Association between Epicardial Adipose Tissue and Atrial Fibrillation in Patients with Transfusion-Dependent β-Thalassemia
Abstract
1. Introduction
2. Methods
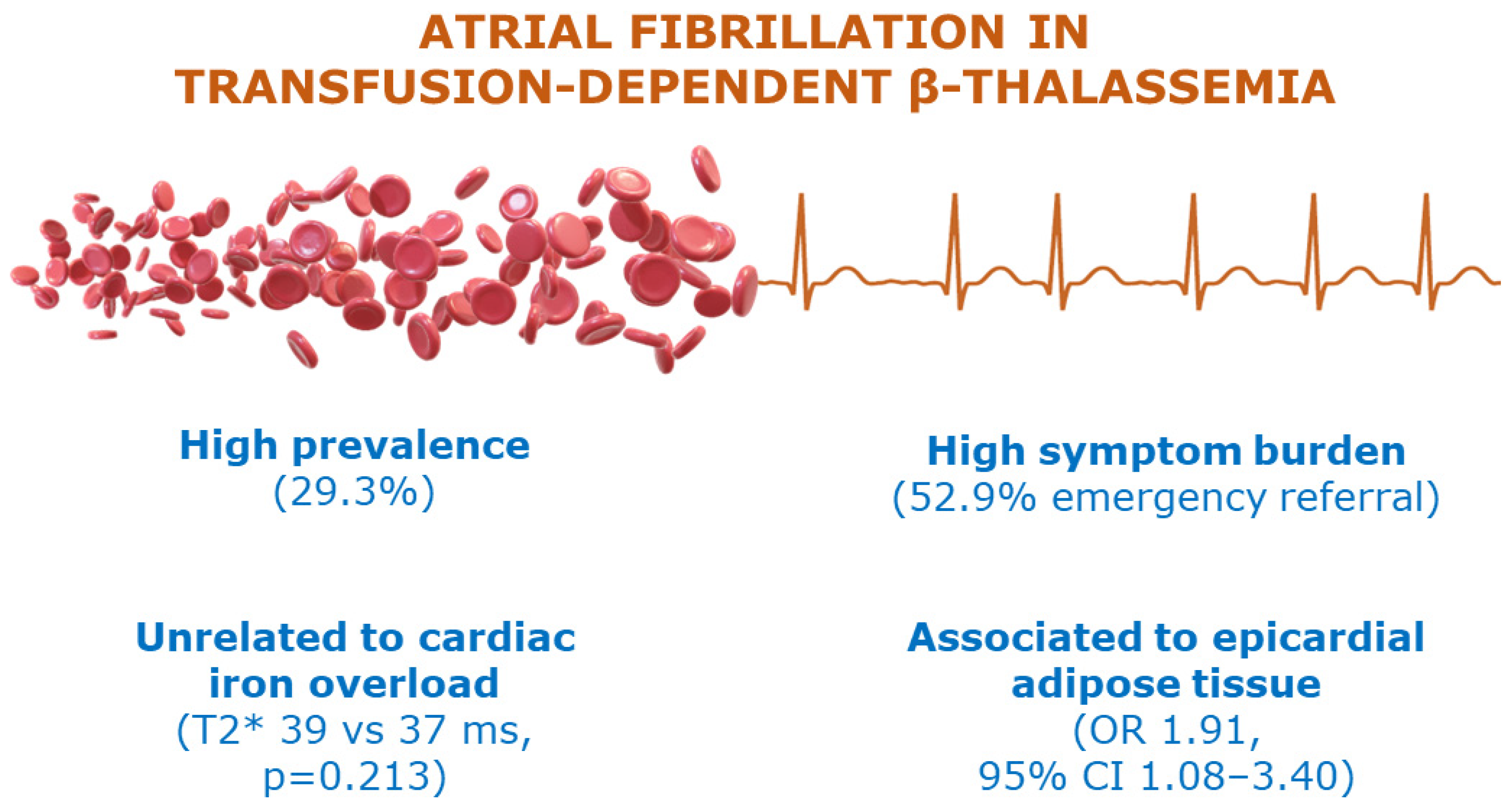
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taher, A.T.; Musallam, K.M.; Cappellini, M.D. β-Thalassemias. N. Engl. J. Med. 2021, 384, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Kattamis, A.; Kwiatkowski, J.L.; Aydinok, Y. Thalassaemia. Lancet 2022, 399, 2310–2324. [Google Scholar] [CrossRef] [PubMed]
- Malagù, M.; Marchini, F.; Fiorio, A.; Sirugo, P.; Clò, S.; Mari, E.; Gamberini, M.R.; Rapezzi, C.; Bertini, M. Atrial Fibrillation in β-Thalassemia: Overview of Mechanism, Significance and Clinical Management. Biology 2022, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Forni, G.L.; Gianesin, B.; Musallam, K.M.; Longo, F.; Rosso, R.; Lisi, R.; Gamberini, M.R.; Pinto, V.M.; Graziadei, G.; Vitucci, A.; et al. Overall and complication-free survival in a large cohort of patients with β-thalassemia major followed over 50 years. Am. J. Hematol. 2023, 98, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Demircan, T.; Onder Sivis, Z.; Tatll Güneş, B.; Karadeniz, C. Evaluation of electrocardiographic markers of cardiac arrhythmic events and their correlation with cardiac iron overload in patients with β-thalassemia major. Cardiol. Young 2020, 30, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.X.; Ganesan, A.N.; Selvanayagam, J.B. Epicardial fat and atrial fibrillation: Current evidence, potential mechanisms, clinical implications, and future directions. Eur. Heart J. 2017, 38, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Al Chekakie, M.O.; Welles, C.C.; Metoyer, R.; Ibrahim, A.; Shapira, A.R.; Cytron, J.; Santucci, P.; Wilber, D.K.; Akar, J.G. Pericardial fat is independently associated with human atrial fibrillation. J. Am. Coll. Cardiol. 2010, 56, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Thanassoulis, G.; Massaro, J.M.; O’Donnell, C.J.; Hoffmann, U.; Levy, D.; Ellinor, P.T.; Wang, T.J.; Schnabel, R.B.; Vasan, R.S.; Fox, C.S.; et al. Pericardial fat is associated with prevalent atrial fibrillation: The Framingham Heart Study. Circ. Arrhythm. Electrophysiol. 2010, 3, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Leo, L.A.; Paiocchi, V.; Schlossbauer, S.; Ho, S.; Faletra, F. The Intrusive Nature of Epicardial Adipose Tissue as Revealed by Cardiac Magnetic Resonance. J. Cardiovasc. Echogr. 2019, 29, 45. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Willens, H.J. Echocardiographic Epicardial Fat: A Review of Research and Clinical Applications. J. Am. Soc. Echocardiogr. 2009, 22, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Restaino, G.; Borsellino, Z.; Caruso, V.; Spasiano, A.; Zuccarelli, A.; Valeri, G.; Taia, P.; Salvatori, C.; Positano, V.; et al. Different patterns of myocardial iron distribution by whole-heart T2∗ magnetic resonance as risk markers for heart complications in thalassemia major. Int. J. Cardiol. 2014, 177, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Marsella, M.; Borgna-Pignatti, C.; Meloni, A.; Caldarelli, V.; Dell’Amico, M.C.; Spasiano, A.; Pitrolo, L.; Cracolici, E.; Valeri, G.; Positano, V.; et al. Cardiac iron and cardiac disease in males and females with transfusion-dependent thalassemia major: A T2* magnetic resonance imaging study. Haematologica 2011, 96, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Kirk, P.; Roughton, M.; Porter, J.B.; Walker, J.M.; Tanner, M.A.; Patel, J.; Wu, D.; Taylor, J.; Westwood, M.A.; Anderson, L.J.; et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation 2009, 120, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, A.G.; Tsiapras, D.P.; Chaidaroglou, A.S.; De Giannis, D.E.; Farmakis, D.; Kremastinos, D.T. The pathophysiological relationship and clinical significance of left atrial function and left ventricular diastolic dysfunction in β-thalassemia major. Am. J. Hematol. 2014, 89, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Marchini, F.; Fiorio, A.; Sirugo, P.; Gamberini, M.R.; Mari, E.; Bertini, M.; Malagù, M. Heavy metal! A case of severe iron overload and supraventricular arrhythmias in a thalassemia major patient. G. Ital. Cardiol. 2022, 23, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, L.; Pavon, A.G.; Angeli, F.; Tuttolomondo, D.; Belmonte, M.; Armillotta, M.; Sansonetti, A.; Foà, A.; Paolisso, P.; Baggiano, A.; et al. The Role of Non-Invasive Multimodality Imaging in Chronic Coronary Syndrome: Anatomical and Functional Pathways. Diagnostics 2023, 13, 2083. [Google Scholar] [CrossRef]
- Taher, A.; Isma’eel, H.; Mehio, G.; Bignamini, D.; Kattamis, A.; Rachmilewitz, E.A.; Cappellini, M.D. Prevalence of thromboembolic events among 8,860 patients with thalassaemia major and intermedia in the Mediterranean area and Iran. Thromb Haemost. 2006, 96, 488–491. [Google Scholar] [CrossRef]
- Karimi, M.; Khanlari, M.; Rachmilewitz, E.A. Cerebrovascular accident in beta-thalassemia major (beta-TM) and beta-thalassemia intermedia (beta-TI). Am. J. Hematol. 2008, 83, 77–79. [Google Scholar] [CrossRef]
- Malagù, M.; Longo, F.; Marchini, F.; Sirugo, P.; Capanni, A.; Clò, S.; Mari, E.; Culcasi, M.; Bertini, M. Non-Vitamin K Antagonist Oral Anticoagulants in Patients with β-Thalassemia. Biology 2023, 12, 491. [Google Scholar] [CrossRef]
- Apostolou, C.; Klonizakis, P.; Mainou, M.; Kapsali, E.; Kafantari, K.; Kotsiafti, A.; Vetsiou, E.; Vakalopoulou, S.; Vlachaki, E. Rivaroxaban Use in Patients with Hemoglobinopathies. Hemoglobin 2017, 41, 223–224. [Google Scholar] [CrossRef]
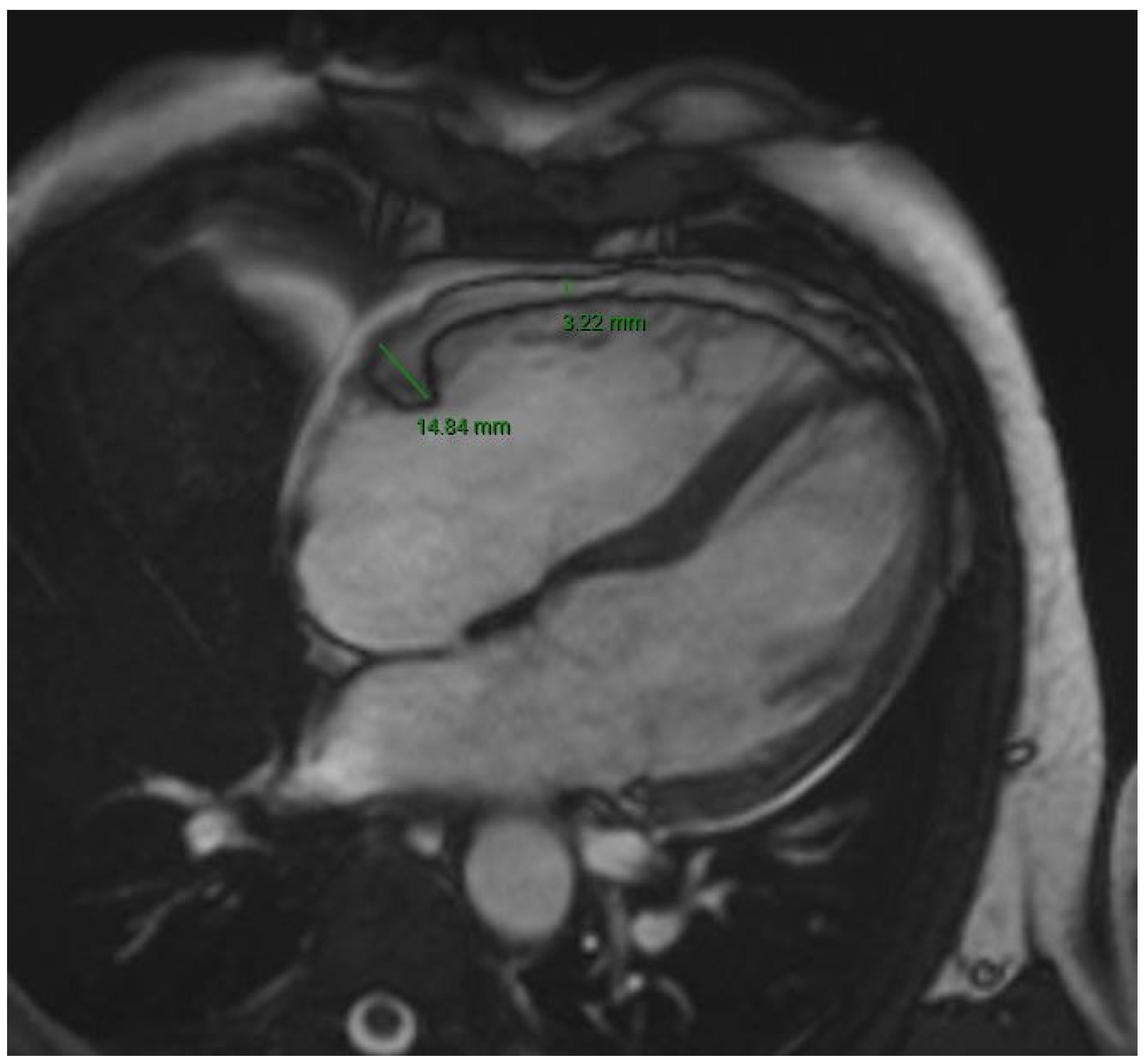
| Variable | Study Population (n = 116) | No AF (n = 82) | AF (n = 34) | p Value |
|---|---|---|---|---|
| Male sex | 60 (51.7%) | 39 (47.6%) | 21 (61.8%) | 0.22 |
| Age (years) | 50 [45–55] | 49 [43–54] | 53 [48–57] | <0.01 |
| BMI (kg/m2) | 22.1 [20.6–24.2] | 22.1 [20.5–24.2] | 22.4 [20.8–24.0] | 0.96 |
| Arterial hypertension | 13 (11.2%) | 7 (8.5%) | 6 (17.6%) | 0.20 |
| Dyslipidemia | 2 (1.7%) | 2 (2.4%) | 0 (0%) | 1.00 |
| Smoking habit | 0.91 | |||
| - Active | 16 (13.8%) | 12 (14.6%) | 4 (11.8%) | |
| - Previous | 21 (18.1%) | 14 (17.1%) | 7 (20.6%) | |
| Altered glucose metabolism | 0.29 | |||
| - Impaired fasting glucose | 2 (1.7%) | 2 (2.4%) | 0 (0%) | |
| - Abnormal OGTT | 3 (2.6%) | 1 (1.2%) | 2 (5.9%) | |
| - Diabetes mellitus | 24 (20.7%) | 19 (23.2%) | 5 (14.7%) | |
| Hyperinsulinism | 1 (0.9%) | 0 (0%) | 1 (2.9%) | 0.29 |
| Hypothyroidism | 32 (27.6%) | 17 (20.7%) | 15 (44.1%) | 0.01 |
| Hyperthyroidism | 1 (0.9%) | 0 (0%) | 1 (2.9%) | 0.29 |
| Hypoparathyroidism | 4 (3.4%) | 2 (2.4%) | 2 (5.9%) | 0.29 |
| GH deficiency | 4 (3.4%) | 3 (3.7%) | 1 (2.9%) | 1.00 |
| Hypogonadism | 33 (28.4%) | 21 (25.6%) | 12 (35.3%) | 0.37 |
| Osteoporosis | 65 (56.0%) | 46 (56.1%) | 19 (55.9%) | 1.00 |
| Ischemic stroke | 3 (2.6%) | 2 (2.4%) | 1 (2.9%) | 1.00 |
| Pulmonary hypertension | 10 (8.6%) | 2 (2.4%) | 8 (23.5%) | <0.01 |
| Splenectomy | 83 (71.6%) | 53 (64.6%) | 30 (88.2%) | 0.01 |
| COPD | 2 (1.7%) | 1 (1.2%) | 1 (2.9%) | 0.50 |
| LIC (mg Fe/g) | 2.77 [1.39–5.38] | 2.85 [1.56–5.88] | 1.84 [1.31–4.03] | 0.13 |
| Heart failure | 5 (4.3%) | 3 (3.7%) | 2 (5.9%) | 0.63 |
| WBC (×103/µL) | 9.5 [7.3–12.9] | 9.6 [6.7–13.1] | 9.4 [7.9–11.6] | 0.67 |
| Frequency of transfusions (days) | 15 [15–21] | 15 [15–21] | 15 [15–20] | 0.32 |
| Pre-transfusion Hb (g/dL) | 9.9 [9.4–10.4] | 9.8 [9.4–10.3] | 10.0 [9.3–10.6] | 0.28 |
| Platelets (×103/µL) | 446 [296–571] | 437 [292–583] | 454 [390–560] | 0.79 |
| Ferritin (ng/mL) | 531 [325–782] | 608 [397–866] | 342 [245–595] | <0.01 |
| Soluble transferrin receptor (mg/dL) | 3.3 [2.6–4.6] | 3.4 [2.6–4.6] | 3.2 [2.4–3.9] | 0.43 |
| Heart rate (bpm) | 72 [65–79] | 72 [65–79] | 74 [64–79] | 0.96 |
| P wave duration (msec) | 85 [75–96] | 85 [75–96] | 90 [77–100] | 0.24 |
| QTc interval, Bazett (msec) | 414 [394–433] | 414 [395–429] | 421 [392–438] | 0.44 |
| LV EDV index (mL/m2) | 61.7 [54.1–72.1] | 62.2 [55.5–73.6] | 61.6 [53.3–66.2] | 0.34 |
| Ejection fraction (%) | 60.0 [57.0–65.0] | 61.0 [60.0–65.0] | 60.0 [55.0–61.5] | 0.01 |
| Interventricular septum thickness (mm) | 0.9 [0.8–1.0] | 0.9 [0.8–1.0] | 0.9 [0.8–1.1] | 0.24 |
| LA volume (mL) | 49 [35–67] | 43 [33–55] | 74 [59–88] | <0.01 |
| RA volume (mL) | 45 [32–58] | 40 [26–48] | 61 [49–81] | <0.01 |
| Diastolic dysfunction | 20 (21.3%) | 11 (15.9%) | 9 (36%) | <0.01 |
| TAPSE (cm) | 2.4 [2.2–2.7] | 2.4 [2.2–2.7] | 2.4 [2.3–3.2] | 0.36 |
| T2* (ms) | 39.0 [35.0–41.0] | 39.0 [36.0–41.3] | 37.0 [33.5–41.0] | 0.21 |
| RA EAT (mm) | 4.0 [3.3–4.7] | 4.0 [3.2–4.4] | 4.4 [3.9–5.0] | 0.02 |
| RAVG EAT (mm) | 13.0 [11.2–15.0] | 13.1 [11.7–14.7] | 12.2 [10.5–16.1] | 0.69 |
| RV EAT (mm) | 4.6 [3.6–5.6] | 4.3 [3.6–5.5] | 5.0 [4.4–6.2] | 0.04 |
| LA EAT (mm) | 4.4 [3.6–5.5] | 4.0 [3.4–5.2] | 5.0 [4.2–6.0] | <0.01 |
| LAVG EAT (mm) | 12.4 [10.7–13.7] | 12.3 [10.8–13.5] | 12.6 [9.7–14.8] | 0.64 |
| LV EAT (mm) | 4.4 [3.3–5.2] | 4.3 [3.2–5.3] | 4.5 [3.7–4.8] | 0.79 |
| Variable | Patients with AF (n = 34) |
|---|---|
| AF type | |
| - Paroxysmal | 21 (61.8%) |
| - Persistent | 7 (20.6%) |
| - Permanent | 6 (17.6%) |
| CHA2DS2VASc score | |
| - 0 | 14 (41.2%) |
| - 1 | 9 (26.5%) |
| - 2 | 8 (23.5%) |
| - 3 | 2 (5.9%) |
| - 4 | 1 (2.9%) |
| - >4 | 0 (0%) |
| AF ablation | 10 (29.4%) |
| Cardioversion | 22 (64.7%) |
| - Electrical | 8 (23.5%) |
| - Pharmacological | 5 (14.7%) |
| - Both | 9 (26.5%) |
| At least one emergency room referral for AF | 18 (52.9%) |
| Number of AF episodes within last year | |
| - 0 | 23 (67.6%) |
| - 1 | 7 (20.6%) |
| - 2 | 1 (2.9%) |
| - >2 | 3 (8.8%) |
| EHRA symptom scale | |
| - 1 | 21 (61.8%) |
| - 2a | 8 (23.5%) |
| - 2b | 2 (5.9%) |
| - 3 | 3 (8.8%) |
| Other arrhythmias | 21 (61.8%) |
| - Typical atrial flutter | 6 (17.6%) |
| - Atypical atrial flutter | 7 (20.6%) |
| - Paroxysmal SVT | 2 (5.9%) |
| - Atrial tachycardia | 5 (14.7%) |
| Variable | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p Value | Odds Ratio | 95% CI | p Value | |
| Age | 1.05 | 1.00–1.10 | 0.04 | |||
| Hypothyroidism | 3.02 | 1.28–7.15 | 0.01 | 9.95 | 1.99–49.85 | <0.01 |
| Splenectomy | 4.10 | 1.32–12.80 | 0.02 | |||
| LA volume | 1.04 | 1.02–1.07 | <0.01 | 1.09 | 1.03–1.15 | <0.01 |
| Diastolic dysfunction | 3.39 | 1.18–9.75 | 0.02 | |||
| LA EAT | 1.61 | 1.14–2.28 | 0.01 | 1.91 | 1.08–3.40 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malagù, M.; Tonet, E.; Orazio, G.; Longo, F.; De Raffele, M.; Sirugo, P.; Capanni, A.; Clò, S.; Berloni, M.L.; Marchini, F.; et al. Association between Epicardial Adipose Tissue and Atrial Fibrillation in Patients with Transfusion-Dependent β-Thalassemia. J. Clin. Med. 2024, 13, 3471. https://doi.org/10.3390/jcm13123471
Malagù M, Tonet E, Orazio G, Longo F, De Raffele M, Sirugo P, Capanni A, Clò S, Berloni ML, Marchini F, et al. Association between Epicardial Adipose Tissue and Atrial Fibrillation in Patients with Transfusion-Dependent β-Thalassemia. Journal of Clinical Medicine. 2024; 13(12):3471. https://doi.org/10.3390/jcm13123471
Chicago/Turabian StyleMalagù, Michele, Elisabetta Tonet, Giovanni Orazio, Filomena Longo, Martina De Raffele, Paolo Sirugo, Andrea Capanni, Stefano Clò, Maria Letizia Berloni, Federico Marchini, and et al. 2024. "Association between Epicardial Adipose Tissue and Atrial Fibrillation in Patients with Transfusion-Dependent β-Thalassemia" Journal of Clinical Medicine 13, no. 12: 3471. https://doi.org/10.3390/jcm13123471
APA StyleMalagù, M., Tonet, E., Orazio, G., Longo, F., De Raffele, M., Sirugo, P., Capanni, A., Clò, S., Berloni, M. L., Marchini, F., Manfrini, M., Mari, E., Soffritti, O., Culcasi, M., Balla, C., Vitali, F., Cossu, A., & Bertini, M. (2024). Association between Epicardial Adipose Tissue and Atrial Fibrillation in Patients with Transfusion-Dependent β-Thalassemia. Journal of Clinical Medicine, 13(12), 3471. https://doi.org/10.3390/jcm13123471








