The Tri-Steps Model of Critical Conditions in Intensive Care: Introducing a New Paradigm for Chronic Critical Illness
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
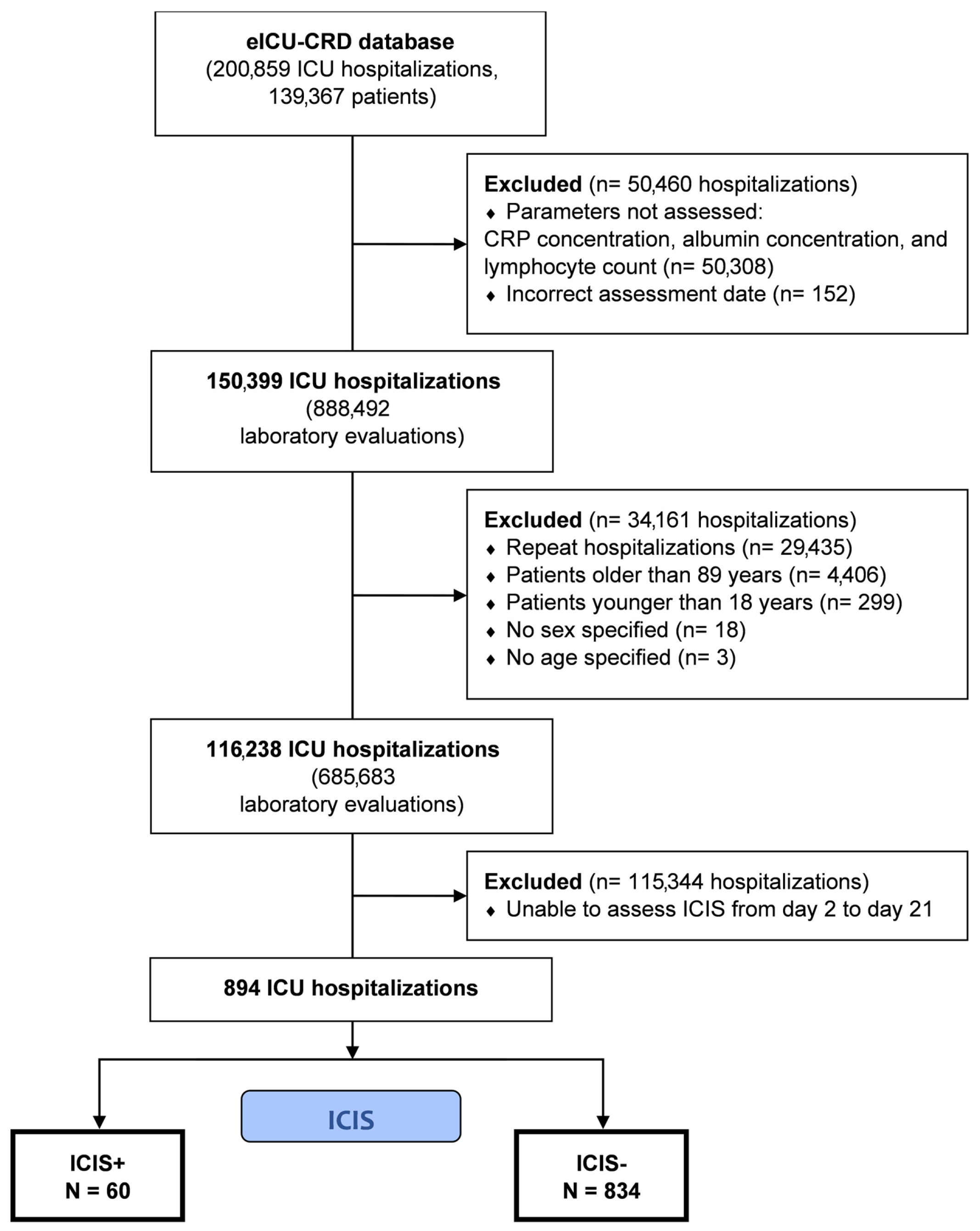
2.2. Selection Criteria
2.3. Data Extraction
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
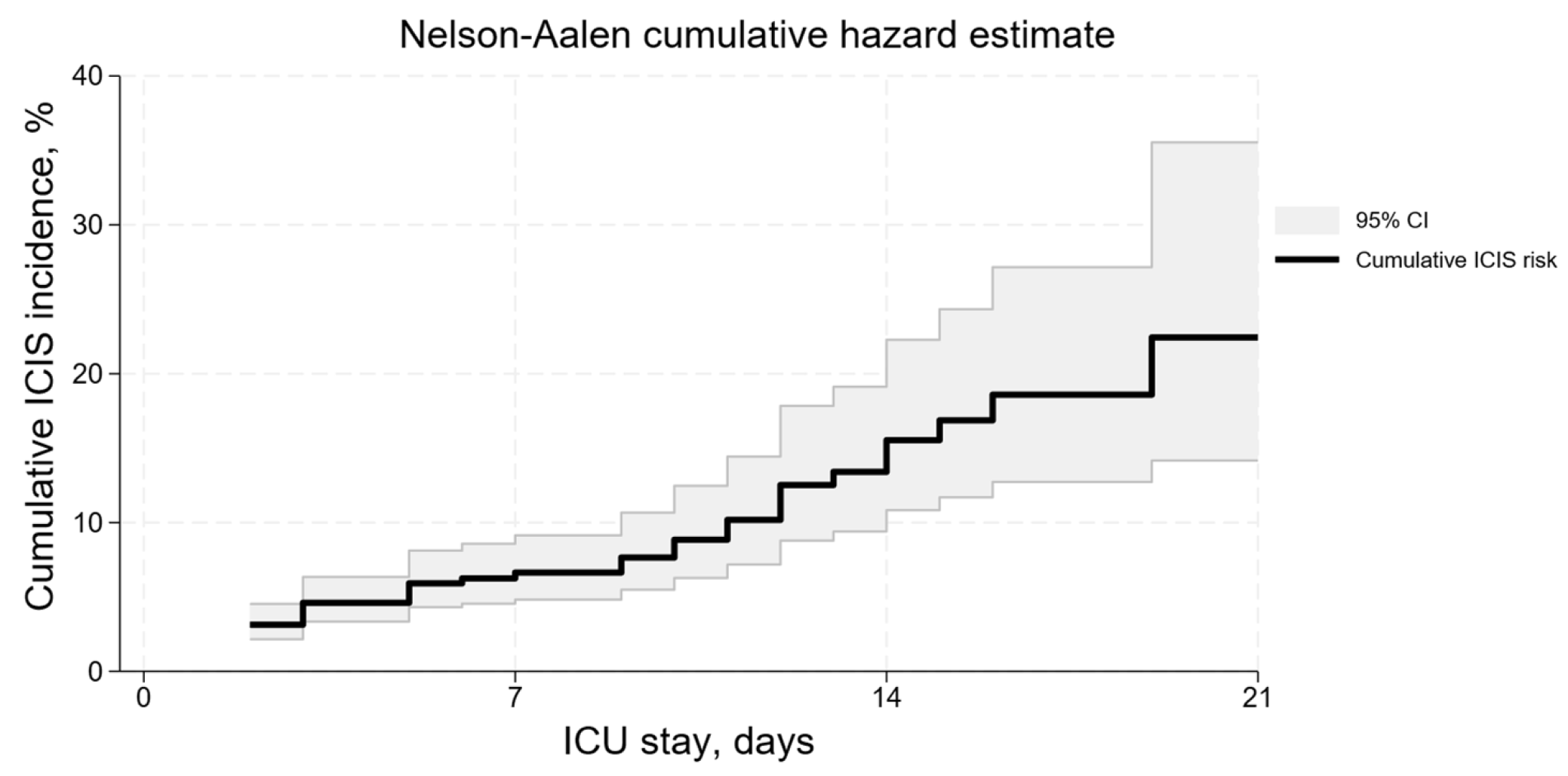
3.2. ICIS Development Risk
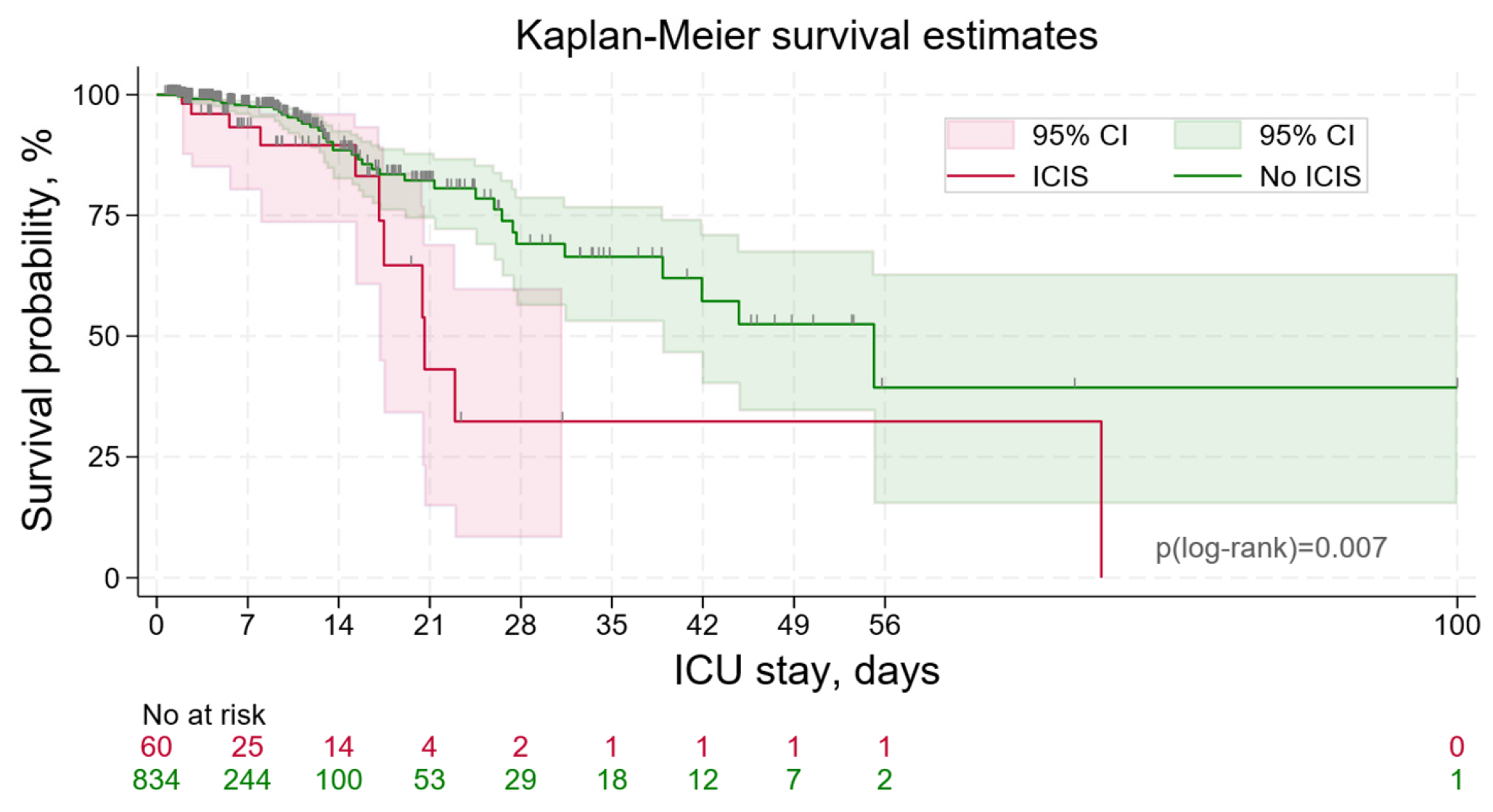
3.3. ICIS Outcomes
3.4. Risk Factors for ICIS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Girard, K.; Raffin, T.A. The Chronically Critically Ill: To Save or Let Die? Respir. Care 1985, 30, 339–347. [Google Scholar] [PubMed]
- Zimmerman, J.E.; Kramer, A.A.; Knaus, W.A. Changes in Hospital Mortality for United States Intensive Care Unit Admissions from 1988 to 2012. Crit. Care 2013, 17, R81. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, N.R. Chronic Critical Illness: The Growing Challenge to Health Care. Respir. Care 2012, 57, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Mira, J.C.; Gentile, L.F.; Mathias, B.J.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Moore, F.A.; Moldawer, L.L. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit. Care Med. 2017, 45, 253–262. [Google Scholar] [CrossRef]
- Nelson, J.E.; Cox, C.E.; Hope, A.A.; Carson, S.S. Chronic Critical Illness. Am. J. Respir. Crit. Care Med. 2010, 182, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Carson, S.S. Definitions and Epidemiology of the Chronically Critically Ill. Respir. Care 2012, 57, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Iwashyna, T.J.; Hodgson, C.L.; Pilcher, D.; Bailey, M.; Bellomo, R. Persistent Critical Illness Characterised by Australian and New Zealand Icu Clinicians. Crit. Care Resusc. 2015, 17, 153–158. [Google Scholar] [CrossRef]
- Gardner, A.K.; Ghita, G.L.; Wang, Z.; Ozrazgat-Baslanti, T.; Raymond, S.L.; Mankowski, R.T.; Brumback, B.A.; Efron, P.A.; Bihorac, A.; Moore, F.A.; et al. The Development of Chronic Critical Illness Determines Physical Function, Quality of Life, and Long-Term Survival among Early Survivors of Sepsis in Surgical ICUs*. Crit. Care Med. 2019, 47, 566–573. [Google Scholar] [CrossRef]
- Stortz, J.A.; Mira, J.C.; Raymond, S.L.; Loftus, T.J.; Ozrazgat-Baslanti, T.; Wang, Z.; Ghita, G.L.; Leeuwenburgh, C.; Segal, M.S.; Bihorac, A.; et al. Benchmarking Clinical Outcomes and the Immunocatabolic Phenotype of Chronic Critical Illness after Sepsis in Surgical Intensive Care Unit Patients. J. Trauma Acute Care Surg. 2018, 84, 342–349. [Google Scholar] [CrossRef]
- Nelson, J.E.; Meier, D.E.; Litke, A.; Natale, D.A.; Siegel, R.E.; Morrison, R.S. The Symptom Burden of Chronic Critical Illness. Crit. Care Med. 2004, 32, 1527–1534. [Google Scholar] [CrossRef]
- Carson, S.S.; Bach, P.B. The Epidemiology and Costs of Chronic Critical Illness. Crit. Care Clin. 2002, 18, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Carson, S.S.; Kahn, J.M.; Hough, C.L.; Seeley, E.J.; White, D.B.; Douglas, I.S.; Cox, C.E.; Caldwell, E.; Bangdiwala, S.I.; Garrett, J.M.; et al. A Multicenter Mortality Prediction Model for Patients Receiving Prolonged Mechanical Ventilation. Crit. Care Med. 2012, 40, 1171–1176. [Google Scholar] [CrossRef]
- Nierman, D.M. A Structure of Care for the Chronically Critically Ill. Crit. Care Clin. 2002, 18, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Boniatti, M.M.; Friedman, G.; Castilho, R.K.; Vieira, S.R.R.; Fialkow, L.A. Characteristics of Chronically Critically Ill Patients: Comparing Two Definitions. Clinics 2011, 66, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Arguiano, M.J.; Miñambres, E.; Cuenca-Fito, E.; Suberviola, B.; Burón-Mediavilla, F.J.; Ballesteros, M.A. Chronic Critical Illness after Trauma Injury: Outcomes and Experience in a Trauma Center. Acta Chir. Belg. 2023, 123, 618–624. [Google Scholar] [CrossRef]
- MacIntyre, N.R.; Epstein, S.K.; Carson, S.; Scheinhorn, D.; Christopher, K.; Muldoon, S. Management of Patients Requiring Prolonged Mechanical Ventilation: Report of a NAMDRC Consensus Conference. Chest 2005, 128, 3937–3954. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Luippold, R.S.; Sulsky, S.; Shorr, A.F. Prolonged Acute Mechanical Ventilation, Hospital Resource Utilization, and Mortality in the United States. Crit. Care Med. 2008, 36, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Nasraway, S.A.; Button, G.J.; Rand, W.M.; Hudson-Jinks, T.; Gustafson, M. Survivors of Catastrophic Illness: Outcome after Direct Transfer from Intensive Care to Extended Care Facilities. Crit. Care Med. 2000, 28, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.L.; Daly, B.J.; Brennan, P.F.; Harris, S.; Nochomovitz, M.; Dyer, M.A. Outcomes of Long-Term Ventilator Patients: A Descriptive Study. Am. J. Crit. Care 1997, 6, 99–105. [Google Scholar] [CrossRef]
- Parfenov, A.L.; Petrova, M.V.; Pichugina, I.M.; Luginina, E.V. Comorbidity Development in Patients with Severe Brain Injury Resulting in Chronic Critical Condition (Review). Obs. Reanimatol. 2020, 16, 72–89. [Google Scholar] [CrossRef]
- Berikashvili, L.B.; Geize, A.V.; Kornelyuk, R.A.; Plotnikov, G.P. Immune Status in Chronic Critical Illness: A Systematic Review. Ann. Crit. Care 2023, 2023, 133–144. [Google Scholar] [CrossRef]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent Inflammation and Immunosuppression: A Common Syndrome and New Horizon for Surgical Intensive Care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef]
- Hu, D.; Ren, J.; Wang, G.; Gu, G.; Chen, J.; Zhou, B.; Liu, S.; Wu, X.; Li, J. Persistent Inflammation-Immunosuppression Catabolism Syndrome, a Common Manifestation of Patients with Enterocutaneous Fistula in Intensive Care Unit. J. Trauma Acute Care Surg. 2014, 76, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Ogura, K.; Nakano, H.; Naraba, H.; Takahashi, Y.; Sonoo, T.; Hashimoto, H.; Goto, T. Disseminated Intravascular Coagulopathy Is Associated with the Outcome of Persistent Inflammation, Immunosuppression and Catabolism Syndrome. J. Clin. Med. 2020, 9, 2662. [Google Scholar] [CrossRef]
- Hesselink, L.; Hoepelman, R.J.; Spijkerman, R.; de Groot, M.C.H.; van Wessem, K.J.P.; Koenderman, L.; Leenen, L.P.H.; Hietbrink, F. Persistent Inflammation, Immunosuppression and Catabolism Syndrome (PICS) after Polytrauma: A Rare Syndrome with Major Consequences. J. Clin. Med. 2020, 9, 191. [Google Scholar] [CrossRef]
- Chadda, K.R.; Puthucheary, Z. Persistent Inflammation, Immunosuppression, and Catabolism Syndrome (PICS): A Review of Definitions, Potential Therapies, and Research Priorities. Br. J. Anaesth. 2024, 132, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.L.; Ball, L.; Rocco, P.R.M.; Pelosi, P. Physiological and Pathophysiological Consequences of Mechanical Ventilation. Semin. Respir. Crit. Care Med. 2022, 43, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Kundra, P.; Goswami, S. Endothelial Glycocalyx: Role in Body Fluid Homeostasis and Fluid Management. Indian J. Anaesth. 2019, 63, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Astapenko, D.; Benes, J.; Pouska, J.; Lehmann, C.; Islam, S.; Cerny, V. Endothelial Glycocalyx in Acute Care Surgery—What Anaesthesiologists Need to Know for Clinical Practice. BMC Anesthesiol. 2019, 19, 238. [Google Scholar] [CrossRef]
- Aninat, C.; Seguin, P.; Descheemaeker, P.N.; Morel, F.; Malledant, Y.; Guillouzo, A. Catecholamines Induce an Inflammatory Response in Human Hepatocytes. Crit. Care Med. 2008, 36, 848–854. [Google Scholar] [CrossRef]
- Johnson, J.D.; Campisi, J.; Sharkey, C.M.; Kennedy, S.L.; Nickerson, M.; Greenwood, B.N.; Fleshner, M. Catecholamines Mediate Stress-Induced Increases in Peripheral and Central Inflammatory Cytokines. Neuroscience 2005, 135, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakis, S.H.; Simos, P.; Basta, M.; Zaganas, I.; Perysinaki, G.S.; Akoumianakis, I.; Tziraki, C.; Lionis, C.; Vgontzas, A.; Boumpas, D. Maedica-a Journal of Clinical Medicine MAEDICA-a Journal of Clinical Medicine Interactions of Mediterranean Diet, Obesity, Polypharmacy, Depression and Systemic Inflammation with Frailty Status. Maedica J. Clin. Med. 2022, 17, 2022. [Google Scholar]
- Pollard, T.J.; Johnson, A.E.W.; Raffa, J.D.; Celi, L.A.; Mark, R.G.; Badawi, O. The EICU Collaborative Research Database, a Freely Available Multi-Center Database for Critical Care Research. Sci. Data 2018, 5, 180178. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Ogura, K.; Ohbe, H.; Goto, T. Clinical Criteria for Persistent Inflammation, Immunosuppression, and Catabolism Syndrome: An Exploratory Analysis of Optimal Cut-Off Values for Biomarkers. J. Clin. Med. 2022, 11, 5790. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.C.; Brakenridge, S.C.; Stortz, J.A.; Hawkins, R.B.; Darden, D.B.; Ghita, G.L.; Mohr, A.M.; Moldawer, L.L.; Efron, P.A.; Moore, F.A. Abdominal Sepsis Patients Have a High Incidence of Chronic Critical Illness with Dismal Long-Term Outcomes. Am. J. Surg. 2020, 220, 1467–1474. [Google Scholar] [CrossRef]
- Stortz, J.A.; Murphy, T.J.; Raymond, S.L.; Mira, J.C.; Ungaro, R.; Dirain, M.L.; Nacionales, D.C.; Loftus, T.J.; Wang, Z.; Ozrazgat-Baslanti, T.; et al. Evidence for Persistent Immune Suppression in Patients Who Develop Chronic Critical Illness after Sepsis. Shock 2018, 49, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.Y.; Qiu, J.N.; Liu, B.Y.; Li, X.X.; Sun, Y.N.; Liang, Y.J.; Zhao, D.M.; Zhu, R.; Zhang, Z.D.; Ma, X.C. A Retrospective Clinical Study of Sixty-Three Cases with Persistent Inflammation Immunosuppression and Catabolism Syndrome. Zhonghua Nei Ke Za Zhi 2016, 55, 941–944. [Google Scholar]
- Hartl, W.H.; Wolf, H.; Schneider, C.P.; Küchenhoff, H.; Jauch, K.W. Acute and Long-Term Survival in Chronically Critically Ill Surgical Patients: A Retrospective Observational Study. Crit. Care 2007, 11, R55. [Google Scholar] [CrossRef] [PubMed]
- Mankowski, R.T.; Anton, S.D.; Ghita, G.L.; Brumback, B.; Cox, M.C.; Mohr, A.M.; Leeuwenburgh, C.; Moldawer, L.L.; Efron, P.A.; Brakenridge, S.C.; et al. Older Sepsis Survivors Suffer Persistent Disability Burden and Poor Long-Term Survival. J. Am. Geriatr. Soc. 2020, 68, 1962–1969. [Google Scholar] [CrossRef]
- Viglianti, E.M.; Kramer, R.; Admon, A.J.; Sjoding, M.W.; Hodgson, C.L.; Bellomo, R.; Iwashyna, T.J. Late Organ Failures in Patients with Prolonged Intensive Care Unit Stays. J. Crit. Care 2018, 46, 55–57. [Google Scholar] [CrossRef]
- Yeh, D.D.; Fuentes, E.; Quraishi, S.A.; Lee, J.; Kaafarani, H.M.A.; Fagenholz, P.; Butler, K.; DeMoya, M.; Chang, Y.; Velmahos, G. Early Protein Inadequacy Is Associated with Longer Intensive Care Unit Stay and Fewer Ventilator-Free Days: A Retrospective Analysis of Patients with Prolonged Surgical Intensive Care Unit Stay. J. Parenter. Enter. Nutr. 2018, 42, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Darden, D.B.; Brakenridge, S.C.; Efron, P.A.; Ghita, G.L.; Fenner, B.P.; Kelly, L.S.; Mohr, A.M.; Moldawer, L.L.; Moore, F.A. Biomarker Evidence of the Persistent Inflammation, Immunosuppression and Catabolism Syndrome (PICS) in Chronic Critical Illness (CCI) After Surgical Sepsis. Ann. Surg. 2021, 274, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.L.; Bettcher, L.F.; Navarro, S.L.; Pascua, V.; Neto, F.C.; Cuschieri, J.; Raftery, D.; O’Keefe, G.E. Persistent Metabolomic Alterations Characterize Chronic Critical Illness after Severe Trauma. J. Trauma Acute Care Surg. 2021, 90, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Guirgis, F.W.; Black, L.P.; Henson, M.; Labilloy, G.; Smotherman, C.; Hopson, C.; Tfirn, I.; DeVos, E.L.; Leeuwenburgh, C.; Moldawer, L.; et al. A Hypolipoprotein Sepsis Phenotype Indicates Reduced Lipoprotein Antioxidant Capacity, Increased Endothelial Dysfunction and Organ Failure, and Worse Clinical Outcomes. Crit. Care 2021, 25, 341. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Wu, J.; Yu, X.Y.; Luo, S.M.; Wang, J.Z.; Luo, L.; Zheng, X.S.; Han, X.N.; Li, G.Y.; Chen, Y.J.; et al. A Multicenter Cross-Sectional Study on Chronic Critical Illness and Surgery-Related Chronic Critical Illness in China. Chin. J. Gastrointest. Surg./Zhonghua Wei Chang Wai Ke Za Zhi 2019, 22, 1027–1033. [Google Scholar]
- Chen, L.; Cao, R.; Wang, J.; Lu, X.; Mu, E. Analysis of clinical characteristics of patients with chronic critical illness after sepsis. Chin. Crit. Care Med. 2021, 33, 1414–1417. [Google Scholar] [CrossRef]
- Wang, L.; Chen, R.; Dong, J.; Guo, Z. Predictive value of neutrophil to lymphocyte ratio in the progression of sepsis to chronic critical illness in elderly patients. Chin. Crit. Care Med. 2021, 33, 1291–1295. [Google Scholar]
- Lee, K.; Hong, S.B.; Lim, C.M.; Koh, Y. Sequential Organ Failure Assessment Score and Comorbidity: Valuable Prognostic Indicators in Chronically Critically Ill Patients. Anaesth. Intensive Care 2008, 36, 528–534. [Google Scholar] [CrossRef]
- Loss, S.H.; Marchese, C.B.; Boniatti, M.M.; Wawrzeniak, I.C.; Oliveira, R.P.; Nunes, L.N.; Victorino, J.A. Prediction of Chronic Critical Illness in a General Intensive Care Unit. Rev. Assoc. Med. Bras. 2013, 59, 241–247. [Google Scholar] [CrossRef]
- Aguiar, F.P.; Westphal, G.A.; Dadam, M.M.; Mota, E.C.C.; Pfutzenreuter, F.; França, P.H.C. Characteristics and predictors of chronic critical illness in the intensive care unit. Rev. Bras. Ter. Intensiv. 2019, 31, 511–520. [Google Scholar] [CrossRef]
- Custodero, C.; Wu, Q.; Ghita, G.L.; Anton, S.D.; Brakenridge, S.C.; Brumback, B.A.; Efron, P.A.; Gardner, A.K.; Leeuwenburgh, C.; Moldawer, L.L.; et al. Prognostic Value of NT-ProBNP Levels in the Acute Phase of Sepsis on Lower Long-Term Physical Function and Muscle Strength in Sepsis Survivors. Crit. Care 2019, 23, 230. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Ren, J.; Ren, H.; Hong, Z.; Wang, G.; Gu, G.; Wu, X. Early Active Irrigation-Suction Drainage among Enterocutaneous Fistulas Patients with Chronic Critical Illness: A Retrospective Cohort Study. Am. Surg. 2020, 86, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.B.; Stortz, J.A.; Holden, D.C.; Wang, Z.; Raymond, S.L.; Cox, M.C.; Brakenridge, S.C.; Moore, F.A.; Moldawer, L.L.; Efron, P.A. Persistently Increased Cell-Free DNA Concentrations Only Modestly Contribute to Outcome and Host Response in Sepsis Survivors with Chronic Critical Illness. Surgery 2020, 167, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Brakenridge, S.C.; Moore, F.A.; Mercier, N.R.; Cox, M.; Wu, Q.; Moldawer, L.L.; Mohr, A.M.; Efron, P.A.; Smith, R.S. Persistently Elevated Glucagon-Like Peptide-1 Levels among Critically Ill Surgical Patients after Sepsis and Development of Chronic Critical Illness and Dismal Long-Term Outcomes. J. Am. Coll. Surg. 2019, 229, 58–67.e1. [Google Scholar] [CrossRef] [PubMed]
- Sereika, S.M.; Clochesy, J.M. Left Ventricular Dysfunction and Duration of Mechanical Ventilatory Support in the Chronically Critically Ill: A Survival Analysis. Heart Lung J. Acute Crit. Care 1996, 25, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.A. Patient Perception of Fatigue While Undergoing Long-Term Mechanical Ventilation: Incidence and Associated Factors. Heart Lung J. Acute Crit. Care 1998, 27, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Mira, J.C.; Cuschieri, J.; Ozrazgat-Baslanti, T.; Wang, Z.; Ghita, G.L.; Loftus, T.J.; Stortz, J.A.; Raymond, S.L.; Lanz, J.D.; Hennessy, L.V.; et al. The Epidemiology of Chronic Critical Illness after Severe Traumatic Injury at Two Level-One Trauma Centers. Crit. Care Med. 2017, 45, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Wendlandt, B.; Ceppe, A.; Choudhury, S.; Cox, C.E.; Hanson, L.C.; Danis, M.; Tulsky, J.A.; Nelson, J.E.; Carson, S.S. Modifiable Elements of ICU Supportive Care and Communication Are Associated with Surrogates’ PTSD Symptoms. Intensive Care Med. 2019, 45, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.E.; Hanson, L.C.; Keller, K.L.; Carson, S.S.; Cox, C.E.; Tulsky, J.A.; White, D.B.; Chai, E.J.; Weiss, S.P.; Danis, M. The Voice of Surrogate Decision-Makers: Family Responses to Prognostic Information in Chronic Critical Illness. Am. J. Respir. Crit. Care Med. 2017, 196, 864–872. [Google Scholar] [CrossRef]
- Carson, S.S.; Cox, C.E.; Wallenstein, S.; Hanson, L.C.; Danis, M.; Tulsky, J.A.; Chai, E.; Nelson, J.E. Effect of Palliative Care-Led Meetings for Families of Patients with Chronic Critical Illness: A Randomized Clinical Trial. JAMA-J. Am. Med. Assoc. 2016, 316, 51–62. [Google Scholar] [CrossRef]
- Wendlandt, B.; Ceppe, A.; Cox, C.E.; Hanson, L.C.; Nelson, J.E.; Carson, S.S. The Association between Patient Health Status and Surrogate Decision Maker Post-Traumatic Stress Disorder Symptoms in Chronic Critical Illness. Ann. Am. Thorac. Soc. 2021, 18, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Farley, K.J.; Eastwood, G.M.; Bellomo, R. A Feasibility Study of Functional Status and Follow-up Clinic Preferences of Patients at High Risk of Post Intensive Care Syndrome. Anaesth. Intensive Care 2016, 44, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Wendlandt, B.; Ceppe, A.; Choudhury, S.; Nelson, J.E.; Cox, C.E.; Hanson, L.C.; Danis, M.; Tulsky, J.A.; Carson, S.S. Risk Factors for Post-Traumatic Stress Disorder Symptoms in Surrogate Decision-Makers of Patients with Chronic Critical Illness. Ann. Am. Thorac. Soc. 2018, 15, 1451–1458. [Google Scholar] [CrossRef]
- Mankowski, R.T.; Anton, S.D.; Ghita, G.L.; Brumback, B.; Darden, D.B.; Bihorac, A.; Leeuwenburgh, C.; Moldawer, L.L.; Efron, P.A.; Brakenridge, S.C.; et al. Older Adults Demonstrate Biomarker Evidence of the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome (PICS) after Sepsis. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2022, 77, 188–196. [Google Scholar] [CrossRef]
- Boniatti, M.M.; Giustina, A.D.; Marin, L.G.; França, J.; dos Santos, M.C.; Vidart, J.; Pellegrini, J.A.S.; Lincho, C.S.; Rodrigues Filho, E.M. Mortality in Chronically Critically Ill Patients: Expanding the Use of the ProVent Score. J. Crit. Care 2015, 30, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Ingraham, N.E.; Tignanelli, C.J.; Menk, J.; Chipman, J.G. Pre- and Peri-Operative Factors Associated with Chronic Critical Illness in Liver Transplant Recipients. Surg. Infect. 2020, 21, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Sauter, W.; Starrost, U.; Pohl, M.; Mehrholz, J. Time to Decannulation and Associated Risk Factors in the Postacute Rehabilitation of Critically Ill Patients with Intensive Care Unit-Acquired Weakness: A Cohort Study. Eur. J. Phys. Rehabil. Med. 2017, 53, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.M.; Werner, R.M.; David, G.; Ten Have, T.R.; Benson, N.M.; Asch, D.A. Effectiveness of Long-Term Acute Care Hospitalization in Elderly Patients with Chronic Critical Illness. Med. Care 2013, 51, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Donahoe, M.P.; Zullo, T.G.; Hoffman, L.A. Caregivers of the Chronically Critically Ill after Discharge from the Intensive Care Unit: Six Months’ Experience. Am. J. Crit. Care 2011, 20, 12–23. [Google Scholar] [CrossRef]
- Thomas, S.; Burridge, J.H.; Pohl, M.; Oehmichen, F.; Mehrholz, J. Recovery of Sit-to-Stand Function in Patients with Intensivecare-Unit-Acquired Muscle Weakness: Results from the General Weakness Syndrome Therapy Cohort Study. J. Rehabil. Med. 2016, 48, 793–798. [Google Scholar] [CrossRef]
- Lamas, D.J.; Owens, R.L.; Nace, R.N.; Massaro, A.F.; Pertsch, N.J.; Moore, S.T.; Bernacki, R.E.; Block, S.D. Conversations about Goals and Values Are Feasible and Acceptable in Long-Term Acute Care Hospitals: A Pilot Study. J. Palliat. Med. 2017, 20, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Frengley, J.D.; Sansone, G.R.; Kaner, R.J. Chronic Comorbid Illnesses Predict the Clinical Course of 866 Patients Requiring Prolonged Mechanical Ventilation in a Long-Term, Acute-Care Hospital. J. Intensive Care Med. 2020, 35, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Iwashyna, T.J.; Hodgson, C.L.; Pilcher, D.; Bailey, M.; van Lint, A.; Chavan, S.; Bellomo, R. Timing of Onset and Burden of Persistent Critical Illness in Australia and New Zealand: A Retrospective, Population-Based, Observational Study. Lancet Respir. Med. 2016, 4, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Chadda, K.R.; Blakey, E.E.; Davies, T.W.; Puthucheary, Z. Risk factors, biomarkers, and mechanisms for persistent inflammation, immunosuppression, and catabolism syndrome (PICS): A systematic review and meta-analysis. Br. J. Anaesth. 2024, in press. [CrossRef] [PubMed]

| Parameters | ICIS, N = 60 | No ICIS, N = 834 | p Value | |
|---|---|---|---|---|
| Sex | Male | 37, 57% | 448, 54% | 0.7 1 |
| Female | 26, 43% | 386, 46% | ||
| Age, years | 63.5 (IQR 56.3–74.0) | 63.5 (IQR 52.0–73.0) | 0.5 2 | |
| BMI, kg/m2 | 26.2 (IQR 21.0–32.7) | 27.5 (IQR 23.2–33.5) | 0.18 2 | |
| Body weight on admission, kg | 79 (IQR 62–99) | 81 (IQR 67–97) | 0.4 2 | |
| Body weight at discharge, kg | 78 (IQR 48–98) | 79 (IQR 65–96) | 0.3 2 | |
| Change in body weight, kg | 0.4 (IQR −50.2–6.3) | 0.0 (IQR −4.1–3.2) | 0.8 2 | |
| APACHE IV, score | 71 (IQR 46–91) | 59 (IQR 45–76) | 0.017 2 | |
| Mechanical ventilation (MV) | 27, 45% | 273, 33% | 0.052 1 | |
| Duration of MV, days | 5 (IQR 2–13) | 4 (IQR 2–10) | 0.5 2 | |
| Use of vasoactive drugs | 16, 27% | 117, 14% | 0.008 1 | |
| Lactate level on admission, mmol/L | 1.8 (IQR 1.3–3.0) | 1.5 (IQR 1.0–2.4) | 0.068 2 | |
| Main reasons for ICU admission | ||||
| Sepsis | 14, 23% | 250, 30% | 0.3 1 | |
| Cardiac arrest | 1, 1.7% | 20, 2.4% | 0.9 3 | |
| Respiratory arrest | 0, 0% | 15, 1.8% | 0.6 3 | |
| Coma | 0, 0% | 17, 2.0% | 0.6 3 | |
| Bleeding | 2, 3.3% | 20, 2.4% | 0.7 3 | |
| Cardiogenic shock | 0, 0% | 4, 0.5% | 0.9 3 | |
| Hypovolemia | 0, 0% | 8, 1.0% | 0.9 3 | |
| Pancreatitis | 4, 6.7% | 12, 1.4% | 0.018 3 | |
| Acute renal failure | 2, 3.3% | 21, 2.5% | 0.7 3 | |
| Congestive heart failure | 2, 3.3% | 34, 4.1% | 0.9 3 | |
| Pneumonia | 5, 8.3% | 30, 3.6% | 0.079 3 | |
| Weaning from MV | 1, 1.7% | 28, 3.4% | 0.7 3 | |
| Acute myocardial infarction | 0, 0% | 17, 2.0% | 0.6 3 | |
| Seizures | 0, 0% | 12, 1.4% | 0.9 3 | |
| Supraventricular arrhythmias | 1, 1.7% | 17, 2.0% | 0.9 3 | |
| Stroke | 0, 0% | 15, 1.8% | 0.6 3 | |
| Cardiac surgery | 1, 1.7% | 13, 1.6% | 0.9 3 | |
| Hospitalization outcomes | ||||
| Hospital mortality | 11, 18.3% | 41, 4.9% | <0.001 3 | |
| ICU mortality | 11, 18.3% | 41, 4.9% | <0.001 3 | |
| Duration of hospitalization, days | 13 (IQR 9–19) | 11 (IQR 6–18) | 0.041 2 | |
| Length of ICU stay, days | 6.0 (IQR 2.7–13.4) | 3.4 (IQR 1.8–8.3) | <0.001 2 | |
| Discharge location | Home | 17, 45% | 357, 50% | 0.5 1 |
| Other (rehabilitation, other hospital) | 21, 55% | 354, 50% | ||
| Parameters | AUROC (95% CI) | Hazard Ratio (95% CI) | p Value | |
|---|---|---|---|---|
| APACHE IV | 0.598 (0.512–0.684) | APACHE IV ≥ 65 * | 2.36 (1.33–4.18) | 0.003 |
| No cutoff point | 1.013 (1.003–1.022) | 0.012 | ||
| Pancreatitis | - | 3.07 (0.94–10.1) * | 0.063 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Likhvantsev, V.V.; Berikashvili, L.B.; Yadgarov, M.Y.; Yakovlev, A.A.; Kuzovlev, A.N. The Tri-Steps Model of Critical Conditions in Intensive Care: Introducing a New Paradigm for Chronic Critical Illness. J. Clin. Med. 2024, 13, 3683. https://doi.org/10.3390/jcm13133683
Likhvantsev VV, Berikashvili LB, Yadgarov MY, Yakovlev AA, Kuzovlev AN. The Tri-Steps Model of Critical Conditions in Intensive Care: Introducing a New Paradigm for Chronic Critical Illness. Journal of Clinical Medicine. 2024; 13(13):3683. https://doi.org/10.3390/jcm13133683
Chicago/Turabian StyleLikhvantsev, Valery V., Levan B. Berikashvili, Mikhail Ya. Yadgarov, Alexey A. Yakovlev, and Artem N. Kuzovlev. 2024. "The Tri-Steps Model of Critical Conditions in Intensive Care: Introducing a New Paradigm for Chronic Critical Illness" Journal of Clinical Medicine 13, no. 13: 3683. https://doi.org/10.3390/jcm13133683
APA StyleLikhvantsev, V. V., Berikashvili, L. B., Yadgarov, M. Y., Yakovlev, A. A., & Kuzovlev, A. N. (2024). The Tri-Steps Model of Critical Conditions in Intensive Care: Introducing a New Paradigm for Chronic Critical Illness. Journal of Clinical Medicine, 13(13), 3683. https://doi.org/10.3390/jcm13133683








