Agreement between CT-Angiography and Digital Subtraction Angiography in Predicting Angiographic Vasospasm in Patients with Subarachnoid Hemorrhage
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Radiological Assessment
2.3. Statistical Analysis
3. Results
3.1. Population
3.2. Radiological Assessment
3.2.1. At Admission
3.2.2. Vasospasm Period
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorsch, N.W.; King, M.T. A Review of Cerebral Vasospasm in Aneurysmal Subarachnoid Haemorrhage Part I: Incidence and Effects. J. Clin. Neurosci. 1994, 1, 19–26. [Google Scholar] [CrossRef]
- Janjua, N.; Mayer, S.A. Cerebral Vasospasm after Subarachnoid Hemorrhage. Curr. Opin. Crit. Care 2003, 9, 113–119. [Google Scholar] [CrossRef]
- Yao, G.-E.; Li, Q.; Jiang, X.-J.; Liu, J.; Li, J.-L.; Zhang, L.-L.; Li, L.-L.; Zhang, J.; Xie, P. Vasospasm after Subarachnoid Hemorrhage: A 3D Rotational Angiography Study. Acta Neurochir. Suppl. 2011, 110, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.N.; Mehta, V.; Russin, J.; Amar, A.P.; Rajamohan, A.; Mack, W.J. Advanced Imaging Modalities in the Detection of Cerebral Vasospasm. Neurol. Res. Int. 2013, 2013, 415960. [Google Scholar] [CrossRef]
- Samagh, N.; Bhagat, H.; Jangra, K. Monitoring Cerebral Vasospasm: How Much Can We Rely on Transcranial Doppler. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, X.; Wu, B. Neuroimaging Research on Cerebrovascular Spasm and Its Current Progress. Acta Neurochir. Suppl. 2011, 110, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Bederson, J.B.; Connolly, E.S.; Batjer, H.H.; Dacey, R.G.; Dion, J.E.; Diringer, M.N.; Duldner, J.E.; Harbaugh, R.E.; Patel, A.B.; Rosenwasser, R.H.; et al. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage: A Statement for Healthcare Professionals from a Special Writing Group of the Stroke Council, American Heart Association. Stroke 2009, 40, 994–1025. [Google Scholar] [CrossRef]
- Soumah, M.; Brami, J.; Simonato, D.; Chousterman, B.; Guillonnet, A.; Bernat, A.-L.; Houdart, E.; Labeyrie, M.-A. Computed Tomography Angiography for Quantification of Cerebral Vasospasm Following Aneurysmal Subarachnoid Hemorrhage. Diagn. Interv. Imaging 2022, 103, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.L. Delayed Neurological Deterioration after Subarachnoid Haemorrhage. Nat. Rev. Neurol. 2014, 10, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Vergouwen, M.D.I.; Ilodigwe, D.; Macdonald, R.L. Cerebral Infarction after Subarachnoid Hemorrhage Contributes to Poor Outcome by Vasospasm-Dependent and -Independent Effects. Stroke 2011, 42, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Pradilla, G.; Chaichana, K.L.; Hoang, S.; Huang, J.; Tamargo, R.J. Inflammation and Cerebral Vasospasm after Subarachnoid Hemorrhage. Neurosurg. Clin. N. Am. 2010, 21, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Vergouwen, M.D.I. Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage Vasospasm versus Delayed Cerebral Ischemia as an Outcome Event in Clinical Trials and Observational Studies. Neurocrit. Care 2011, 15, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Hébert, J.; Roncarolo, F.; Tampieri, D.; Cortes, M. delPilar 320-Row Multidetector Computed Tomographic Angiogram in the Evaluation of Cerebral Vasospasm After Aneurysmal Subarachnoid Hemorrhage: A Pilot Study. J. Comput. Assist. Tomogr. 2015, 39, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.D.; Rosen, D.S.; Bardo, D.; Macdonald, R.L. Arterial Diameters on Catheter and Computed Tomographic Angiography. World Neurosurg. 2010, 73, 165–173; discussion e25. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.B.; Ashforth, R.; Steinke, D.E.; Findlay, J.M. CT Angiography for the Detection of Cerebral Vasospasm in Patients with Acute Subarachnoid Hemorrhage. AJNR Am. J. Neuroradiol. 2000, 21, 1011–1015. [Google Scholar] [PubMed]
- Joo, S.P.; Kim, T.S.; Kim, Y.S.; Moon, K.S.; Lee, J.K.; Kim, J.H.; Kim, S.H. Clinical Utility of Multislice Computed Tomographic Angiography for Detection of Cerebral Vasospasm in Acute Subarachnoid Hemorrhage. Minim. Invasive Neurosurg. 2006, 49, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Otawara, Y.; Ogasawara, K.; Ogawa, A.; Sasaki, M.; Takahashi, K. Evaluation of Vasospasm after Subarachnoid Hemorrhage by Use of Multislice Computed Tomographic Angiography. Neurosurgery 2002, 51, 939–942; discussion 942–943. [Google Scholar] [CrossRef] [PubMed]
- Kerkeni, H.; Schatlo, B.; Dan-Ura, H.; Remonda, L.; Muroi, C.; Diepers, M.; Fandino, J.; Fathi, A.-R. Proximal Arterial Diameters on CT Angiography and Digital Subtraction Angiography Correlate Both at Admission and in the Vasospasm Period after Aneurysmal Subarachnoid Hemorrhage. Acta Neurochir. Suppl. 2015, 120, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Letourneau-Guillon, L.; Farzin, B.; Darsaut, T.E.; Kotowski, M.; Guilbert, F.; Chagnon, M.; Diouf, A.; Roy, D.; Weill, A.; Lemus, M.; et al. Reliability of CT Angiography in Cerebral Vasospasm: A Systematic Review of the Literature and an Inter- and Intraobserver Study. AJNR Am. J. Neuroradiol. 2020, 41, 612–618. [Google Scholar] [CrossRef]
- Hosmann, A.; Wang, W.; Dodier, P.; Bavinzski, G.; Engel, A.; Herta, J.; Plöchl, W.; Reinprecht, A.; Gruber, A. The Impact of Intra-Arterial Papaverine-Hydrochloride on Cerebral Metabolism and Oxygenation for Treatment of Delayed-Onset Post-Subarachnoid Hemorrhage Vasospasm. Neurosurgery 2020, 87, 712–719. [Google Scholar] [CrossRef]
- Hosmann, A.; Rauscher, S.; Wang, W.-T.; Dodier, P.; Bavinzski, G.; Knosp, E.; Gruber, A. Intra-Arterial Papaverine-Hydrochloride and Transluminal Balloon Angioplasty for Neurointerventional Management of Delayed-Onset Post-Aneurysmal Subarachnoid Hemorrhage Vasospasm. World Neurosurg. 2018, 119, e301–e312. [Google Scholar] [CrossRef] [PubMed]
- Hosmann, A.; Angelmayr, C.; Hopf, A.; Rauscher, S.; Brugger, J.; Ritscher, L.; Bohl, I.; Schnackenburg, P.; Engel, A.; Plöchl, W.; et al. Detrimental Effects of Intrahospital Transport on Cerebral Metabolism in Patients Suffering Severe Aneurysmal Subarachnoid Hemorrhage. J. Neurosurg. 2021, 135, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Lubrica, R.J.; Song, M.; Vandse, R.; Boling, W.; Pillai, P. The Role of Transcranial Doppler in Cerebral Vasospasm: A Literature Review. Acta Neurochir. Suppl. 2020, 127, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Darsaut, T.E.; Keough, M.B.; Chan, A.M.; Farzin, B.; Findlay, J.M.; Chow, M.M.; Chagnon, M.; Zehr, J.; Gevry, G.; Raymond, J. Transcranial Doppler Velocities and Angiographic Vasospasm after SAH: A Diagnostic Accuracy Study. AJNR Am. J. Neuroradiol. 2022, 43, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Ran, Y.; Chen, R.; Zhang, F.; Lei, X.; Wang, X.; Li, T.; Zhu, J.; Zhang, Y.; Cheng, J.; et al. Use of PETRA-MRA to Assess Intracranial Arterial Stenosis: Comparison with TOF-MRA, CTA, and DSA. Front. Neurol. 2022, 13, 1068132. [Google Scholar] [CrossRef] [PubMed]
- Burth, S.; Meis, J.; Kronsteiner, D.; Heckhausen, H.; Zweckberger, K.; Kieser, M.; Wick, W.; Ulfert, C.; Möhlenbruch, M.; Ringleb, P.; et al. Outcome Analysis for Patients with Subarachnoid Hemorrhage and Vasospasm Including Endovascular Treatment. Neurol. Res. Pract. 2023, 5, 57. [Google Scholar] [CrossRef]
- Claassen, J.; Park, S. Spontaneous Subarachnoid Haemorrhage. Lancet 2022, 400, 846–862. [Google Scholar] [CrossRef]
- Neifert, S.N.; Chapman, E.K.; Martini, M.L.; Shuman, W.H.; Schupper, A.J.; Oermann, E.K.; Mocco, J.; Macdonald, R.L. Aneurysmal Subarachnoid Hemorrhage: The Last Decade. Transl. Stroke Res. 2021, 12, 428–446. [Google Scholar] [CrossRef]

| Age [years] | 48 ± 11 * | |
| sex | Female | 36 |
| Male | 15 | |
| Hunt and Hess Grade | 3 ± 1 * | |
| Type of SAH | Aneurysmal subarachnoid hemorrhage | 49 |
| Prepontine subarachnoid hemorrhage | 2 | |
| Ruptured aneurysm | - Middle cerebral artery | 9 |
| - Anterior cerebral artery | 0 | |
| - Anterior communicating artery | 19 | |
| - Internal carotid artery | 3 | |
| - Basilar artery | 2 | |
| - Posterior communicating artery | 6 | |
| - Posterior inferior cerebellar artery | 3 | |
| - Anterior choroidal artery | 3 | |
| - Pericallosal artery | 2 | |
| - Superior cerebellar artery | 1 | |
| - No aneurysm | 2 | |
| Type of intervention | Coiling | 15 |
| Clipping | 31 | |
| Coiling and clipping | 1 | |
| No intervention | 4 | |
| Timing of intervention (days after SAH) | 1 ± 3 * | |
| Sedation | Deeply sedated | 40 |
| Awake | 9 | |
| State of wakefulness not documented | 2 | |
| Mean maximum transcranial Doppler velocity [cm/s] | 180 ± 36 |
| Segment | ICA | M1 | A1 | VA | BA | P1 |
|---|---|---|---|---|---|---|
| At time of admission | ||||||
| CTA diameter [mm] | 2.2 ± 0.41 | 1.48 ± 0.39 | 1.09 ± 0.29 | 1.57 ± 0.35 | 1.89 ± 0.51 | 1.16 ± 0.35 |
| DSA diameter [mm] | 2.45 ± 0.51 | 1.71 ± 0.47 | 1.28 ± 0.37 | 2.09 ± 0.52 | 2.08 ± 0.56 | 1.34 ± 0.40 |
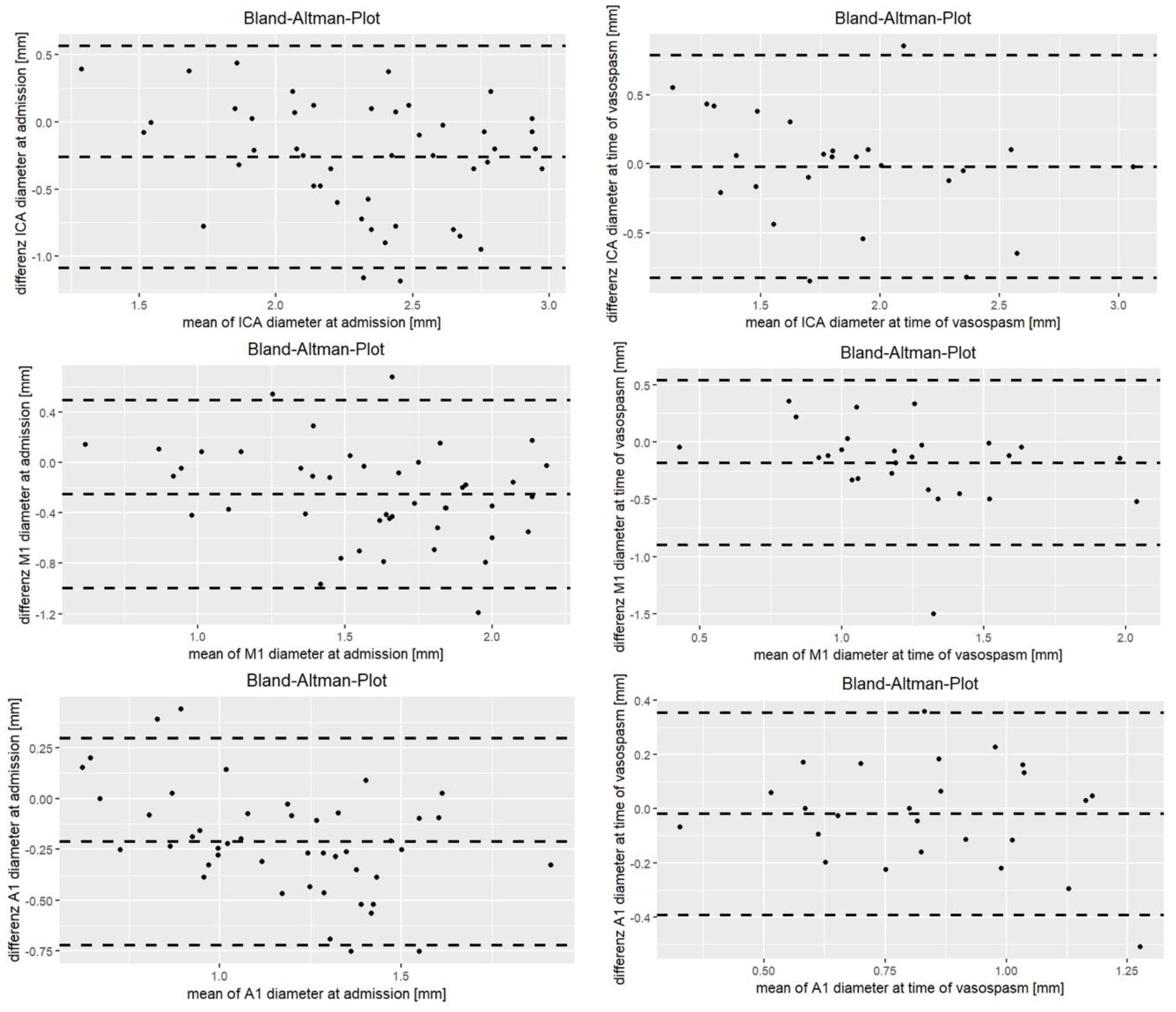
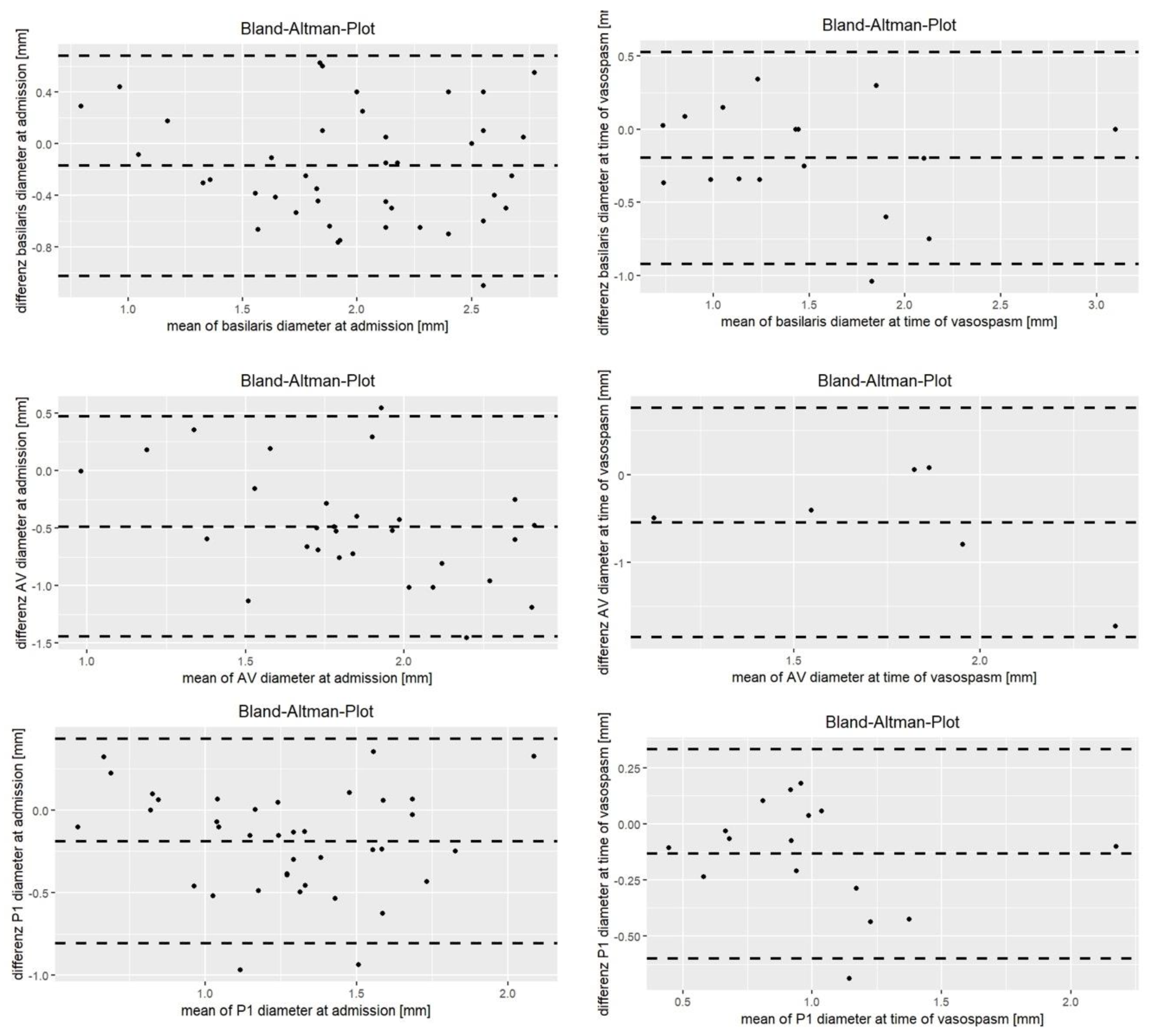
| Difference CTA—DSA [mm] | −0.26 ± 0.42 (95% CI −0.39; −0.14) p < 0.001 | −0.25 (±0.38) (95% CI 0.37; −0.13) p < 0.001 | −0.21 ± 0.26 (95% CI −0.29; −0.13) p < 0.001 | −0.49 (±0.49) (95% CI −0.67; −0.30) p < 0.001 | −0.17 (±0.43) (95% CI −0.31; −0.04) p = 0.01 | −0.19 (±0.32) (95% CI −0.29; −0.09) p < 0.001 |
| Intraclass correlation coefficient | 0.51 (95% CI 0.18, 0.72) | 0.53 (95% CI 0.18, 0.74) | 0.55 (95% CI 0.12, 0.77 | 0.23 (95% CI −0.09, 0.54) | 0.65 (95% CI 0.42, 0.80) | 0.58 (95% CI 0.24, 0.77) |
| At time of suspected vasospasm | ||||||
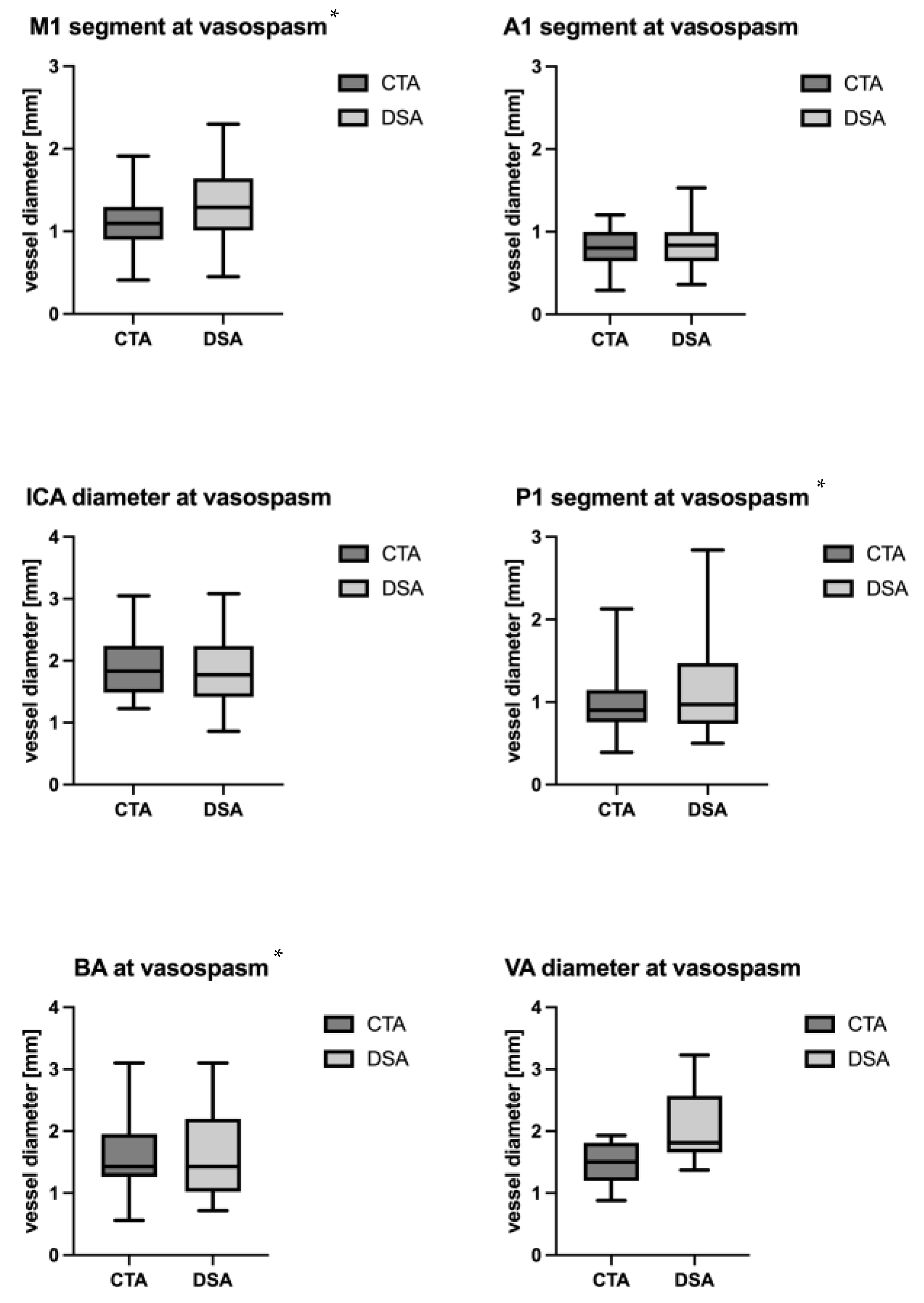
| CTA diameter [mm] mean ± SD | 1.89 ± 0.47 | 1.12 ± 0.33 | 0.82 ± 0.22 | 1.48 ± 0.33 | 1.56 ± 0.58 | 0.96 ± 0.35 |
| DSA diameter [mm] mean ± SD | 1.85 ± 0.57 | 1.33 ± 0.45 | 0.83 ± 0.28 | 2.05 ± 0.65 | 1.58 ± 0.67 | 1.17 ± 0.61 |
| Difference betweeen CTA and DSA [mm] | −0.02 ± 0.41 (95% CI −0.19; 0.15) p = 0.79 | −0.18 ± 0.37 (95% CI −0.33; −0.03) p = 0.02 | −0.02 ± 0.41 (95% CI −0.10; 0.06) p = 0.62 | −0.55 ± 0.67 (95% CI −1.25; 0.15) p = 0.1 | −0.20 ± 0.37 (95% CI −0.39; −0.01) p = 0.04 | −0.13 ± 0.24 (95% CI −0.26; −0.01) p = 0.04 |
| Intraclass correlation coefficient | 0.69 (95% CI 0.41, 0.85) | 0.53 (95% CI 0.18, 0.76) | 0.72 (95% CI 0.46, 0.87) | 0.16 (95% CI −0.33, 0.77) | 0.80 (95% CI 0.51, 0.93) | 0.80 (95% CI 0.48, 0.93) |
| VSP Grading at Time of Suspected Vasospasm in DSA and CTA | |||||
|---|---|---|---|---|---|
| No Vasospasm | Mild | Moderate | Severe | ||
| ICA (n = 23) | DSA | 9% (2) | 61% (14) | 30% (7) | 0% (0) |
| CTA | 8.5% (2) | 69.5% (16) | 22% (5) | 0% (0) | |
| p-value | p = 0.95 | ||||
| M1 (n = 24) | DSA | 16% (4) | 42% (10) | 42% (10) | 0% (0) |
| CTA | 4% (1) | 42% (10) | 54% (13) | 0% (0) | |
| p-value | p = 0.008 | ||||
| A1 (n = 23) | DSA | 4% (1) | 46% (11) | 46% (11) | 0% (0) |
| CTA | 0% (0) | 52% (12) | 48% (11) | 0% (0) | |
| p-value | p = 0.75 | ||||
| VA (n = 6) | DSA | 33% (2) | 50% (3) | 16.6% (1) | 0% (0) |
| CTA | 0% (0) | 66.6% (4) | 33.3% (2) | 0% (0) | |
| p-value | p = 0.12 | ||||
| BA (n = 15) | DSA | 26.6% (4) | 33.3% (5) | 40% (6) | 0% (0) |
| CTA | 13.3% (2) | 46.6% (7) | 40% (6) | 0% (0) | |
| p-value | p = 0.06 | ||||
| P1 (n = 14) | DSA | 7% (1) | 57% (8) | 36% (5) | 0% (0) |
| CTA | 12% (2) | 50% (7) | 43% (6) | 7% (1) | |
| p-value | p = 0.06 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moser, M.M.; Gramss, L.; Marik, W.; Weber, M.; Hirschmann, D.; Wang, W.-T.; Dodier, P.; Kasprian, G.; Bavinzski, G.; Rössler, K.; et al. Agreement between CT-Angiography and Digital Subtraction Angiography in Predicting Angiographic Vasospasm in Patients with Subarachnoid Hemorrhage. J. Clin. Med. 2024, 13, 3743. https://doi.org/10.3390/jcm13133743
Moser MM, Gramss L, Marik W, Weber M, Hirschmann D, Wang W-T, Dodier P, Kasprian G, Bavinzski G, Rössler K, et al. Agreement between CT-Angiography and Digital Subtraction Angiography in Predicting Angiographic Vasospasm in Patients with Subarachnoid Hemorrhage. Journal of Clinical Medicine. 2024; 13(13):3743. https://doi.org/10.3390/jcm13133743
Chicago/Turabian StyleMoser, Miriam M., Leon Gramss, Wolfgang Marik, Michael Weber, Dorian Hirschmann, Wei-Te Wang, Philippe Dodier, Gregor Kasprian, Gerhard Bavinzski, Karl Rössler, and et al. 2024. "Agreement between CT-Angiography and Digital Subtraction Angiography in Predicting Angiographic Vasospasm in Patients with Subarachnoid Hemorrhage" Journal of Clinical Medicine 13, no. 13: 3743. https://doi.org/10.3390/jcm13133743
APA StyleMoser, M. M., Gramss, L., Marik, W., Weber, M., Hirschmann, D., Wang, W.-T., Dodier, P., Kasprian, G., Bavinzski, G., Rössler, K., & Hosmann, A. (2024). Agreement between CT-Angiography and Digital Subtraction Angiography in Predicting Angiographic Vasospasm in Patients with Subarachnoid Hemorrhage. Journal of Clinical Medicine, 13(13), 3743. https://doi.org/10.3390/jcm13133743






