Robotic Mitral Valve Repair: Impact of Experience on Results and Complex Mitral Disease Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Technique
2.2. Statistical Analysis
3. Results
3.1. Preoperative and Operative Data
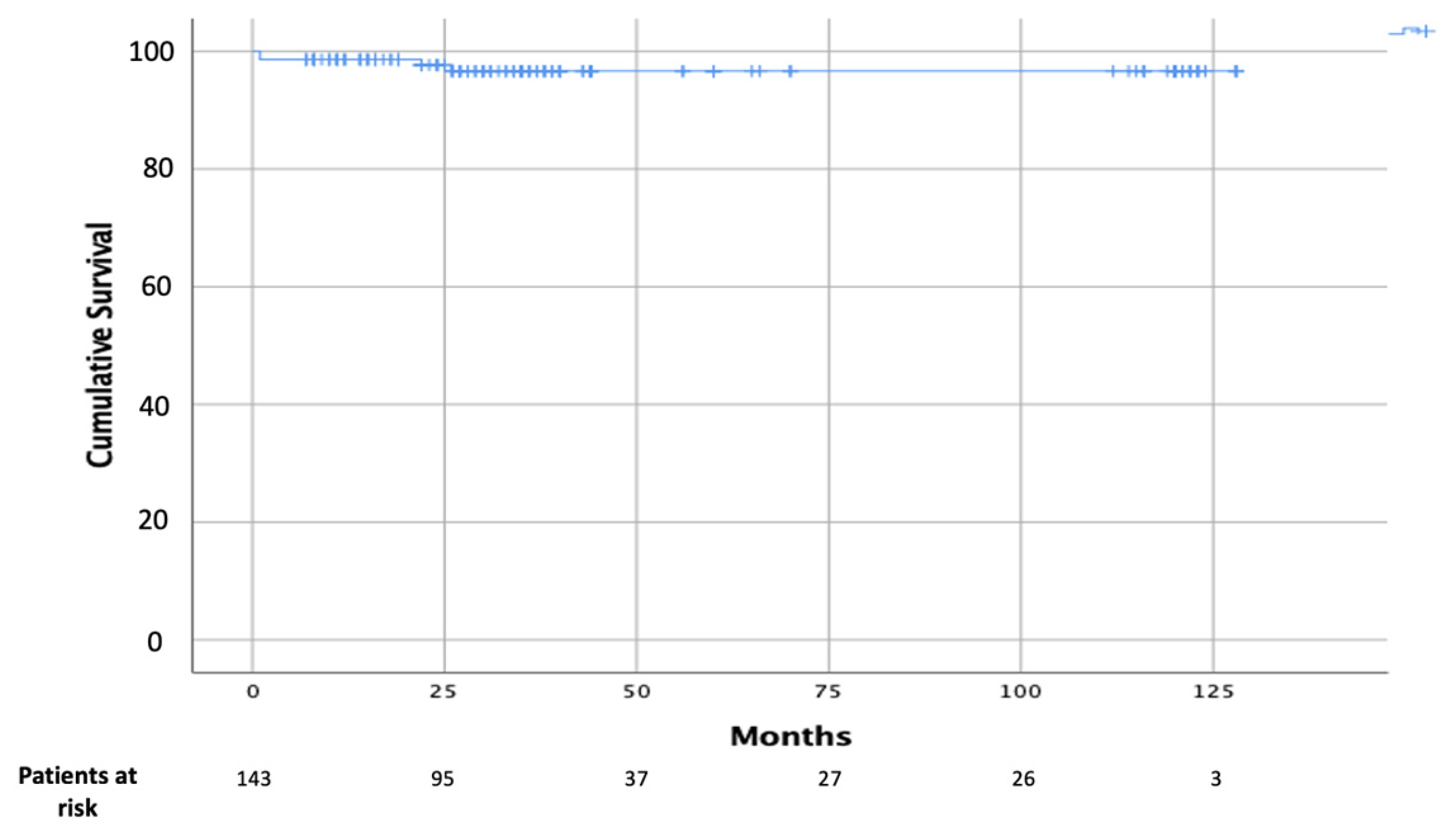
3.2. Postoperative and Follow-Up Data
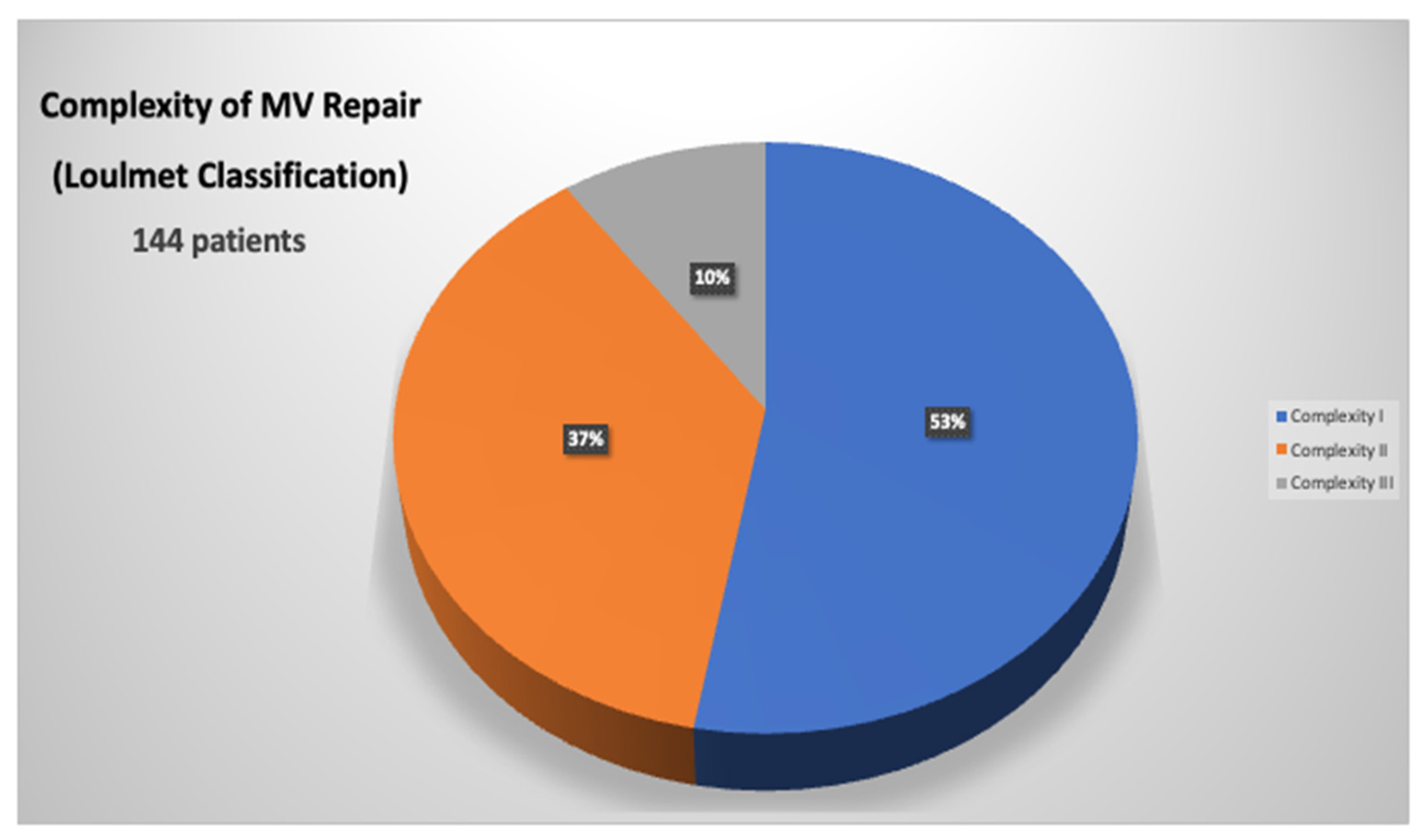
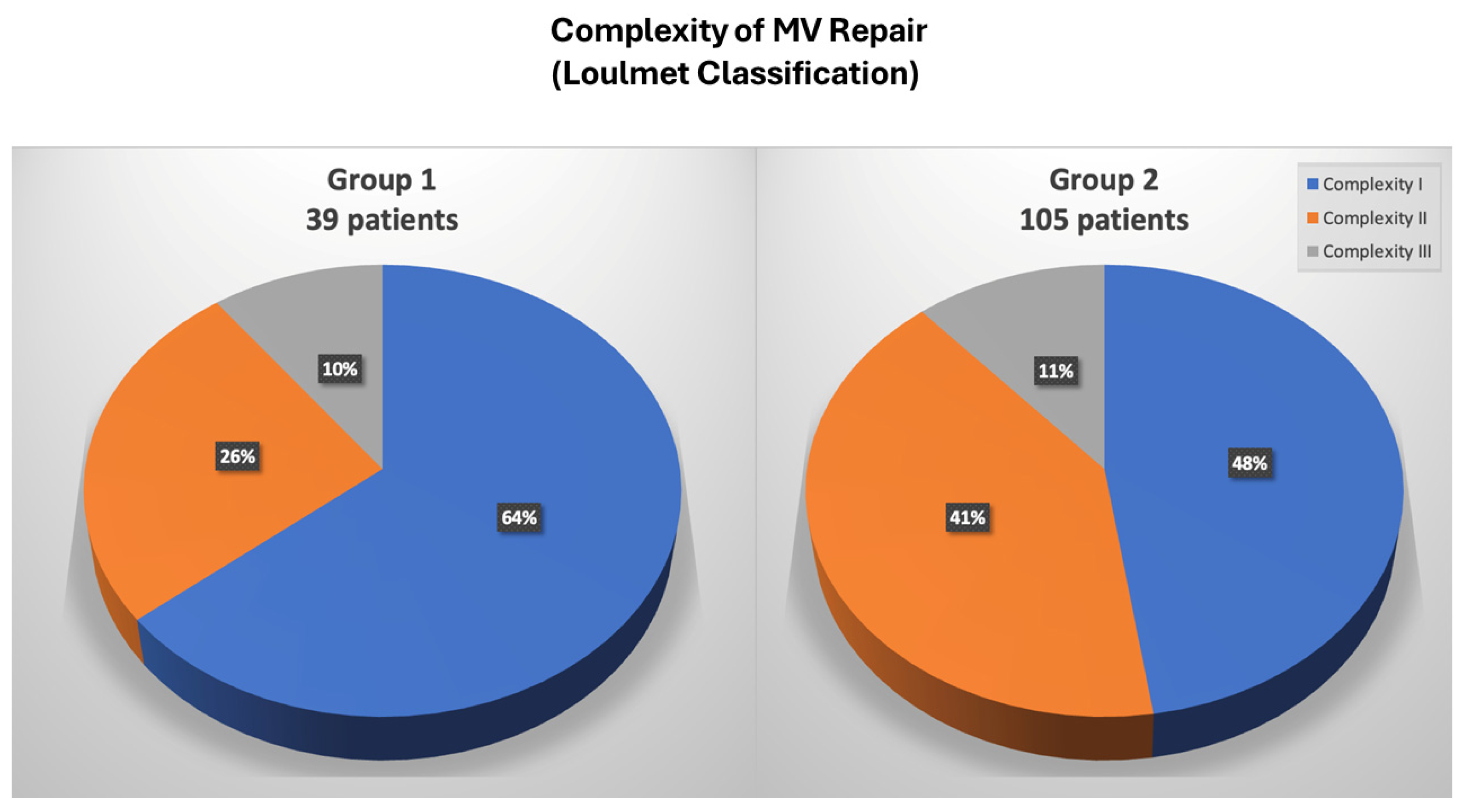
3.3. Mitral Valve Repair Complexity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Autschbach, R.; Onnasch, J.F.; Falk, V.; Walther, T.; Krüger, M.; Schilling, L.O.; Mohr, F.W. The Leipzig experience with robotic valve surgery. J. Card. Surg. 2000, 15, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wolfenden, H.; Liou, K.; Pathan, F.; Gupta, S.; Nienaber, T.A.; Chandrakumar, D.; Indraratna, P.; Yan, T.D. A meta-analysis of robotic vs. conventional mitral valve surgery. Ann. Cardiothorac. Surg. 2015, 4, 305–314. [Google Scholar] [PubMed]
- Mihaljevic, T.; Jarrett, C.M.; Gillinov, A.M.; Williams, S.J.; DeVilliers, P.A.; Stewart, W.J.; Svensson, L.G.; Sabik, J.F.; Blackstone, E.H. Robotic repair of posterior mitral valve prolapse versus conventional approaches: Potential realized. J. Thorac. Cardiovasc. Surg. 2011, 141, 72–80.e4. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.A.; Moss, E.; Binongo, J.; Miller, J.S.; Macheers, S.K.; Sarin, E.L.; Herzog, A.M.; Thourani, V.H.; Guyton, R.A.; Halkos, M.E. The expanding role of endoscopic robotics in mitral valve surgery: 1,257 consecutive procedures. Ann. Thorac. Surg. 2015, 100, 1675–1681; discussion 1681–1682. [Google Scholar] [CrossRef] [PubMed]
- Nifong, L.W.; Rodriguez, E.; Chitwood, W.R., Jr. 540 consecutive robotic mitral valve repairs including concomitant atrial fibrillation cryoablation. Ann. Thorac. Surg. 2012, 94, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.M.; Burkhart, H.M.; Daly, R.C.; Dearani, J.A.; Park, S.J.; Sundt, T.M.; Li, Z.; Enriquez-Sarano, M.; Schaff, H.V. Robotic mitral valve repair for all prolapse subsets using techniques identical to open valvuloplasty: Establishing the benchmark against which percutaneous interventions should be judged. J. Thorac. Cardiovasc. Surg. 2011, 142, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.M.; Dearani, J.A.; Mihaljevic, T.; Chitwood, W.R., Jr.; Murphy, D.A.; Trento, A.; Javadikasgari, H.; Burkhart, H.M.; Nifong, W.L.; Daly, R.C.; et al. Mitral valve repair using robotic technology: Safe, effective, and durable. J. Thorac. Cardiovasc. Surg. 2016, 151, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.M.; Taggarse, A.; Burkhart, H.M.; Daly, R.C.; Mauermann, W.; Nishimura, R.A.; Li, Z.; Dearani, J.A.; Michelena, H.I.; Enriquez-Sarano, M. Robotic mitral valve repair for simple and complex degenerative disease: Midterm clinical and echocardiographic quality outcomes. Circulation 2015, 132, 1961–1968. [Google Scholar] [CrossRef]
- Yoo, J.S.; Kim, J.B.; Jung, S.-H.; Kim, D.-H.; Choo, S.J.; Chung, C.H.; Lee, J.W. Mitral durability after robotic mitral valve repair: Analysis of 200 consecutive mitral regurgitation repairs. J. Thorac. Cardiovasc. Surg. 2014, 148, 2773–2779. [Google Scholar] [CrossRef]
- Ailawadi, G.; Agnihotri, A.K.; Mehall, J.R.; Wolfe, J.A.; Hummel, B.W.; Fayers, T.M.; Farivar, R.S.; Grossi, E.A.; Guy, T.S.; Hargrove, W.C.; et al. Minimally invasive mitral valve surgery I: Patient selection, evaluation, and planning. Innovations 2016, 11, 243–250. [Google Scholar]
- Wolfe, J.A.; Malaisrie, S.C.; Farivar, R.S.; Khan, J.H.; Hargrove, W.C.; Moront, M.G.; Ryan, W.H.; Ailawadi, G.; Agnihotri, A.K.; Hummel, B.W.; et al. Minimally invasive mitral valve surgery II: Surgical technique and postoperative management. Innovations 2016, 11, 251–259. [Google Scholar] [PubMed]
- Lehr, E.J.; Guy, T.S.; Smith, R.L.; Grossi, E.A.; Shemin, R.J.; Rodriguez, E.; Ailawadi, G.; Agnihotri, A.K.; Fayers, T.M.; Hargrove, W.C.; et al. Minimally invasive mitral valve surgery III: Training and robotic-assisted approaches. Innovations 2016, 11, 260–267. [Google Scholar] [PubMed]
- Loulmet, D.F.; Ranganath, N.K.; Neuburger, P.J.; Nampiaparampil, R.G.; Galloway, A.C.; Grossi, E.A. Can complex mitral valve repair be performed with robotics? An institution’s experience utilizing a dedicated team approach in 500 patients. Eur. J. Cardiothorac. Surg. 2019, 56, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Kakuta, T.; Kawamoto, N.; Shimahara, Y.; Yajima, S.; Tadokoro, N.; Kitamura, S.; Kobayashi, J.; Fukushima, S. Benefits of robotically-assisted surgery for complex mitral valve repair. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 417–425. [Google Scholar] [CrossRef]
- Suri, R.M.; Thompson, J.E.; Burkhart, H.M.; Huebner, M.; Borah, B.J.; Li, Z.; Michelena, H.I.; Visscher, S.L.; Roger, V.L.; Daly, R.C.; et al. Improving affordability through innovation in the surgical treatment of mitral valve disease. Mayo Clin. Proc. 2013, 88, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Mihaljevic, T.; Koprivanac, M.; Kelava, M.; Goodman, A.; Jarrett, C.; Williams, S.J.; Gillinov, A.M.; Bajwa, G.; Mick, S.L.; Bonatti, J.; et al. Value of robotically assisted surgery for mitral valve disease. JAMA Surg. 2014, 149, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Coyan, G.; Wei, L.M.; Althouse, A.; Roberts, H.G.; Schauble, D.; Murashita, T.; Cook, C.C.; Rankin, J.S.; Badhwar, V. Robotic mitral valve operations by experienced surgeons are cost-neutral and durable at 1 year. J. Thorac. Cardiovasc. Surg. 2018, 156, 1040–1047. [Google Scholar] [CrossRef]
- Borger, M.A.; Kaeding, A.F. Minimally invasive mitral valve repair in Barlow’s disease: Early and long-term results. J. Thorac. Cardiovasc. Surg. 2014, 148, 1379–1385. [Google Scholar] [CrossRef]
- McClure, R.S.; Athanasopoulos, L.V. One thousand minimally invasive mitral valve operations: Early outcomes, late outcomes, and echocardiographic follow-up. J. Thorac. Cardiovasc. Surg. 2013, 145, 1199–1206. [Google Scholar] [CrossRef]
- Gillinov, A.M.; Mihaljevic, T.; Javadikasgari, H.; Suri, R.M.; Mick, S.L.; Navia, J.L.; Desai, M.Y.; Bonatti, J.; Khosravi, M.; Idrees, J.J.; et al. Early results of robotically assisted mitral valve surgery: Analysis of the first 1000 cases. J. Thorac. Cardiovasc. Surg. 2018, 155, 82–91. [Google Scholar] [CrossRef]
- Roach, A.; Trento, A.; Emerson, D.; Gill, G.; Rowe, G.; Peiris, A.; Hussaini, A.; Cheng, W.; Ramzy, D.; Egorova, N.; et al. Durable Robotic Mitral Repair of Degenerative Primary Regurgitation With Long-Term Follow-Up. Ann. Thorac. Surg. 2022, 114, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Bush, B.; Nifong, L.W.; Alwair, H.; Chitwood, W.R., Jr. Robotic mitral valve surgery-current status and future directions. Ann. Cardiothorac. Surg. 2013, 2, 814–817. [Google Scholar] [PubMed]
- Rodriguez, E.; Nifong, L.W. Pathway for surgeons and programs to establish and maintain a successful robot-assisted adult cardiac surgery program. J. Thorac. Cardiovasc. Surg. 2016, 152, 9–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Güllü, A.; Senay, S.; Kocyigit, M.; Zencirci, E.; Akyol, A.; Degirmencioglu, A.; Karakus, G.; Ersin, E.; Karabiber, A.; Alhan, C. An analysis of the learning curve for robotic-assisted mitral valve repair. J. Card. Surg. 2021, 36, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Piperata, A.; Busuttil, O.; d’Ostrevy, N.; Jansens, J.L.; Taymoor, S.; Cuko, B.; Modine, T.; Pernot, M.; Labrousse, L. Starting A New Robotic Surgery Program for Mitral Valve Repair. Lessons Learned from The First Nine Months. J. Clin. Med. 2021, 10, 5439. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Fontana, G.P.; De Robertis, M.A.; Mirocha, J.; Czer, L.S.; Kass, R.M.; Trento, A. Is robotic mitral valve repair a reproducible approach? J. Thorac. Cardiovasc. Surg. 2010, 139, 628–633. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramzy, D.; Trento, A.; Cheng, W.; De Robertis, M.A.; Mirocha, J.; Ruzza, A.; Kass, R.M. Three hundred robotic-assisted mitral valve repairs: The Cedars-Sinai experience. J. Thorac. Cardiovasc. Surg. 2014, 147, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Isaacs, A.J.; Jalbert, J.; Osakwe, N.C.; Salemi, A.; Girardi, L.N.; Sedrakyan, A. A population-based analysis of robotic-assisted mitral valve repair. Ann. Thorac. Surg. 2015, 99, 1546–1553. [Google Scholar] [CrossRef]
- Loulmet, D.F.; Ranganath, N.K.; Neragi-Miandoab, S.; Koeckert, M.S.; Galloway, A.C.; Grossi, E.A. Advanced experience allows robotic mitral valve repair in the presence of extensive mitral annular calcification. J. Thorac. Cardiovasc. Surg. 2021, 161, 80–88. [Google Scholar] [CrossRef]
| All (144 PTS) | Group 1 (39 PTS) | Group 2 (105 PTS) | p Value | |
|---|---|---|---|---|
| Age, years (mean ± SD) | 58 ± 10 | 56 ± 10 | 59 ± 10 | ns |
| Male, n (%) | 131 (91) | 36 (92) | 95 (90.5) | ns |
| Hypertension, n (%) | 78 (54) | 22 (56) | 56 (53) | ns |
| Diabetes Mellitus, n (%) | 3 (2.5) | 1 (2.5) | 3 (2.5) | ns |
| Extracardiac Arteriopathy, n (%) | 3 (2) | 2 (5) | 1 (1) | ns |
| COPD, n (%) | 6 (4) | 1 (2.5) | 5 (4.5) | ns |
| EuroSCORE II (mean ± SD) | 0.8 ± 0.5 | 0.7 ± 0.6 | 0.8 ± 0.6 | ns |
| STS Score (mean ± SD) | 0.6 ± 0.3 | 0.6 ± 0.2 | 0.6 ± 0.4 | ns |
| MV pathology | ||||
| Barlow disease, n (%) | 13 (9) | 3 (7) | 10 (9) | ns |
| Posterior Leaflet pathology, n (%) | 136 (94) | 35 (90) | 101 (96) | ns |
| Anterior Leaflet pathology, n (%) | 10 (7) | 1 (2.5) | 9 (8.5) | 0.03 |
| LVEF > 55%, n (%) | 141 (98) | 37 (95) | 104 (99) | ns |
| All (144 PTS) | Group 1 (39 PTS) | Group 2 (105 PTS) | p Value | |
|---|---|---|---|---|
| Transareolar approach, n (%) | 14 (10) | 4 (10) | 10 (9.5) | ns |
| Jugular-femoral venous cannulation, n (%) | 72 (50) | 39 (100) | 33 (31) | <0.001 |
| Femoral venous cannulation, n (%) | 72 (50) | 0 (0) | 72 (69) | < 0.001 |
| Femoral arterial cannulation, n (%) | 143 (99.3) | 39 (100) | 104 (99) | ns |
| Axillary arterial cannulation, n (%) | 1 (0.7) | 0 (0) | 1 (1) | ns |
| Endoclamp, n (%) | 28 (19) | 28 (72) | 0 (0) | <0.001 |
| External clamp, n (%) | 116 (80) | 11 (28) | 105 (100) | <0.001 |
| CPB time, min (mean ± SD) | 128 ± 40 | 155 ± 44 | 121 ± 36 | 0.002 |
| Cross-clamp time, min (mean ± sd) | 78 ± 42 | 112 ± 25 | 68 ± 39 | <0.001 |
| Conversion to sternotomy, n (%) | 1 (0.7) | 0 (0) | 1 (1) | ns |
| Triangular resection PL, n (%) | 68 (60) | 16 (41) | 70 (67) | 0.002 |
| Quadrangular resection PL, n (%) | 35 (24) | 4 (10) | 31 (29) | 0.01 |
| Commissuroplasty, n (%) | 5 (3.5) | 0 (0) | 5 (5) | ns |
| Sliding plasty, n (%) | 50 (35) | 3 (7) | 47 (45) | <0.001 |
| Neochordae, n (%) | 39 (27) | 24 (65) | 15 (14) | <0.001 |
| Flexible band, n (%) | 144 (100) | 39 (100) | 105 (100) | ns |
| All (144 PTS) | Group 1 (39 PTS) | Group 2 (105 PTS) | p Value | |
|---|---|---|---|---|
| In-hospital Mortality, n (%) | 1 (0.7) | 0 (0) | 1 (1) | ns |
| AMI, n (%) | 1 (0.7) | 1 (2.5) | 0 (0) | ns |
| Stroke major, n (%) | 1 (0.7) | 1 (2.5) | 0 (0) | ns |
| Revision for bleeding, n (%) | 4 (2.5) | 0 (0) | 4 (4) | ns |
| Blood transfusion, n (%) | 24 (16) | 13 (33) | 11 (10) | <0.001 |
| ICU stay, hours median (IQR) | 24 (48) | 24 (20–30) | 27 (24–49) | ns |
| Ventilation time, hours–median (IQR) | 6 (1–15) | 7 (5–19) | 6 (1–15) | ns |
| In-Hospital stay, days–median (IQR) | 8 (7–12) | 9 (8–12) | 8 (7–12) | ns |
| MR > 1+, n (%) | 0 (0) | 0 (0) | 0 (0) | ns |
| LVEF > 55%, n (%) | 139 (96) | 36 (92) | 103 (98) | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lio, A.; Russo, M.; Sangiorgi, B.; Nicolò, F.; Chirichilli, I.; Irace, F.; Ranocchi, F.; Musumeci, F. Robotic Mitral Valve Repair: Impact of Experience on Results and Complex Mitral Disease Treatment. J. Clin. Med. 2024, 13, 3744. https://doi.org/10.3390/jcm13133744
Lio A, Russo M, Sangiorgi B, Nicolò F, Chirichilli I, Irace F, Ranocchi F, Musumeci F. Robotic Mitral Valve Repair: Impact of Experience on Results and Complex Mitral Disease Treatment. Journal of Clinical Medicine. 2024; 13(13):3744. https://doi.org/10.3390/jcm13133744
Chicago/Turabian StyleLio, Antonio, Marco Russo, Beatrice Sangiorgi, Francesca Nicolò, Ilaria Chirichilli, Francesco Irace, Federico Ranocchi, and Francesco Musumeci. 2024. "Robotic Mitral Valve Repair: Impact of Experience on Results and Complex Mitral Disease Treatment" Journal of Clinical Medicine 13, no. 13: 3744. https://doi.org/10.3390/jcm13133744
APA StyleLio, A., Russo, M., Sangiorgi, B., Nicolò, F., Chirichilli, I., Irace, F., Ranocchi, F., & Musumeci, F. (2024). Robotic Mitral Valve Repair: Impact of Experience on Results and Complex Mitral Disease Treatment. Journal of Clinical Medicine, 13(13), 3744. https://doi.org/10.3390/jcm13133744







