Update on Transcatheter Interventions in Adults with Congenital Heart Disease
Abstract
1. Introduction
2. Ostium Secundum ASD Closure
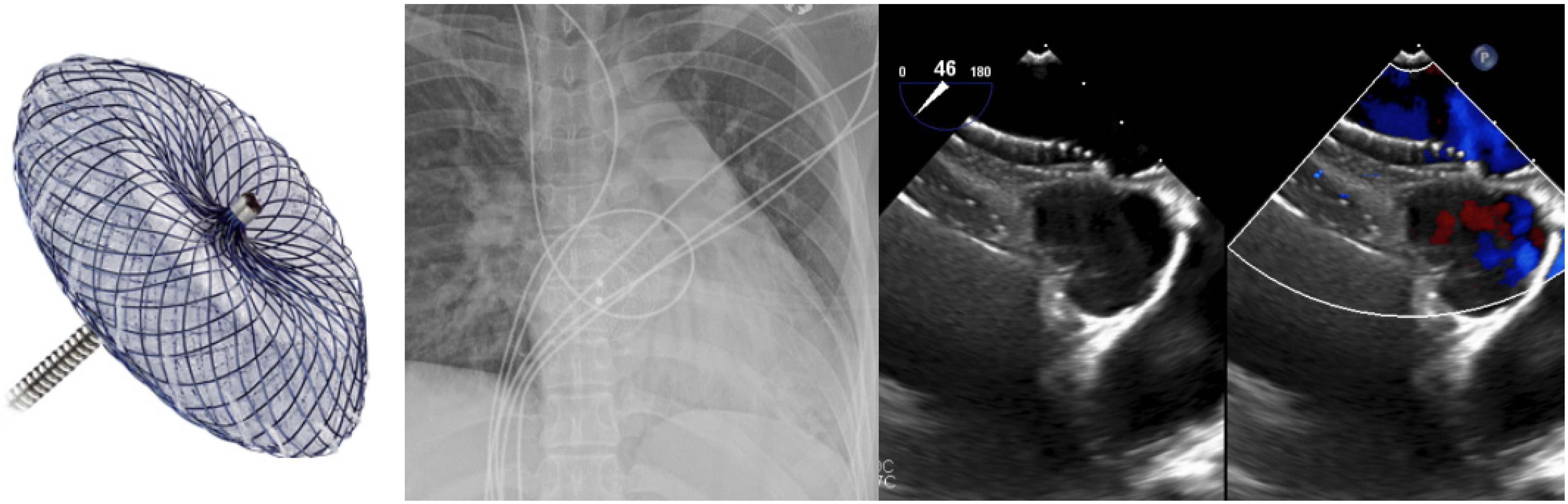
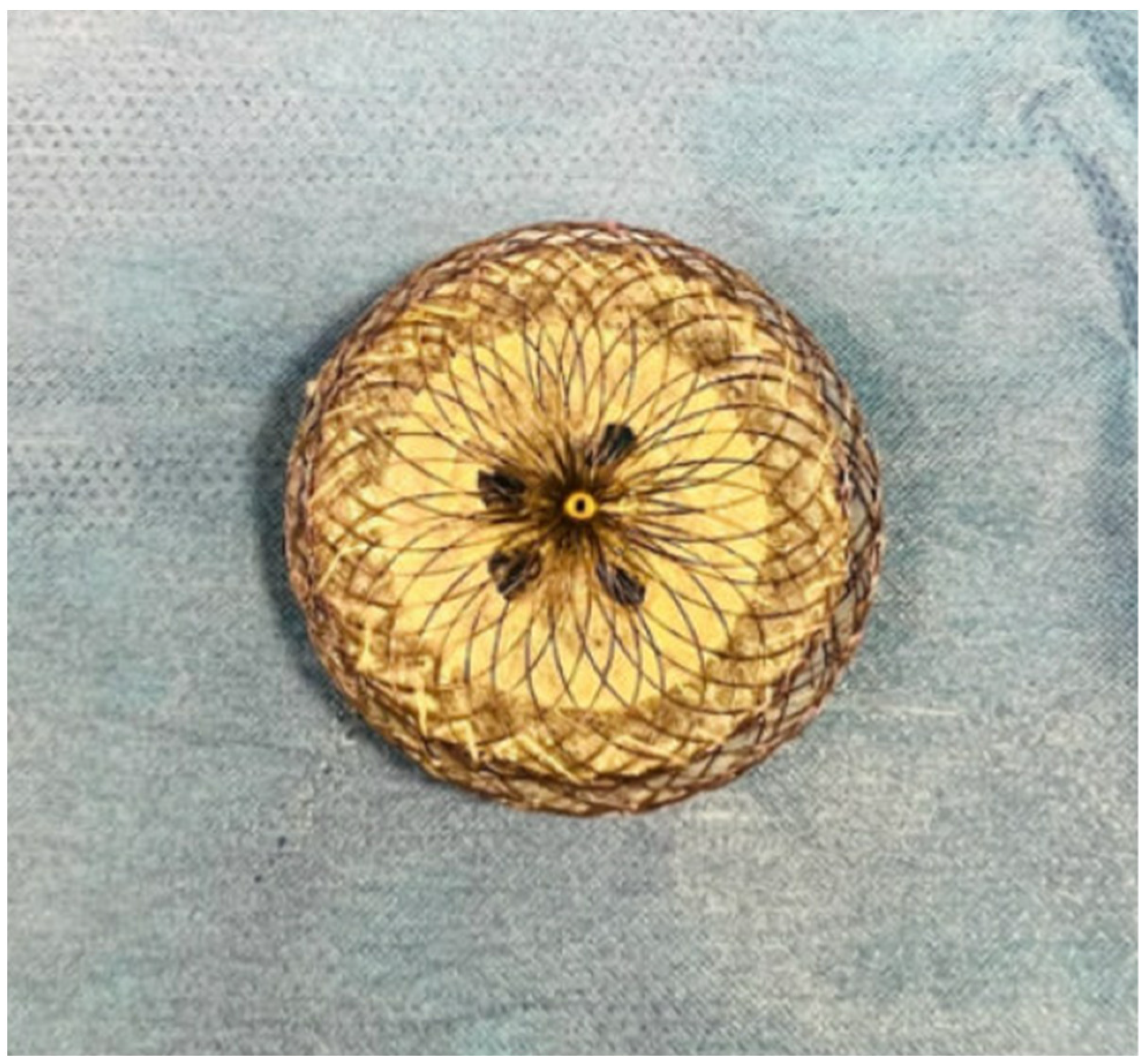
3. Secundum ASD Device Fenestration/Atrial Flow Regulator for Elevated Left Atrial Pressure
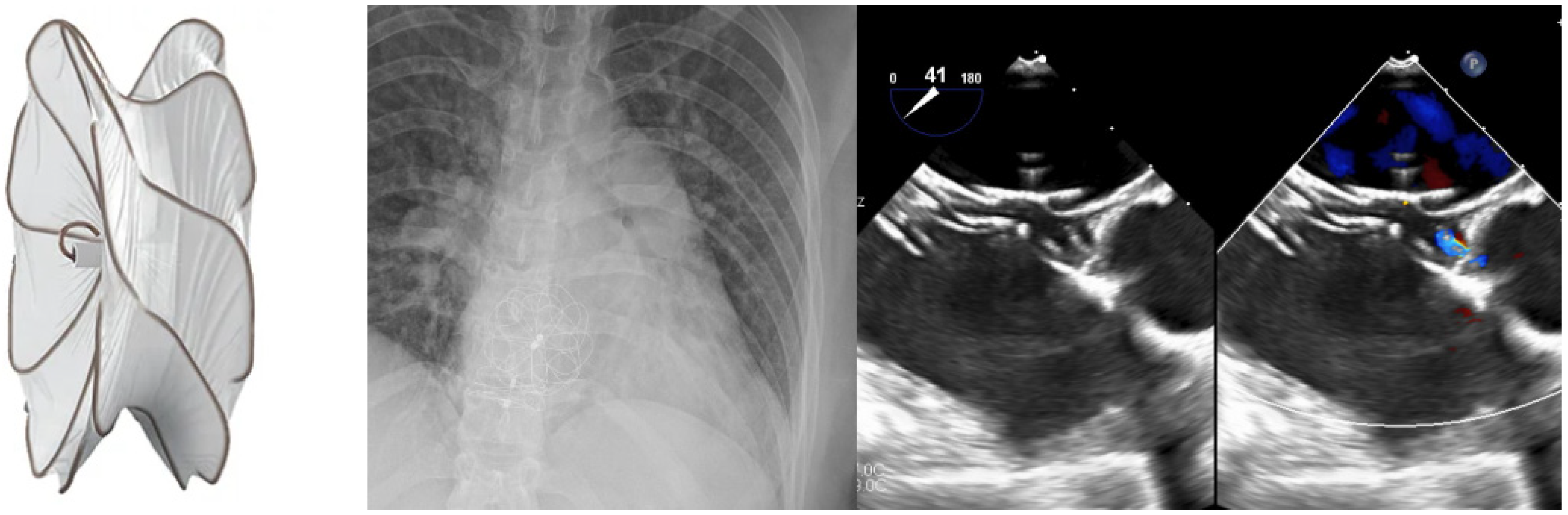
4. Superior Sinus Venosus ASD with Partial Anomalous Pulmonary Venous Connection-Covered Stenting of the Superior Vena Cava
5. VSD Closure
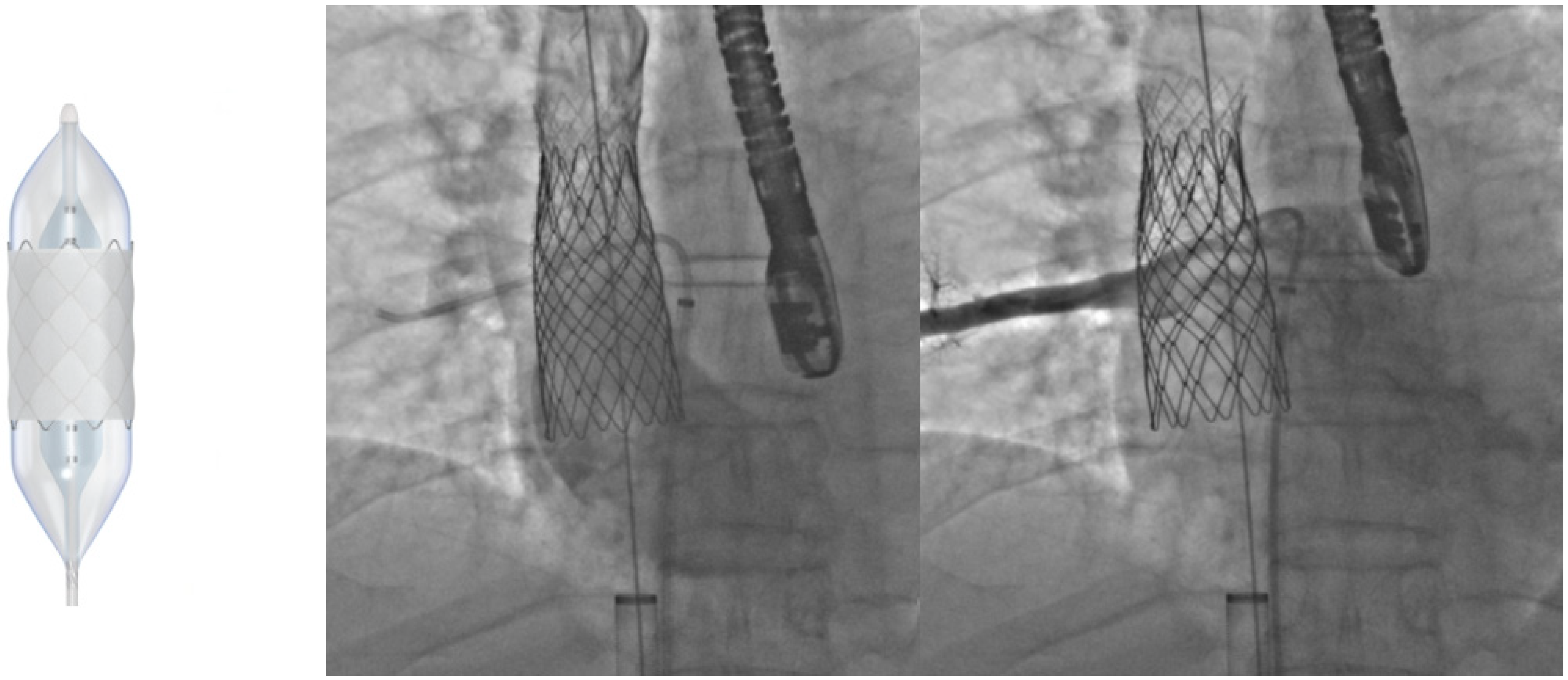
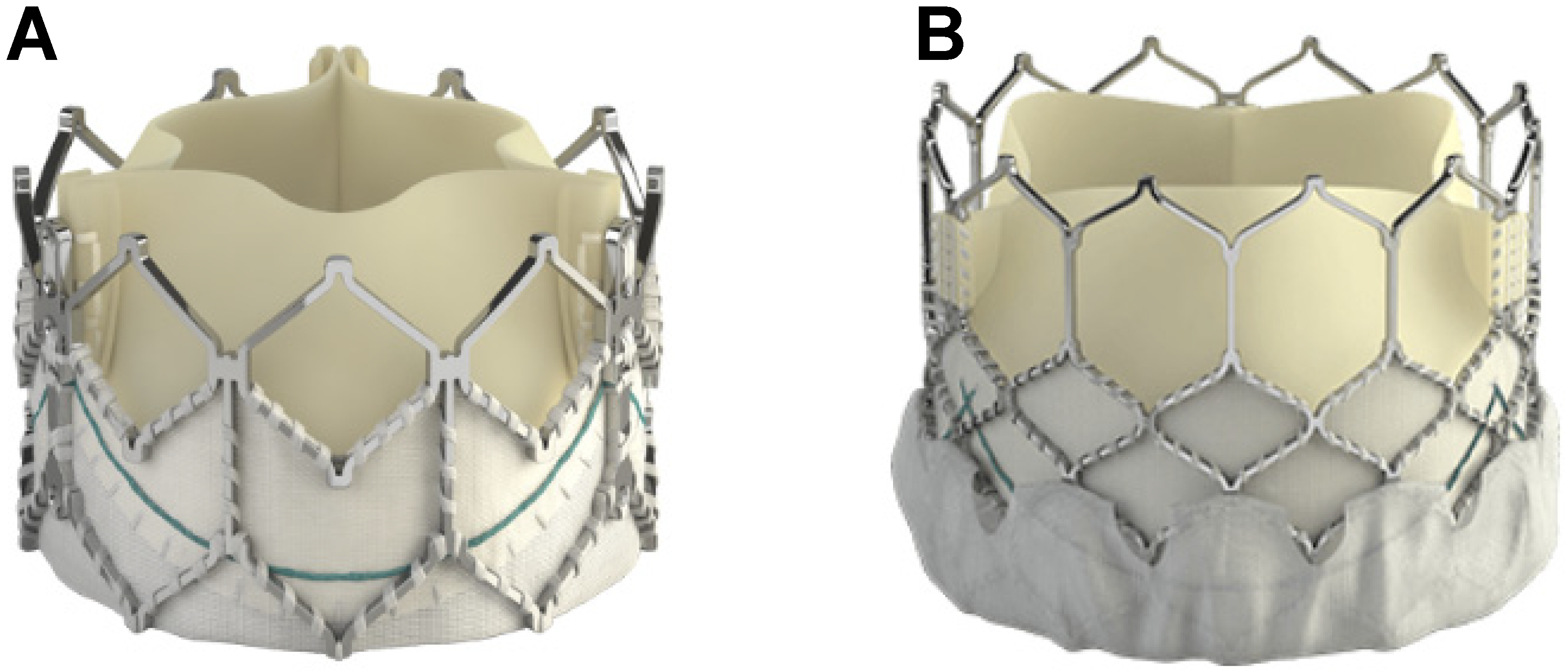
6. Transcatheter Pulmonary Valve Replacement (TCPVR)
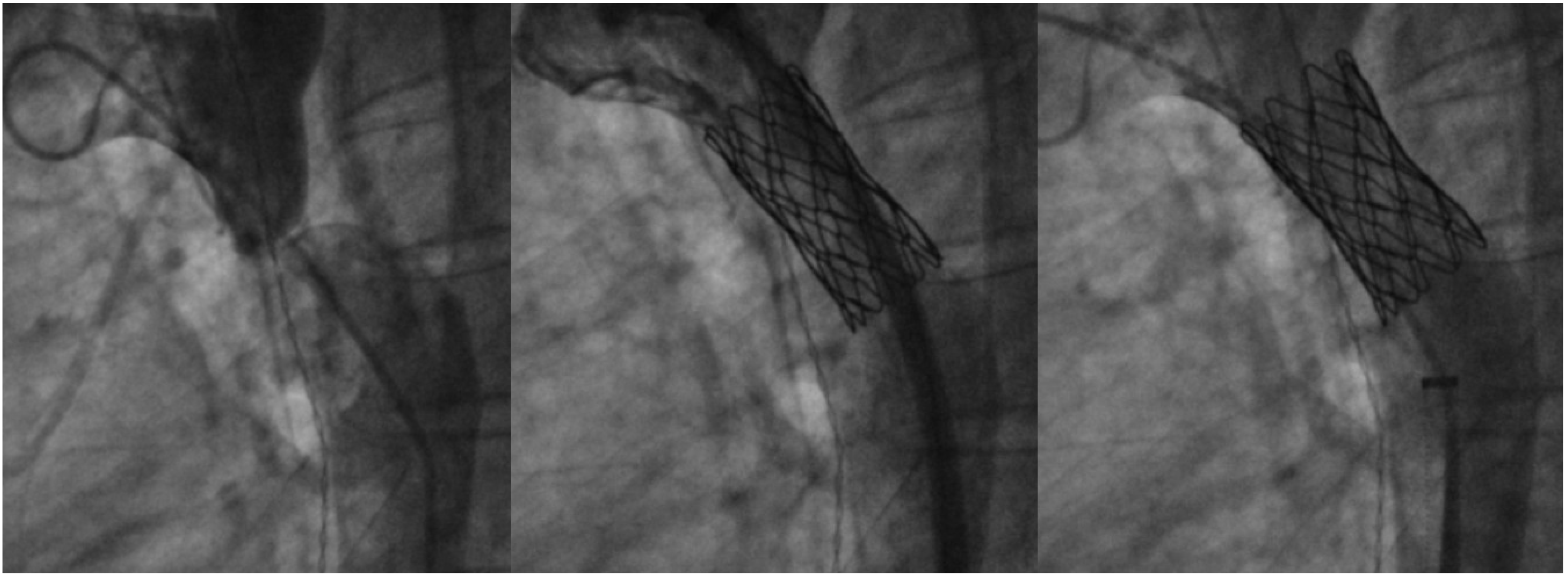
7. Coarctation of Aorta Stenting
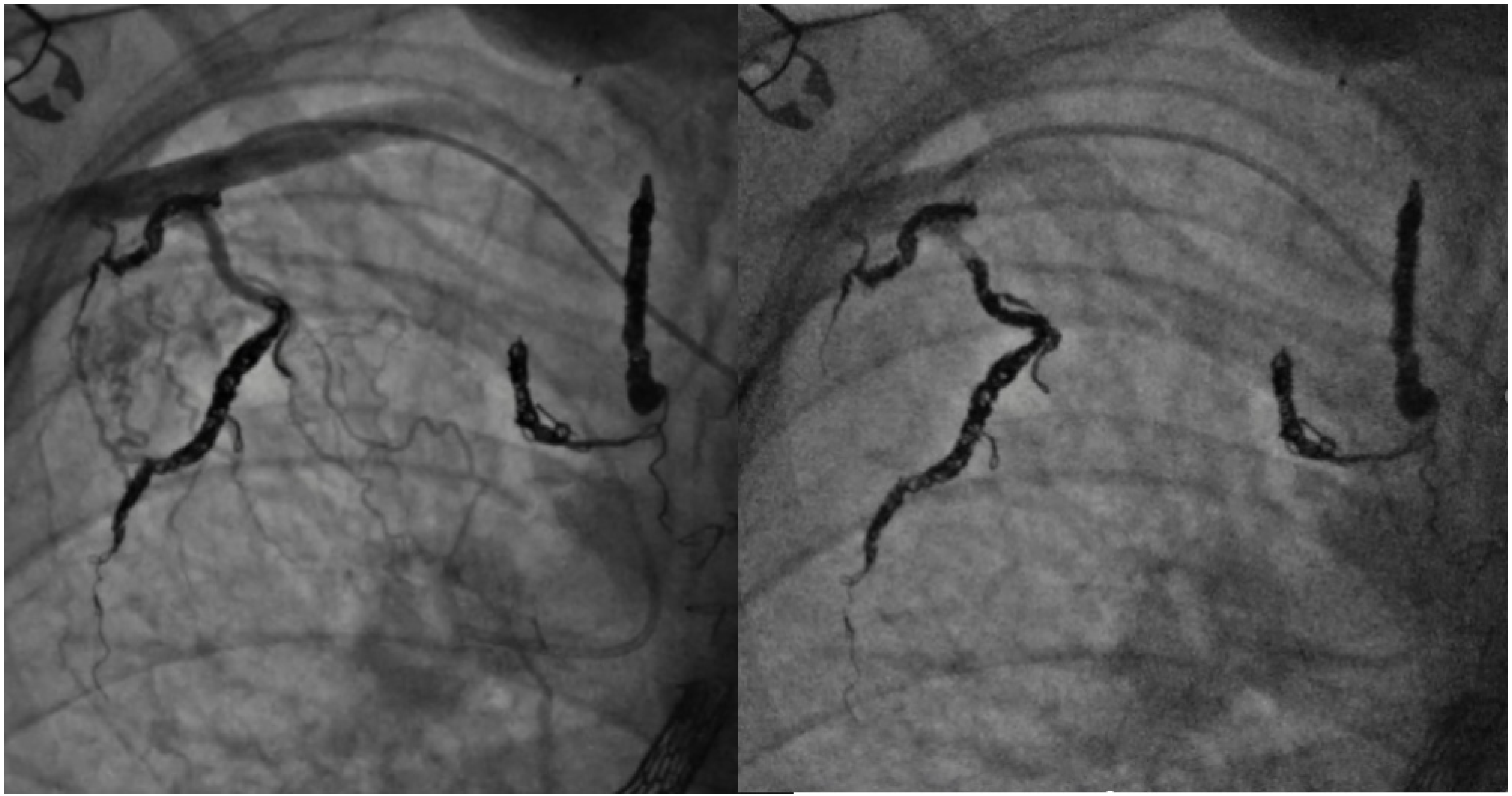
8. Collateral Coil Embolization
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fraisse, A.; Chessa, M. Catheter interventions for Adult Congenital Heart Disease: A European perspective presented by Alain Fraisse and Massimo Chessa. Eur. Heart J. 2019, 40, 231–233. [Google Scholar] [CrossRef]
- Marelli, A.J.; Ionescu-Ittu, R.; Mackie, A.S.; Guo, L.; Dendukuri, N.; Kaouache, M. Lifetime Prevalence of Congenital Heart Disease in the General Population From 2000 to 2010. Circulation 2014, 130, 749–756. [Google Scholar] [CrossRef]
- Brida, M.; Diller, G.P.; Nashat, H.; Barracano, R.; Kempny, A.; Uebing, A.; Rigby, M.L.; A Gatzoulis, M. Cardiac catheter intervention complexity and safety outcomes in adult congenital heart disease. Heart 2020, 106, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, M.G.; Formigari, R.; Perrone, M.A.; Pomiato, E.; Fanisio, F.; Panebianco, M.; Barracano, R.; Guccione, P.; Palmieri, R.; Raponi, M.; et al. Changes in the Cath Lab in the Treatment of Adult Patients with Congenital Heart Disease: A 12-Year Experience in a Single Referral Center with the Establishment of a Dedicated Working Group. J. Cardiovasc. Dev. Dis. 2023, 10, 314. [Google Scholar] [CrossRef]
- Akagi, T. Current concept of transcatheter closure of atrial septal defect in adults. J. Cardiol. 2015, 65, 17–25. [Google Scholar] [CrossRef]
- Alnasser, S.; Lee, D.; Austin, P.C.; Labos, C.; Osten, M.; Lightfoot, D.T.; Kutty, S.; Shah, A.; Meier, L.; Benson, L.; et al. Long term outcomes among adults post transcatheter atrial septal defect closure: Systematic review and meta-analysis. Int. J. Cardiol. 2018, 270, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Tan, J.L.; Li, W.; Dimopoulos, K.; Spence, M.S.; Chow, P.; Mullen, M.J. The Impact of Transcatheter Atrial Septal Defect Closure in the Older Population: A Prospective Study. JACC Cardiovasc. Interv. 2010, 3, 276–281. [Google Scholar] [CrossRef][Green Version]
- Patel, A.; Lopez, K.; Banerjee, A.; Joseph, A.; Cao, Q.L.; Hijazi, Z.M. Transcatheter Closure of Atrial Septal Defects in Adults ≥40 Years of Age: Immediate and Follow-Up Results: Journal of Interventional Cardiology. J. Intervent. Cardiol. 2007, 20, 82–88. [Google Scholar] [CrossRef]
- Kotowycz, M.A.; Therrien, J.; Ionescu-Ittu, R.; Owens, C.G.; Pilote, L.; Martucci, G.; Tchervenkov, C.; Marelli, A.J. Long-Term Outcomes After Surgical Versus Transcatheter Closure of Atrial Septal Defects in Adults. JACC Cardiovasc. Interv. 2013, 6, 497–503. [Google Scholar] [CrossRef]
- King, T.D.; Thompson, S.L.; Steiner, C.; Mills, N.L. Secundum Atrial Septal Defect: Nonoperative Closure During Cardiac Catheterization. JAMA 1976, 235, 2506–2509. [Google Scholar] [CrossRef]
- Kang, S.L.; Benson, L. Interventions in Congenital Heart Disease: A Review of Recent Developments: Part II. Struct. Heart 2021, 5, 570–581. [Google Scholar] [CrossRef]
- Jalal, Z.; Hascoet, S.; Baruteau, A.E.; Iriart, X.; Kreitmann, B.; Boudjemline, Y.; Thambo, J.-B. Long-term Complications After Transcatheter Atrial Septal Defect Closure: A Review of the Medical Literature. Can. J. Cardiol. 2016, 32, 1315.e11–1315.e18. [Google Scholar] [CrossRef]
- McElhinney, D.B.; Quartermain, M.D.; Kenny, D.; Alboliras, E.; Amin, Z. Relative Risk Factors for Cardiac Erosion Following Transcatheter Closure of Atrial Septal Defects. Circulation 2016, 133, 1738–1746. [Google Scholar] [CrossRef]
- Jung, S.Y.; Choi, J.Y. Transcatheter closure of atrial septal defect: Principles and available devices. J. Thorac. Dis. 2018, 10 (Suppl. S24), S2909–S2922. [Google Scholar] [CrossRef]
- Santoro, G.; Castaldi, B.; Cuman, M.; Di Candia, A.; Pizzuto, A.; Sirico, D.; Cantinotti, M.; Garibaldi, S.; Pak, V.; Di Salvo, G. Trans-catheter atrial septal defect closure with the new GORE® Cardioform ASD occluder: First European experience. Int. J. Cardiol. 2021, 327, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Mohan, A.K.; Bass, J.; Steinberger, J.; Said, S.M.; Qureshi, A.M. Gore Cardioform atrial septal occluder: Deployment procedure and techniques for closing challenging secundum atrial septal defects. Cardiol. Young 2021, 31, 1885–1892. [Google Scholar] [CrossRef]
- Abdelkarim, A.; Levi, D.S.; Tran, B.; Ghobrial, J.; Aboulhosn, J. Fenestrated Transcatheter ASD Closure in Adults with Diastolic Dysfunction and/or Pulmonary Hypertension: Case Series and Review of the Literature. Congenit. Heart Dis. 2016, 11, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Kretschmar, O.; Sglimbea, A.; Corti, R.; Knirsch, W. Shunt reduction with a fenestrated Amplatzer device. Catheter. Cardiovasc. Interv. 2010, 76, 564–571. [Google Scholar] [CrossRef]
- Ewert, P.; Berger, F.; Nagdyman, N.; Kretschmar, O.; Dittrich, S.; Abdul-Khaliq, H.; Lange, P.E. Masked left ventricular restriction in elderly patients with atrial septal defects: A contraindication for closure? Catheter. Cardiovasc. Interv. 2001, 52, 177–180. [Google Scholar] [CrossRef]
- Holzer, R.; Cao, Q.L.; Hijazi, Z.M. Closure of a moderately large atrial septal defect with a self-fabricated fenestrated Amplatzer septal occluder in an 85-year-old patient with reduced diastolic elasticity of the left ventricle. Catheter. Cardiovasc. Interv. 2005, 64, 513–518; discussion 519–521. [Google Scholar] [CrossRef] [PubMed]
- Bruch, L.; Winkelmann, A.; Sonntag, S.; Scherf, F.; Rux, S.; Grad, M.O.; Kleber, F.X. Fenestrated occluders for treatment of ASD in elderly patients with pulmonary hypertension and/or right heart failure. J. Intervent. Cardiol. 2008, 21, 44–49. [Google Scholar] [CrossRef]
- Castaldi, B.; Cuppini, E.; Sirico, D.; Cattapan, I.; Fumanelli, J.; Pozza, A.; Di Salvo, G. Feasibility, Safety, and Efficacy of the Atrial Flow Regulator in Pediatric Patients: A Single-Center Experience. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2 Part B, 101209. [Google Scholar] [CrossRef]
- Paitazoglou, C.; Bergmann, M.W.; Özdemir, R.; Pfister, R.; Bartunek, J.; Kilic, T.; Lauten, A.; Schmeisser, A.; Zoghi, M.; Anker, S.D.; et al. One-year results of the first-in-man study investigating the Atrial Flow Regulator for left atrial shunting in symptomatic heart failure patients: The PRELIEVE study. Eur. J. Heart Fail 2021, 23, 800–810. [Google Scholar] [CrossRef]
- Manuri, L.; Calaciura, R.E.; De Zorzi, A.; Oreto, L.; Raponi, M.; Lehner, A.; Haas, N.; Agati, S. Atrial flow regulator for failing Fontan circulation: An initial European experience. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 761–764. [Google Scholar] [CrossRef]
- Piccinelli, E.; Testa, A.; Butera, G. Versatility of Atrial Flow Regulator Device in Congenital Heart Disease: A Case Series. Pediatr. Cardiol. 2023. [Google Scholar] [CrossRef]
- Abdullah, H.A.M.; Alsalkhi, H.A.; Khalid, K.A. Transcatheter closure of sinus venosus atrial septal defect with anomalous pulmonary venous drainage: Innovative technique with long-term follow-up. Catheter. Cardiovasc. Interv. 2020, 95, 743–747. [Google Scholar] [CrossRef]
- Brancato, F.; Stephenson, N.; Rosenthal, E.; Hansen, J.H.; Jones, M.I.; Qureshi, S.; Austin, C.; Speggiorin, S.; Caner, S.; Butera, G. Transcatheter versus surgical treatment for isolated superior sinus venosus atrial septal defect. Catheter. Cardiovasc. Interv. 2023, 101, 1098–1107. [Google Scholar] [CrossRef]
- Garg, G.; Tyagi, H.; Radha, A.S. Transcatheter closure of sinus venosus atrial septal defect with anomalous drainage of right upper pulmonary vein into superior vena cava--an innovative technique. Catheter. Cardiovasc. Interv. 2014, 84, 473–477. [Google Scholar] [CrossRef]
- Brancato, F.; Rosenthal, E.; Hansen, J.; Duong, P.; Jones, M.I.; Qureshi, S.; Kabir, S.; Butera, G. Trans-catheter treatments of superior sinus venosus atrial septal defects. Prog. Pediatr. Cardiol. 2021, 61, 101342. [Google Scholar] [CrossRef]
- Thakkar, A.N.; Chinnadurai, P.; Breinholt, J.P.; Lin, C.H. Transcatheter closure of a sinus venosus atrial septal defect using 3D printing and image fusion guidance. Catheter. Cardiovasc. Interv. 2018, 92, 353–357. [Google Scholar] [CrossRef]
- Hansen, J.H.; Duong, P.; Jivanji, S.G.M.; Jones, M.; Kabir, S.; Butera, G.; Qureshi, S.A.; Rosenthal, E. Transcatheter Correction of Superior Sinus Venosus Atrial Septal Defects as an Alternative to Surgical Treatment. J. Am. Coll. Cardiol. 2020, 75, 1266–1278. [Google Scholar] [CrossRef]
- Riahi, M.; Velasco Forte, M.N.; Byrne, N.; Hermuzi, A.; Jones, M.; Baruteau, A.-E.; Valverde, I.; Qureshi, S.A.; Rosenthal, E. Early experience of transcatheter correction of superior sinus venosus atrial septal defect with partial anomalous pulmonary venous drainage. EuroIntervention 2018, 14, 868–876. [Google Scholar] [CrossRef]
- Chessa, M.; Butera, G.; Negura, D.; Bussadori, C.; Giamberti, A.; Fesslova, V.; Carminati, M. Transcatheter closure of congenital ventricular septal defects in adult: Mid-term results and complications. Int. J. Cardiol. 2009, 133, 70–73. [Google Scholar] [CrossRef]
- Yang, L.; Tai, B.C.; Khin, L.W.; Quek, S.C. A systematic review on the efficacy and safety of transcatheter device closure of ventricular septal defects (VSD). J. Intervent. Cardiol. 2014, 27, 260–272. [Google Scholar] [CrossRef]
- Kenny, D.; Morgan, G.; Bajwa, A.; Farrow, C.; Parry, A.; Caputo, M.; Tometzki, A.; Martin, R. Evolution of transcatheter closure of perimembranous ventricular septal defects in a single centre. Catheter. Cardiovasc. Interv. 2009, 73, 568–575. [Google Scholar] [CrossRef]
- Wang, J.; Zuo, J.; Yu, S.; Yi, D.; Yang, X.; Zhu, X.; Li, J.; Yang, L.; Xiong, L.; Ge, S.; et al. Effectiveness and Safety of Transcatheter Closure of Perimembranous Ventricular Septal Defects in Adults. Am. J. Cardiol. 2016, 117, 980–987. [Google Scholar] [CrossRef]
- Haddad, R.N.; Daou, L.S.; Saliba, Z.S. Percutaneous closure of restrictive-type perimembranous ventricular septal defect using the new KONAR multifunctional occluder: Midterm outcomes of the first middle-eastern experience. Catheter. Cardiovasc. Interv. 2020, 96, E295–E302. [Google Scholar] [CrossRef]
- Carminati, M.; Butera, G.; Chessa, M.; De Giovanni, J.; Fisher, G.; Gewillig, M.; Peuster, M.; Piechaud, J.F.; Santoro, G.; Sievert, H.; et al. Transcatheter closure of congenital ventricular septal defects: Results of the European Registry. Eur. Heart J. 2007, 28, 2361–2368. [Google Scholar] [CrossRef]
- Lock, J.E.; Block, P.C.; McKay, R.G.; Baim, D.S.; Keane, J.F. Transcatheter closure of ventricular septal defects. Circulation 1988, 78, 361–368. [Google Scholar] [CrossRef]
- Carminati, M.; Butera, G.; Chessa, M.; Drago, M.; Negura, D.; Piazza, L. Transcatheter Closure of Congenital Ventricular Septal Defect with Amplatzer Septal Occluders. Am. J. Cardiol. 2005, 96 (Supp. S1), 52–58. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, Z.M.; Hakim, F.; Al-Fadley, F.; Abdelhamid, J.; Cao, Q.L. Transcatheter closure of single muscular ventricular septal defects using the amplatzer muscular VSD occluder: Initial results and technical considerations. Catheter. Cardiovasc. Interv. 2000, 49, 167–172. [Google Scholar] [CrossRef]
- Chungsomprasong, P.; Durongpisitkul, K.; Vijarnsorn, C.; Soongswang, J.; Lê, T.P. The results of transcatheter closure of VSD using Amplatzer® device and Nit Occlud® Lê coil. Catheter. Cardiovasc. Interv. 2011, 78, 1032–1040. [Google Scholar] [CrossRef]
- Butera, G.; Carminati, M.; Chessa, M.; Piazza, L.; Micheletti, A.; Negura, D.G.; Abella, R.; Giamberti, A.; Frigiola, A. Transcatheter Closure of Perimembranous Ventricular Septal Defects: Early and Long-Term Results. J. Am. Coll. Cardiol. 2007, 50, 1189–1195. [Google Scholar] [CrossRef]
- Santhanam, H.; Yang, L.; Chen, Z.; Tai, B.C.; Rajgor, D.D.; Quek, S.C. A meta-analysis of transcatheter device closure of perimembranous ventricular septal defect. Int. J. Cardiol. 2018, 254, 75–83. [Google Scholar] [CrossRef]
- Tzikas, A.; Ibrahim, R.; Velasco-Sanchez, D.; Freixa, X.; Alburquenque, M.; Khairy, P.; Bass, J.L.; Ramirez, J.; Aguirre, D.; Miro, J. Transcatheter closure of perimembranous ventricular septal defect with the Amplatzer(®) membranous VSD occluder 2: Initial world experience and one-year follow-up. Catheter. Cardiovasc. Interv. 2014, 83, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, L.; Wan, Y.; Zuo, J.; Zhang, J.; Chen, W.; Li, J.; Sun, L.; Yu, S.; Liu, J.; et al. Transcatheter device closure of perimembranous ventricular septal defects: Mid-term outcomes. Eur. Heart J. 2010, 31, 2238–2245. [Google Scholar] [CrossRef]
- Godart, F.; Baudelet, J.B.; Delarue, A.; Polge, A.S.; Domanski, O.; Bichali, S.; Houeijeh, A. Transcatheter Closure of Perimembranous Ventricular Septal Defects Including Multifenestrated and Gerbode-Type Defects Using the Lifetech Konar Device. J. Clin. Med. 2023, 12, 6370. [Google Scholar] [CrossRef]
- McElhinney, D.B.; Hennesen, J.T. The Melody® valve and Ensemble® delivery system for transcatheter pulmonary valve replacement. Ann. N. Y Acad. Sci. 2013, 1291, 77–85. [Google Scholar] [CrossRef]
- Alkashkari, W.; Alsubei, A.; Hijazi, Z.M. Transcatheter Pulmonary Valve Replacement: Current State of Art. Curr. Cardiol. Rep. 2018, 20, 27. [Google Scholar] [CrossRef]
- Bonhoeffer, P.; Boudjemline, Y.; Saliba, Z.; Merckx, J.; Aggoun, Y.; Bonnet, D.; Acar, P.; Le Bidois, J.; Sidi, D.; Kachaner, J. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 2000, 356, 1403–1405. [Google Scholar] [CrossRef]
- Kheiwa, A.; Divanji, P.; Mahadevan, V.S. Transcatheter pulmonary valve implantation: Will it replace surgical pulmonary valve replacement? Expert. Rev. Cardiovasc. Ther. 2018, 16, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kenny, D.; Rhodes, J.F.; Fleming, G.A.; Kar, S.; Zahn, E.M.; Vincent, J.; Shirali, G.S.; Gorelick, J.; Fogel, M.A.; Fahey, J.T.; et al. 3-Year Outcomes of the Edwards SAPIEN Transcatheter Heart Valve for Conduit Failure in the Pulmonary Position From the COMPASSION Multicenter Clinical Trial. JACC Cardiovasc. Interv. 2018, 11, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, I.; Aboulhosn, J.; Salem, M.; Levi, D. Aortic root compression during transcatheter pulmonary valve replacement. Catheter. Cardiovasc. Interv. 2016, 88, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Teixeira, R.; Lopes, J.; Costa, M.; Pires, A.; Gonçalves, L. Transcatheter Versus Surgical Pulmonary Valve Replacement: A Systemic Review and Meta-Analysis. Ann. Thorac. Surg. 2020, 110, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, Z.L.; Jones, T.K.; Verrier, E.; Stout, K.K.; Krieger, E.V.; Karamlou, T. Early outcomes in patients undergoing transcatheter versus surgical pulmonary valve replacement. Heart 2017, 103, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, M.J.; McElhinney, D.B.; Jones, T.K.; Levi, D.S.; Asnes, J.; Gray, R.G.; Cabalka, A.K.; Fujimoto, K.; Qureshi, A.M.; Justino, H.; et al. 1-Year Outcomes in a Pooled Cohort of Harmony Transcatheter Pulmonary Valve Clinical Trial Participants. JACC Cardiovasc. Interv. 2023, 16, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.D.; Levi, D.S.; Cheatham, J.P.; Qureshi, S.A.; Shahanavaz, S.; Zahn, E.M. Transcatheter Pulmonary Valve Replacement: A Review of Current Valve Technologies. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100452. [Google Scholar] [CrossRef]
- Morgan, G.; Prachasilchai, P.; Promphan, W.; Rosenthal, E.; Sivakumar, K.; Kappanayil, M.; Sakidjan, I.; Walsh, K.P.; Kenny, D.; Thomson, J.; et al. Medium-term results of percutaneous pulmonary valve implantation using the Venus P-valve: International experience. EuroIntervention J. Eur. Collab. Work. Group. Interv. Cardiol. Eur. Soc. Cardiol. 2019, 14, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Garay, F.; Pan, X.; Zhang, Y.J.; Wang, C.; Springmuller, D. Early experience with the Venus p-valve for percutaneous pulmonary valve implantation in native outflow tract. Neth. Heart J. 2017, 25, 76–81. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, G.B.; Kim, S.H.; Jang, S.I.; Choi, J.Y.; Kang, I.S.; Kim, Y.H. Mid-term outcomes of the Pulsta transcatheter pulmonary valve for the native right ventricular outflow tract. Catheter. Cardiovasc. Interv. 2021, 98, E724–E732. [Google Scholar] [CrossRef]
- Morgan, G.J.; Sadeghi, S.; Salem, M.M.; Wilson, N.; Kay, J.; Rothman, A.; Galindo, A.; Martin, M.H.; Gray, R.; Ross, M.; et al. SAPIEN valve for percutaneous transcatheter pulmonary valve replacement without “pre-stenting”: A multi-institutional experience. Catheter. Cardiovasc. Interv. 2019, 93, 324–329. [Google Scholar] [CrossRef]
- Wilson, W.M.; Benson, L.N.; Osten, M.D.; Shah, A.; Horlick, E.M. Transcatheter Pulmonary Valve Replacement With the Edwards Sapien System: The Toronto Experience. JACC Cardiovasc. Interv. 2015, 8, 1819–1827. [Google Scholar] [CrossRef]
- Shahanavaz, S.; Zahn, E.M.; Levi, D.S.; Aboulhousn, J.A.; Hascoet, S.; Qureshi, A.M.; Porras, D.; Morgan, G.J.; Heaton, H.B.; Martin, M.H.; et al. Transcatheter Pulmonary Valve Replacement With the Sapien Prosthesis. J. Am. Coll. Cardiol. 2020, 76, 2847–2858. [Google Scholar] [CrossRef]
- Sabbak, N.; Denby, K.; Kumar, A.; Goldar, G.; Ghobrial, J. Intravascular Lithotripsy for Severe RVOT Calcification to Optimize Transcatheter Pulmonary Valve Replacement. JACC Case Rep. 2023, 19, 101926. [Google Scholar] [CrossRef]
- Lee, M.G.Y.; Russo, J.J.; Norman, S.; Binny, S.D.; Joshi, S.B.; Eastaugh, L.; Grigg, L.E.; Wilson, W.M. Novel Use of Intravascular Lithotripsy for Percutaneous Relief of Critical Right Ventricle-to-Pulmonary Artery Conduit Stenosis. JACC Cardiovasc. Interv. 2023, 16, 2670–2672. [Google Scholar] [CrossRef]
- Salcher, M.; Naci, H.; Law, T.J.; Kuehne, T.; Schubert, S.; Kelm, M.; Morley-Fletcher, E.; Hennemuth, A.; Manset, D.; Mcguire, A.; et al. Balloon Dilatation and Stenting for Aortic Coarctation: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Interv. 2016, 9, e003153. [Google Scholar] [CrossRef]
- Golden, A.B.; Hellenbrand, W.E. Coarctation of the aorta: Stenting in children and adults. Catheter. Cardiovasc. Interv. 2007, 69, 289–299. [Google Scholar] [CrossRef]
- Hartman, E.M.J.; Groenendijk, I.M.; Heuvelman, H.M.; Roos-Hesselink, J.W.; Takkenberg, J.J.M.; Witsenburg, M. The effectiveness of stenting of coarctation of the aorta: A systematic review. EuroIntervention J. Eur. Collab. Work. Group. Interv. Cardiol. Eur. Soc. Cardiol. 2015, 11, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Singh, S.; Mukhopadhyay, S.; Kaul, U.A. Self- and balloon-expandable stent implantation for severe native coarctation of aorta in adults. Am. Heart J. 2003, 146, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.; Holzer, R.J. Comparing balloon angioplasty, stenting and surgery in the treatment of aortic coarctation. Expert. Rev. Cardiovasc. Ther. 2009, 7, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, J.P. Stenting of coarctation of the aorta. Catheter. Cardiovasc. Interv. 2001, 54, 112–125. [Google Scholar] [CrossRef]
- Kenny, D.; Margey, R.; Turner, M.S.; Tometzki, A.J.; Walsh, K.P.; Martin, R.P. Self-expanding and balloon expandable covered stents in the treatment of aortic coarctation with or without aneurysm formation. Catheter. Cardiovasc. Interv. 2008, 72, 65–71. [Google Scholar] [CrossRef]
- Holzer, R.J.; Gauvreau, K.; McEnaney, K.; Watanabe, H.; Ringel, R. Long-Term Outcomes of the Coarctation of the Aorta Stent Trials. Circ. Cardiovasc. Interv. 2021, 14, e010308. [Google Scholar] [CrossRef]
- Sugiyama, H.; Yoo, S.J.; Williams, W.; Benson, L.N. Characterization and treatment of systemic venous to pulmonary venous collaterals seen after the Fontan operation. Cardiol. Young 2003, 13, 424–430. [Google Scholar] [CrossRef]
- Heinemann, M.; Breuer, J.; Steger, V.; Steil, E.; Sieverding, L.; Ziemer, G. Incidence and impact of systemic venous collateral development after Glenn and Fontan procedures. Thorac. Cardiovasc. Surg. 2001, 49, 172–178. [Google Scholar] [CrossRef]
- Andrews, R.E.; Tulloh, R.M.R.; Anderson, D.R. Coil occlusion of systemic venous collaterals in hypoplastic left heart syndrome. Heart 2002, 88, 167–169. [Google Scholar] [CrossRef][Green Version]
- Tan, W.; Reardon, L.; Lin, J.; Lluri, G.; Venkatesh, P.; Bravo-Jaimes, K.; Biniwale, R.; Van Arsdell, G.; Ponder, R.D.; Aboulhosn, J. Occlusion of aortopulmonary and venovenous collaterals prior to heart or combined heart-liver transplantation in Fontan patients: A single-center experience. Int. J. Cardiol. Congenit. Heart Dis. 2021, 6, 100260. [Google Scholar] [CrossRef]
- Kanter, K.R.; Vincent, R.N.; Raviele, A.A. Importance of acquired systemic-to-pulmonary collaterals in the Fontan operation. Ann. Thorac. Surg. 1999, 68, 969–974. [Google Scholar] [CrossRef]
- Bradley, S.M. Management of aortopulmonary collateral arteries in Fontan patients: Routine occlusion is not warranted. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2002, 5, 55–67. [Google Scholar] [CrossRef]
- Grosse-Wortmann, L.; Drolet, C.; Dragulescu, A.; Kotani, Y.; Chaturvedi, R.; Lee, K.-J.; Mertens, L.; Taylor, K.; La Rotta, G.; van Arsdell, G.; et al. Aortopulmonary collateral flow volume affects early postoperative outcome after Fontan completion: A multimodality study. J. Thorac. Cardiovasc. Surg. 2012, 144, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.M.; McCall, M.M.; Sistino, J.J.; Radtke, W.A.K. Aortopulmonary collateral flow in the Fontan patient: Does it matter? Ann. Thorac. Surg. 2001, 72, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.B.; Radtke, W.; Fellows, K.E.; Keane, J.F.; Lock, J.E. Coil embolization to occlude aortopulmonary collateral vessels and shunts in patients with congenital heart disease. J. Am. Coll. Cardiol. 1989, 13, 100–108. [Google Scholar] [CrossRef]
- Kanter, K.R.; Vincent, R.N. Management of aortopulmonary collateral arteries in Fontan patients: Occlusion improves clinical outcome. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2002, 5, 48–54. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, A.; Aboulhosn, J.A. Update on Transcatheter Interventions in Adults with Congenital Heart Disease. J. Clin. Med. 2024, 13, 3685. https://doi.org/10.3390/jcm13133685
Li A, Aboulhosn JA. Update on Transcatheter Interventions in Adults with Congenital Heart Disease. Journal of Clinical Medicine. 2024; 13(13):3685. https://doi.org/10.3390/jcm13133685
Chicago/Turabian StyleLi, Angela, and Jamil A. Aboulhosn. 2024. "Update on Transcatheter Interventions in Adults with Congenital Heart Disease" Journal of Clinical Medicine 13, no. 13: 3685. https://doi.org/10.3390/jcm13133685
APA StyleLi, A., & Aboulhosn, J. A. (2024). Update on Transcatheter Interventions in Adults with Congenital Heart Disease. Journal of Clinical Medicine, 13(13), 3685. https://doi.org/10.3390/jcm13133685





