outcome
2.5. Secondary Outcomes and Follow-up
2.6. Statistical Analysis
3. Results
3.1. Study Cohort
3.2. ROC Analysis and Pearson Correlation
3.3. Univariate and Multivariate Logistic Regression
3.4. Cohorts with Preserved and Enlarged LA Diameter
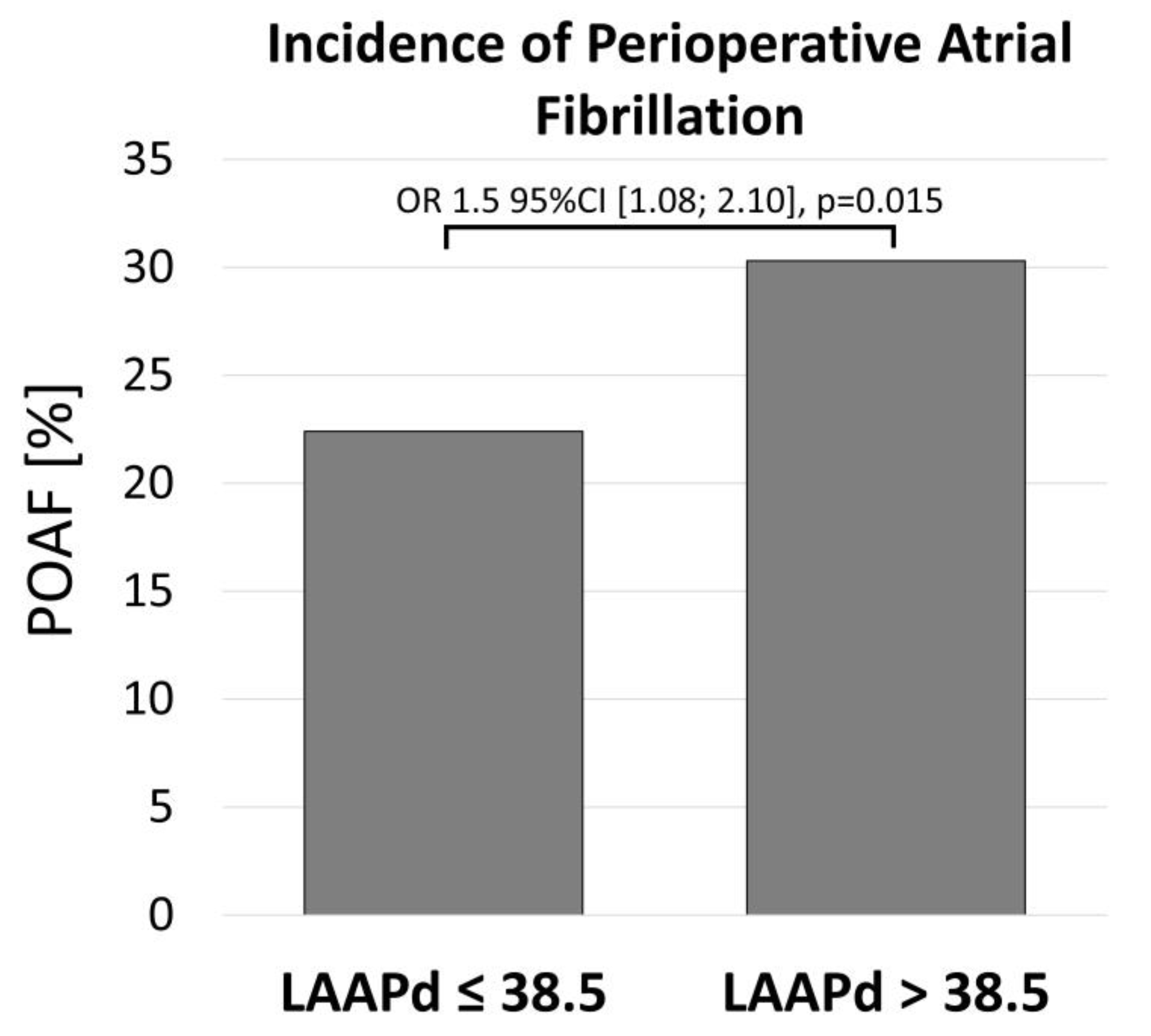
3.5. Incidence of POAF
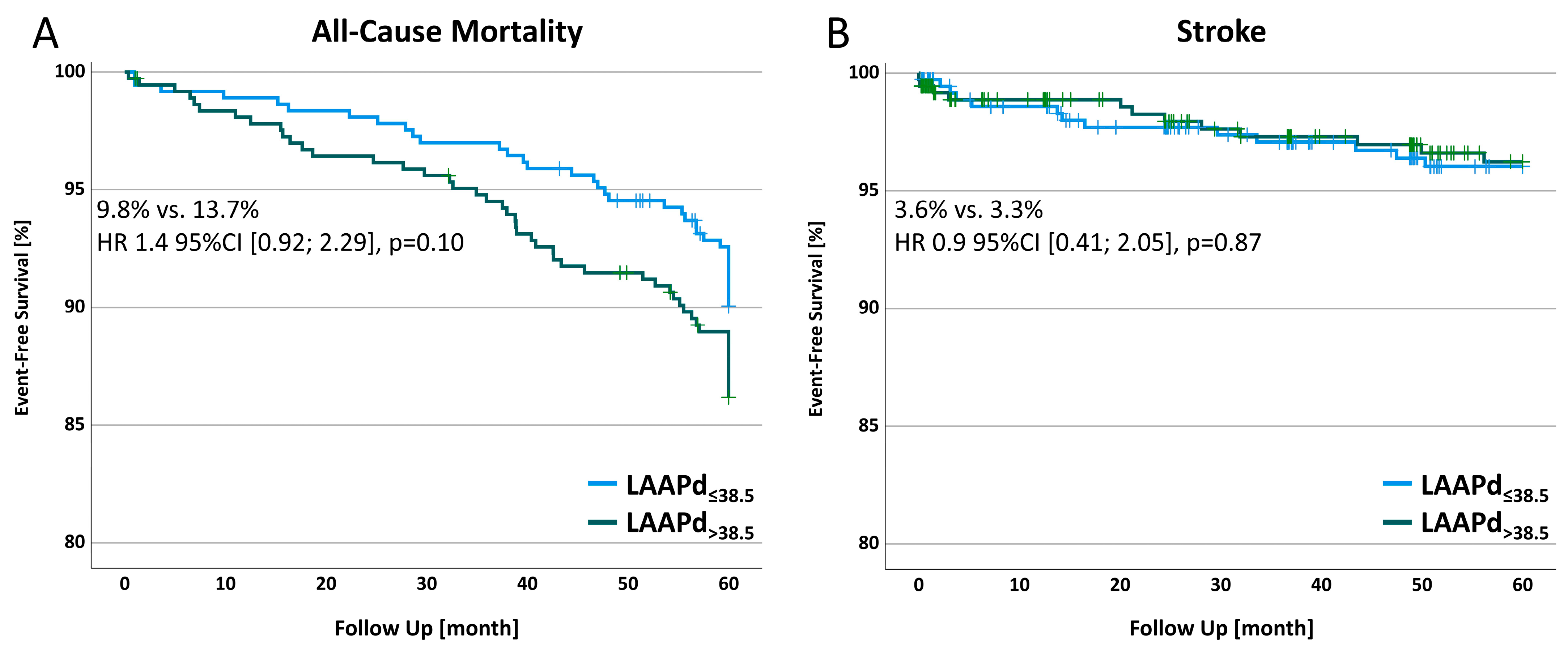
3.6. All-Cause Mortality and Stroke in a 5-Year Follow-up
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 95%CI | 95% confidence interval |
| AF | Atrial fibrillation |
| AHT | Arterial hypertension |
| ASA | American Society of Anesthesiologists |
| BMI | Body mass index |
| Ca | Calcium |
| CAD | Coronary artery disease |
| CCS | Canadian Cardiovascular Society |
| CHA2DS2-VASc | congestive heart failure, hypertension, age >75 (doubled), diabetes, stroke (doubled), vascular disease, age 65 to 74 and sex category (female) |
| COPD | Chronic obstructive pulmonary disease |
| CRT | Controlled randomized trial |
| DVT | Deep vein thrombosis |
| ECLS | Extracorporeal life support |
| LA | Left atrium |
| LAA | Left atrial appendage |
| LVEF | Left ventricular ejection fraction |
| HLP | Hyperlipidemia |
| HR | Hazard ratio |
| IABP | Intra-aortic balloon pump |
| NYHA | New York Heart Association |
| OPCAB | Off-pump coronary artery bypass grafting |
| OR | Odds Ratio |
| POAF | Postoperative atrial fibrillation |
| Preop. | Preoperative |
| PS | Propensity Score |
| ROC | Receiver operating characteristic |
| SD | Standard deviation |
| SMD | Standardized mean difference |
| SR | Sinus rhythm |
| STROBE | STrengthening the Reporting of OBservational studies in Epidemiology |
| TSH | Thyroid-stimulating hormone |
References
- Lubitz, S.A.; Yin, X.; Rienstra, M.; Schnabel, R.B.; Walkey, A.J.; Magnani, J.W.; Rahman, F.; McManus, D.D.; Tadros, T.M.; Levy, D.; et al. Long-Term Outcomes of Secondary Atrial Fibrillation in the Community. Circulation 2015, 131, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, D.; Aguilar, M.; Heijman, J.; Guichard, J.-B.; Nattel, S. Postoperative atrial fibrillation: Mechanisms, manifestations and management. Nat. Rev. Cardiol. 2019, 16, 417–436. [Google Scholar] [CrossRef]
- Bhave, P.D.; Goldman, L.E.; Vittinghoff, E.; Maselli, J.; Auerbach, A. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. Am. Heart J. 2012, 164, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, R.; Sanjanwala, R.; Le, M.-L.; Yamashita, M.H.; Arora, R.C. Postoperative Atrial Fibrillation After Cardiac Surgery: A Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2021, 111, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Gialdini, G.; Nearing, K.; Bhave, P.D.; Bonuccelli, U.; Iadecola, C.; Healey, J.S.; Kamel, H. Perioperative Atrial Fibrillation and the Long-term Risk of Ischemic Stroke. JAMA 2014, 312, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef] [PubMed]
- Oldgren, J.; Healey, J.S.; Ezekowitz, M.; Commerford, P.; Avezum, A.; Pais, P.; Zhu, J.; Jansky, P.; Sigamani, A.; Morillo, C.A.; et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: The RE-LY Atrial Fibrillation Registry. Circulation 2014, 129, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Stefano, P.L.; Bugetti, M.; Del Monaco, G.; Popescu, G.; Pieragnoli, P.; Ricciardi, G.; Perrotta, L.; Checchi, L.; Rondine, R.; Bevilacqua, S.; et al. Overweight and aging increase the risk of atrial fibrillation after cardiac surgery independently of left atrial size and left ventricular ejection fraction. J. Cardiothorac. Surg. 2020, 15, 316. [Google Scholar] [CrossRef]
- Osranek, M.; Fatema, K.; Qaddoura, F.; Al-Saileek, A.; Barnes, M.E.; Bailey, K.R.; Gersh, B.J.; Tsang, T.S.; Zehr, K.J.; Seward, J.B. Left atrial volume predicts the risk of atrial fibrillation after cardiac surgery: A prospective study. J. Am. Coll. Cardiol. 2006, 48, 779–786. [Google Scholar] [CrossRef]
- St-Onge, S.; Perrault, L.P.; Demers, P.; Boyle, E.M.; Gillinov, A.M.; Cox, J.; Melby, S. Pericardial Blood as a Trigger for Postoperative Atrial Fibrillation After Cardiac Surgery. Ann. Thorac. Surg. 2018, 105, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, I. Predictors Affecting Postoperative Atrial Fibrillation in Patients After Coronary Artery Bypass Graft. Clin. Nurs. Res. 2020, 29, 543–550. [Google Scholar] [CrossRef] [PubMed]
- El-Chami, M.F.; Kilgo, P.D.; Elfstrom, K.M.; Halkos, M.; Thourani, V.; Lattouf, O.M.; Delurgio, D.B.; Guyton, R.A.; Leon, A.R.; Puskas, J.D. Prediction of New Onset Atrial Fibrillation After Cardiac Revascularization Surgery. Am. J. Cardiol. 2012, 110, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.S.M.; Abhayaratna, W.P.; Barnes, M.E.; Miyasaka, Y.; Gersh, B.J.; Bailey, K.R.; Cha, S.S.; Seward, J.B. Prediction of Cardiovascular Outcomes With Left Atrial Size. J. Am. Coll. Cardiol. 2006, 47, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.S.M.; Barnes, M.E.; Bailey, K.R.; Leibson, C.L.; Montgomery, S.C.; Takemoto, Y.; Diamond, P.M.; Marra, M.A.; Gersh, B.J.; Wiebers, D.O.; et al. Left atrial volume: Important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin. Proc. 2001, 76, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Kinugawa, S.; Post, H.; Kaminski, P.M.; Zhang, X.; Xu, X.; Huang, H.; Recchia, F.A.; Ochoa, M.; Wolin, M.S.; Kaley, G.; et al. Coronary Microvascular Endothelial Stunning After Acute Pressure Overload in the Conscious Dog Is Caused by Oxidant Processes. Circulation 2003, 108, 2934–2940. [Google Scholar] [CrossRef] [PubMed]
- Kalifa, J.; Jalife, J.; Zaitsev, A.V.; Bagwe, S.; Warren, M.; Moreno, J.; Berenfeld, O.; Nattel, S. Intra-Atrial Pressure Increases Rate and Organization of Waves Emanating From the Superior Pulmonary Veins During Atrial Fibrillation. Circulation 2003, 108, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Staack, T.; Röcken, C.; Arndt, M.; Geller, J.C.; Huth, C.; Ansorge, S.; Klein, H.U.; Lendeckel, U. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J. Am. Coll. Cardiol. 2000, 35, 1669–1677. [Google Scholar] [CrossRef]
- Gaudino, M.; Angelini, G.D.; Antoniades, C.; Bakaeen, F.; Benedetto, U.; Calafiore, A.M.; Di Franco, A.; Di Mauro, M.; Fremes, S.E.; Girardi, L.N.; et al. Off-Pump Coronary Artery Bypass Grafting: 30 Years of Debate. J. Am. Heart Assoc. 2018, 7, e009934. [Google Scholar] [CrossRef]
- Gercek, M.; Borgermann, J.; Gummert, J.; Gercek, M. Five-year-outcome of new-onset perioperative atrial fibrillation after left atrial appendage amputation concomitant with cardiac surgery. Clin. Res. Cardiol. 2023, 112, 1800–1811. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. A comparison of 12 algorithms for matching on the propensity score. Stat. Med. 2014, 33, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Goodarzynejad, H.; Mortazavi, S.H.; Bina, P.; Jalali, A.; Salehi Omran, A.; Ahmadi Tafti, S.H.; Abbasi, K.; Hossein Sabet, A.; Sadeghian, S. Left Atrial Size; a Missing Component in Scoring Systems for Predicting Atrial Fibrillation Following Coronary Artery Bypass Surgery. Acta Cardiol. Sin. 2020, 36, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Bramer, S.; van Straten, A.H.; Soliman Hamad, M.A.; Berreklouw, E.; van den Broek, K.C.; Maessen, J.G. Body mass index predicts new-onset atrial fibrillation after cardiac surgery. Eur. J. Cardiothorac. Surg. 2011, 40, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Bang, C.N.; Gislason, G.H.; Greve, A.M.; Bang, C.A.; Lilja, A.; Torp-Pedersen, C.; Andersen, P.K.; Køber, L.; Devereux, R.B.; Wachtell, K. New-Onset Atrial Fibrillation is Associated With Cardiovascular Events Leading to Death in a First Time Myocardial Infarction Population of 89 703 Patients With Long-Term Follow-Up: A Nationwide Study. J. Am. Heart Assoc. 2014, 3, e000382. [Google Scholar] [CrossRef]
- Magne, J.; Salerno, B.; Mohty, D.; Serena, C.; Rolle, F.; Piccardo, A.; Echahidi, N.; Le Guyader, A.; Aboyans, V. Echocardiography is useful to predict postoperative atrial fibrillation in patients undergoing isolated coronary bypass surgery: A prospective study. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef]
- Khankirawatana, B.; Khankirawatana, S.; Porter, T. How should left atrial size be reported? Comparative assessment with use of multiple echocardiographic methods. Am. Heart J. 2004, 147, 369–374. [Google Scholar] [CrossRef]
- Lester, S.J.; Ryan, E.W.; Schiller, N.B.; Foster, E. Best method in clinical practice and in research studies to determine left atrial size. Am. J. Cardiol. 1999, 84, 829–832. [Google Scholar] [CrossRef]
- Pritchett, A.M.; Jacobsen, S.J.; Mahoney, D.W.; Rodeheffer, R.J.; Bailey, K.R.; Redfield, M.M. Left atrial volume as an index ofleft atrial size: A population-based study. J. Am. Coll. Cardiol. 2003, 41, 1036–1043. [Google Scholar] [CrossRef]
- Klopotowski, M.; Kwapiszewska, A.; Kukula, K.; Jamiolkowski, J.; Dabrowski, M.; Derejko, P.; Oreziak, A.; Baranowski, R.; Spiewak, M.; Marczak, M.; et al. Clinical and echocardiographic parameters as risk factors for atrial fibrillation in patients with hypertrophic cardiomyopathy. Clin. Cardiol. 2018, 41, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Losi, M.-A.; Betocchi, S.; Aversa, M.; Lombardi, R.; Miranda, M.; D’Alessandro, G.; Cacace, A.; Tocchetti, C.-G.; Barbati, G.; Chiariello, M. Determinants of atrial fibrillation development in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2004, 94, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Zacà, V.; Galderisi, M.; Mondillo, S.; Focardi, M.; Ballo, P.; Guerrini, F. Left atrial enlargement as a predictor of recurrences in lone paroxysmal atrial fibrillation. Can. J. Cardiol. 2007, 23, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Kaireviciute, D.; Aidietis, A.; Lip, G.Y.H. Atrial fibrillation following cardiac surgery: Clinical features and preventative strategies. Eur. Heart J. 2009, 30, 410–425. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.B.; Exner, D.V.; Wyse, D.G.; Connolly, C.J.; Prystai, G.D.; Bayes, A.J.; Kidd, W.T.; Kieser, T.; Burgess, J.J.; Ferland, A.; et al. Prophylactic Oral Amiodarone for the Prevention of Arrhythmias That Begin Early After Revascularization, Valve Replacement, or RepairPAPABEAR: A Randomized Controlled Trial. JAMA 2005, 294, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Sanna, T.; Ballman, K.V.; Robinson, N.B.; Hameed, I.; Audisio, K.; Rahouma, M.; Di Franco, A.; Soletti, G.J.; Lau, C.; et al. Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: An adaptive, single-centre, single-blind, randomised, controlled trial. Lancet 2021, 398, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, R.P.; Belley-Cote, E.P.; Paparella, D.; Healey, J.S.; Brady, K.; Sharma, M.; Reents, W.; Budera, P.; Baddour, A.J.; Fila, P.; et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N. Engl. J. Med. 2021, 384, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Gercek, M.; Borgermann, J.; Gercek, M.; Gummert, J. Left atrial appendage amputation concomitant with cardiac surgery in patients with sinus rhythm. Eur. J. Cardiothorac. Surg. 2023, 63, ezad088. [Google Scholar] [CrossRef]
- El-Chami, M.F.; Kilgo, P.; Thourani, V.; Lattouf, O.M.; Delurgio, D.B.; Guyton, R.A.; Leon, A.R.; Puskas, J.D. New-onset atrial fibrillation predicts long-term mortality after coronary artery bypass graft. J. Am. Coll. Cardiol. 2010, 55, 1370–1376. [Google Scholar] [CrossRef]
- Phan, K.; Ha, H.S.; Phan, S.; Medi, C.; Thomas, S.P.; Yan, T.D. New-onset atrial fibrillation following coronary bypass surgery predicts long-term mortality: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2015, 48, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.S.M.; Barnes, M.E.; Gersh, B.J.; Bailey, K.R.; Seward, J.B. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am. J. Cardiol. 2002, 90, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Osranek, M.; Bursi, F.; Bailey, K.R.; Grossardt, B.R.; Brown, R.D., Jr.; Kopecky, S.L.; Tsang, T.S.; Seward, J.B. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: Three-decade follow-up. Eur. Heart J. 2005, 26, 2556–2561. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.C.; Huang, C.Y.; Chuang, S.L.; Yu, H.Y.; Chen, Y.S.; Wang, C.H.; Lin, L.Y. Long Term Outcome of Postoperative Atrial Fibrillation After Cardiac Surgery-A Propensity Score-Matched Cohort Analysis. Front. Cardiovasc. Med. 2021, 8, 650147. [Google Scholar] [CrossRef]
- Mathew, J.P.; Parks, R.; Savino, J.S.; Friedman, A.S.; Koch, C.; Mangano, D.T.; Browner, W.S. Atrial Fibrillation Following Coronary Artery Bypass Graft Surgery: Predictors, Outcomes, and Resource Utilization. JAMA 1996, 276, 300–306. [Google Scholar] [CrossRef]


| Variable | p-Value |
|---|---|
| Univariate Logistic Regression | |
| Left atrial size (LAAPd) | <0.01 |
| BMI | 0.19 |
| LVEF | 0.07 |
| Age | <0.001 |
| CHA2DS2-VASc-score | <0.01 |
| Multivariate Logistic Regression | |
| Left atrial diameter | 0.02 |
| LVEF | 0.11 |
| Age | 0.01 |
| CHA2DS2-VASc-score | 0.53 |
| Variable | All | Unmatched Cohorts | Propensity Score Matched Cohorts | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LAAPd≤38.5 mm n (%) | LAAPd>38.5 mm n (%) | OR [95%CI] | SMD | p-Value | LAAPd≤38.5 mm n (%) | LAAPd>38.5 mm n (%) | OR [95%CI] | SMD | p-Value | ||
| 933 (100) | 426 (100) | 507 (100) | 366 (100.0) | 366 (100.0) | |||||||
| Preop. MI | 256 (27.4) | 121 (28.4) | 135 (26.6) | 0.9 [0.69–1.22] | −0.040 | 0.55 | 104 (28.4) | 103 (28.1) | 1.0 [0.72–1.36] | −0.006 | 0.94 |
| AHT | 801 (85.9) | 353 (82.9) | 448 (88.4) | 1.6 [1.08–2.27] | 0.172 | 0.016 | 305 (83.3) | 314 (85.8) | 1.2 [0.81–1.81] | 0.077 | 0.36 |
| Smoking | 448 (48) | 210 (49.3) | 238 (47.0) | 0.9 [0.70–1.18] | −0.047 | 0.47 | 180 (49.2) | 185 (50.6) | 1.1 [0.79–1.41] | 0.027 | 0.71 |
| Diabetes | 309 (33.1) | 138 (32.4) | 171 (33.7) | 1.1 [0.81–1.40] | 0.028 | 0.67 | 116 (31.7) | 129 (35.3) | 1.2 [0.86–1.60] | 0.075 | 0.31 |
| HLP | 863 (92.5) | 385 (90.4) | 478 (94.3) | 1.8 [1.07–2.88] | 0.168 | 0.024 | 337 (92.1) | 339 (92.6) | 1.1 [0.63–1.86] | 0.024 | 0.78 |
| DVT | 13 (1.4) | 7 (1.6) | 6 (1.2) | 0.7 [0.24–2.15] | −0.043 | 0.55 | 6 (1.6) | 3 (0.8) | 0.5 [0.12–2.00] | −0.076 | 0.31 |
| Preop. Dialysis | 6 (0.6) | 1 (0.2) | 5 (1.00) | 4.2 [0.49–36.37] | 0.076 | 0.15 | 1 (0.3) | 3 (0.8) | 3.0 [0.30–29.10] | 0.055 | 0.32 |
| COPD | 56 (6) | 20 (4.7) | 36 (7.) | 1.6 [0.88–2.72] | 0.094 | 0.12 | 20 (5.5) | 19 (5.2) | 1.0 [0.50–1.81] | −0.011 | 0.87 |
| Beta-blockers | 619 (66.3) | 278 (65.3) | 341 (67.3) | 1.1 [0.83–1.44] | 0.043 | 0.52 | 238 (65.0) | 238 (65.0) | 1.0 [0.74–1.36] | 0.000 | >0.99 |
| Ca antagonists | 186 (19.9) | 69 (16.2) | 117 (23.1) | 1.6 [1.12–2.16] | 0.163 | <0.01 | 61 (16.7) | 71 (19.4) | 1.2 [0.82–1.76] | 0.065 | 0.34 |
| Sex [female] | 160 (17.1) | 87 (20.4) | 73 (14.4) | 0.7 [0.47–0.92] | −0.171 | 0.015 | 62 (16.9) | 61 (16.7) | 1.0 [0.67–1.44] | −0.008 | 0.92 |
| ASA [class] | 2.9 ± 0.5 | 2.9 ± 0.5 | 2.9 ± 0.5 | −0.11–0.02 | 0.089 | 0.21 | 2.9 ± 0.5 | 2.9 ± 0.5 | −0.11–0.04 | 0.071 | 0.39 |
| NYHA [class] | 2.0 ± 0.9 | 2.1 ± 0.9 | 2.03 ± 0.9 | −0.09–0.13 | −0.030 | 0.65 | 2.0 ± 0.9 | 2.0 ± 0.9 | −0.12–0.12 | −0.003 | 0.97 |
| CCS [class] | 2.6 ± 1.2 | 2.7 ± 1.2 | 2.5 ± 1.2 | 0.01–0.32 | −0.145 | 0.034 | 2.6 ± 1.2 | 2.5 ± 1.2 | −0.10–0.23 | −0.061 | 0.41 |
| LVEF [%] | 59.1 ± 9.1 | 60.0 ± 9.0 | 58.4 ± 9.2 | 0.43–2.78 | −0.176 | <0.01 | 59.4 ± 8.7 | 59.0 ± 9.2 | −0.82–1.64 | −0.045 | 0.51 |
| EurosScore II [%] | 1.5 ± 1.5 | 1.6 ± 1.8 | 1.4 ± 1.2 | −0.04–0.34 | −0.126 | 0.12 | 1.5 ± 1.6 | 1.5 ± 1.3 | −0.24–0.18 | 0.017 | 0.84 |
| CHA2DS2VASc-score [points] | 2.4 ± 1.3 | 2.3 ± 1.3 | 2.4 ± 1.3 | −0.26–0.08 | 0.064 | 0.33 | 2.3 ± 1.3 | 2.4 ± 1.3 | −0.29–0.08 | 0.079 | 0.27 |
| Age [years] | 64.5 ± 9.4 | 64.3 ± 9.5 | 64.6 ± 9.3 | −1.59–0.84 | 0.040 | 0.55 | 64.1 ± 9.5 | 64.4 ± 9.2 | −1.71–1.04 | 0.036 | 0.64 |
| BMI [kg/m2BSA] | 28.7 ± 4.2 | 27.9 ± 4.0 | 29.4 ± 4.3 | −2.07–−0.99 | 0.354 | <0.01 | 28.4 ± 4.0 | 28.8 ± 4.2 | −0.88–0.10 | 0.090 | 0.12 |
| TSH [µU/mL] | 1.4 ± 2.2 | 1.3 ± 1.1 | 1.5 ± 2.8 | −0.55–0.02 | 0.091 | 0.08 | 1.3 ± 1.1 | 1.3 ± 1.3 | −0.17–0.16 | <0.001 | >0.99 |
| Potassium [mmol/L] | 3.9 ± 0.8 | 3.8 ± 0.9 | 3.9 ± 0.7 | −0.21–0.00 | 0.142 | 0.06 | 3.9 ± 0.8 | 3.9 ± 0.7 | −0.16–0.04 | 0.079 | 0.27 |
| Sodium [mmol/L] | 135.2 ± 24.4 | 133.6 ± 28.2 | 136.6 ± 20.5 | −6.14–0.14 | 0.146 | 0.07 | 135.3 ± 24.0 | 136.6 ± 20.6 | −4.20–1.68 | 0.061 | 0.40 |
| Calcium [mmol/L] | 2.3 ± 0.4 | 2.3 ± 0.5 | 2.4 ± 0.4 | −0.11–0.00 | 0.145 | 0.07 | 2.3 ± 0.4 | 2.4 ± 0.4 | −0.08–0.02 | 0.068 | 0.36 |
| Creatinine [mg/dL] | 1.0 ± 0.5 | 1.0 ± 0.3 | 1.1 ± 0.6 | −0.15–−0.02 | 0.132 | 0.014 | 1.0 ± 0.3 | 1.0 ± 0.5 | −0.10–0.02 | 0.064 | 0.22 |
| Variable | LAAPd ≤ 38.5 mm n (%) | LAAPd > 38.5 mm n (%) | OR/HR [95%CI] | p-Value |
|---|---|---|---|---|
| 366 (100) | 366 (100) | |||
| Primary Endpoint | ||||
| POAF | 82 (22.4) | 111 (30.3) | 1.5 [1.08–2.10] | 0.015 |
| Secondary End-Point (5y Follow-Up) | ||||
| All-cause mortality | 36 (9.8) | 50 (13.7) | 1.4 [0.92–2.29] | 0.10 |
| Stroke | 13 (3.6) | 12 (3.3) | 0.9 [0.41–2.05] | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerçek, M.; Börgermann, J.; Gummert, J.; Gerçek, M. Postoperative Atrial Fibrillation Prediction by Left Atrial Size in Coronary Artery Bypass Grafting and Five-Year Survival Outcome. J. Clin. Med. 2024, 13, 3738. https://doi.org/10.3390/jcm13133738
Gerçek M, Börgermann J, Gummert J, Gerçek M. Postoperative Atrial Fibrillation Prediction by Left Atrial Size in Coronary Artery Bypass Grafting and Five-Year Survival Outcome. Journal of Clinical Medicine. 2024; 13(13):3738. https://doi.org/10.3390/jcm13133738
Chicago/Turabian StyleGerçek, Mustafa, Jochen Börgermann, Jan Gummert, and Muhammed Gerçek. 2024. "Postoperative Atrial Fibrillation Prediction by Left Atrial Size in Coronary Artery Bypass Grafting and Five-Year Survival Outcome" Journal of Clinical Medicine 13, no. 13: 3738. https://doi.org/10.3390/jcm13133738
APA StyleGerçek, M., Börgermann, J., Gummert, J., & Gerçek, M. (2024). Postoperative Atrial Fibrillation Prediction by Left Atrial Size in Coronary Artery Bypass Grafting and Five-Year Survival Outcome. Journal of Clinical Medicine, 13(13), 3738. https://doi.org/10.3390/jcm13133738






