Particle Beam Therapy and Surgery as Radical Treatments for Parotid Malignancies—A Single-Center Preliminary Case Study
Abstract
1. Introduction
2. Patients and Methods
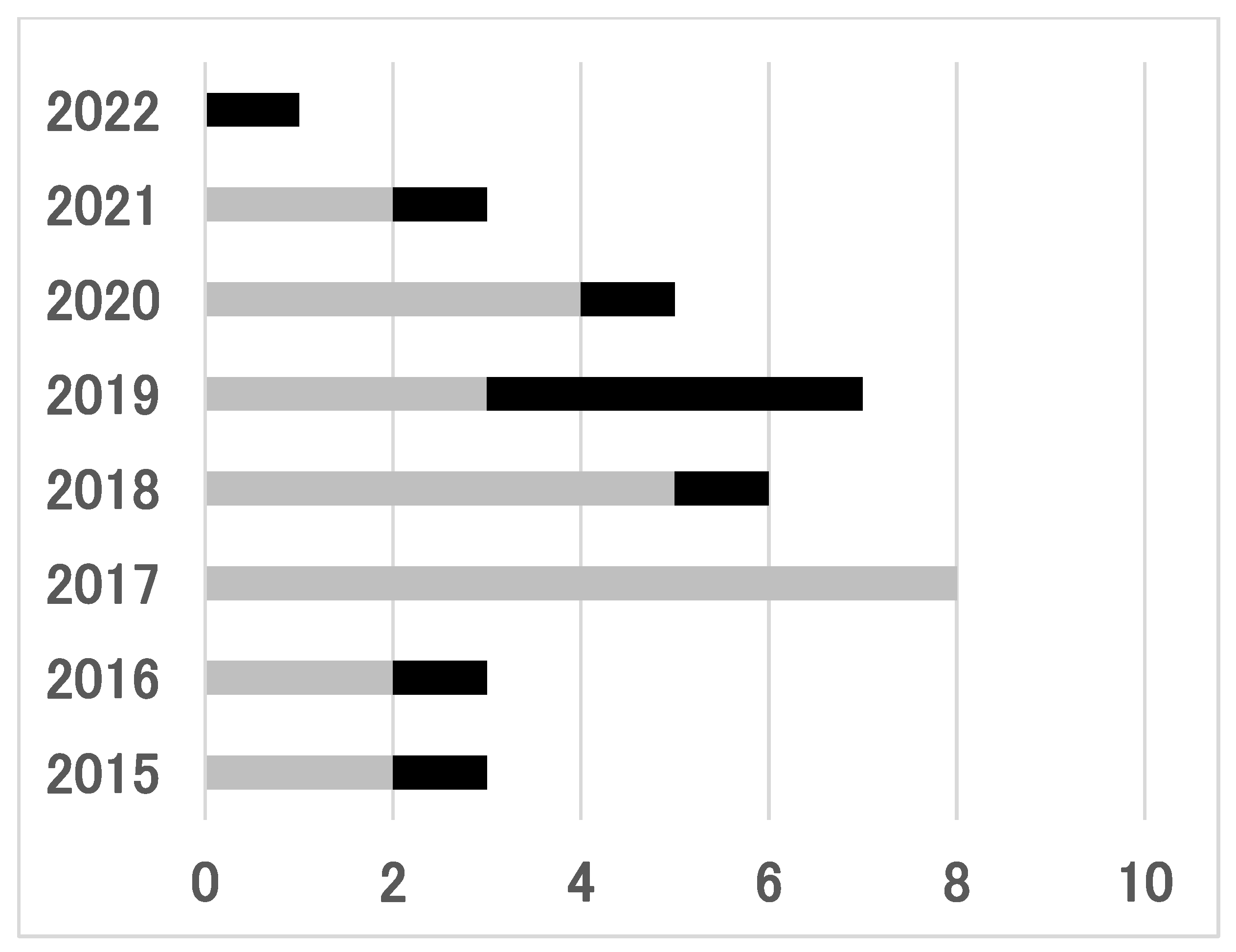
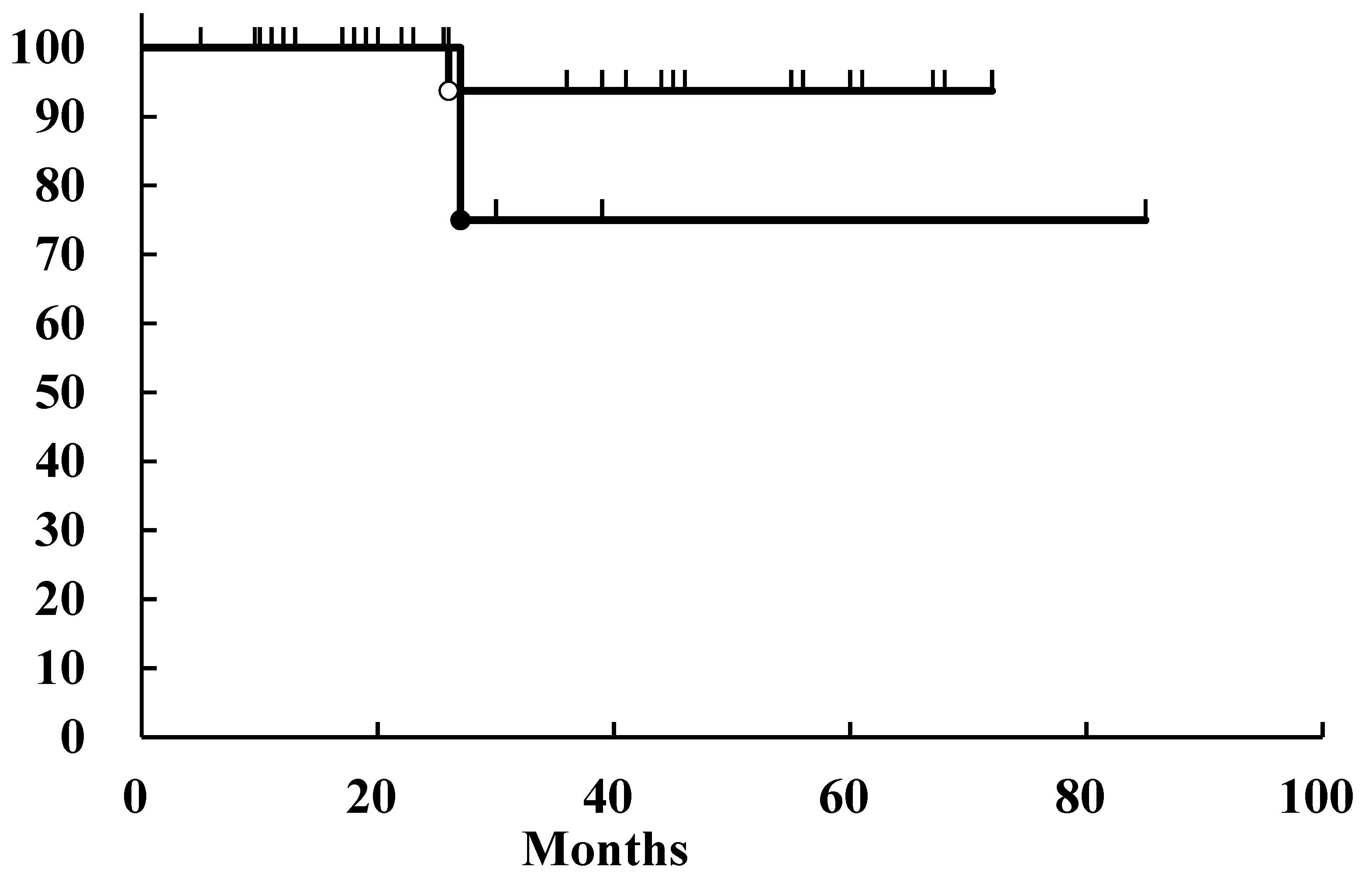
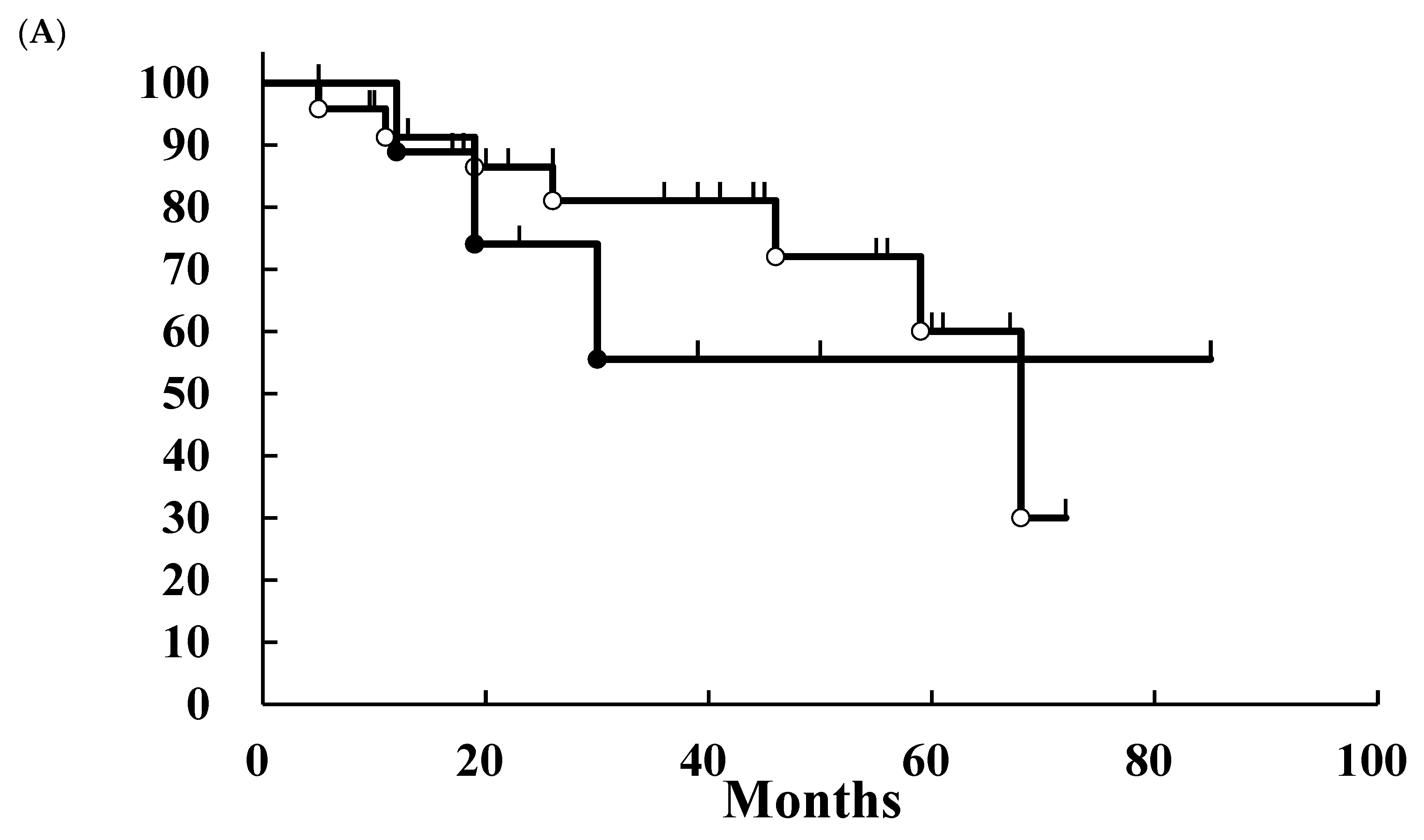
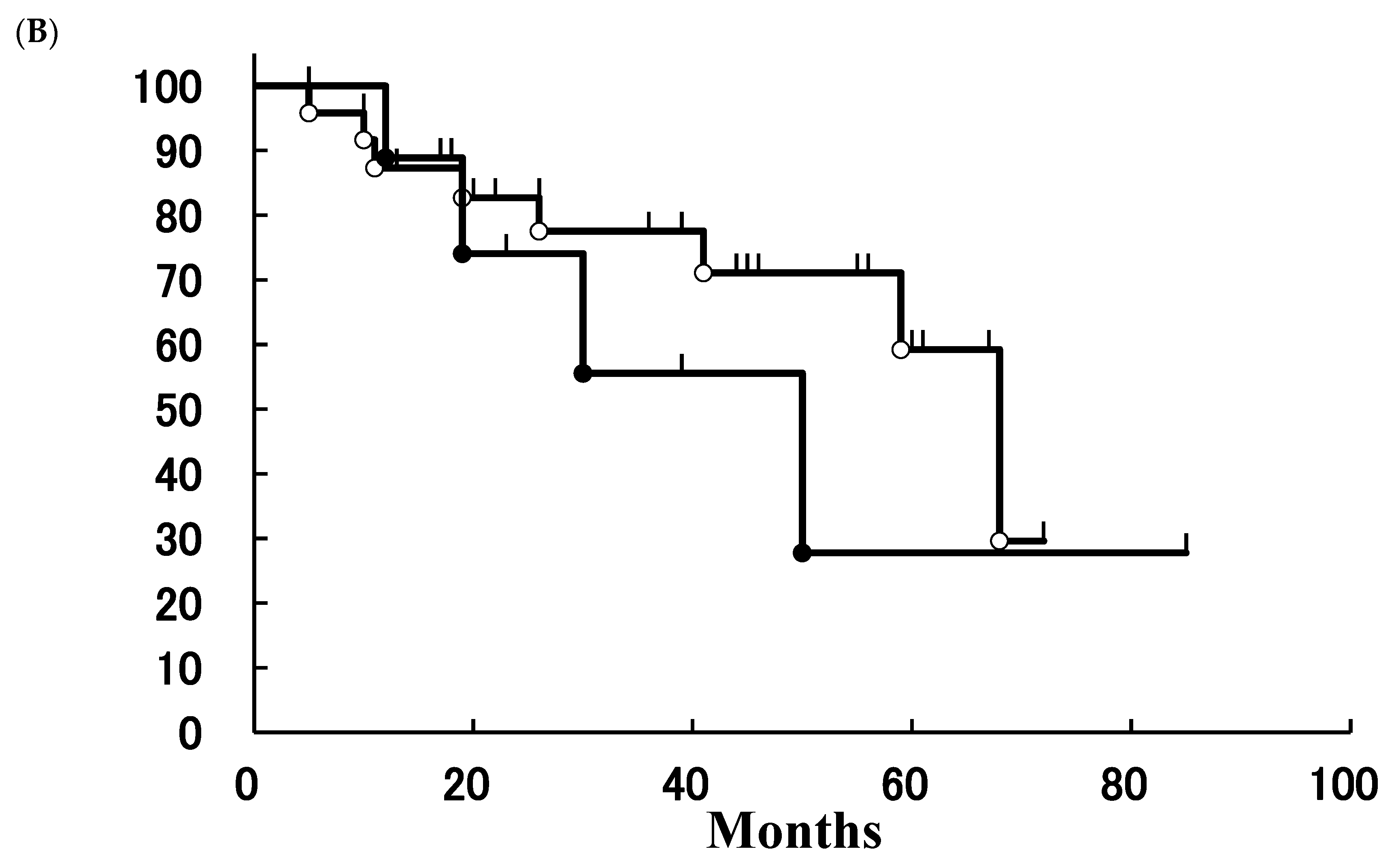
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Naggar, A.K.; Chan, J.K.C.; Takata, T.; Grandis, J.R.; Slootweg, P.J. The fourth edition of the head and neck World Health Organization blue book: Editors’ perspectives. Hum. Pathol. 2017, 66, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Kawata, R.; Kinoshita, I.; Jinnin, T.; Higashino, M.; Terada, T.; Kurisu, Y.; Hirose, Y.; Tochizawa, T. Clinicopathological characteristics of four major histological types of high-grade parotid carcinoma. Int. J. Clin. Oncol. 2023, 28, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Jeannon, J.P.; Calman, F.; Gleeson, M.; McGurk, M.; Morgan, P.; O’Connell, M.; Odell, E.; Simo, R. Management of advanced parotid cancer. A systematic review. Eur. J. Surg. Oncol. 2009, 35, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.L.; Ismaila, N.; Beadle, B.; Caudell, J.J.; Chau, N.; Deschler, D.; Glastonbury, C.; Kaufman, M.; Lamarre, E.; Lau, H.Y.; et al. Management of salivary gland malignancy: ASCO guideline. J. Clin. Oncol. 2021, 39, 1909–1941. [Google Scholar] [CrossRef] [PubMed]
- Morse, E.; Fujiwara, R.J.T.; Judson, B.; Prasad, M.L.; Mehra, S. Positive surgical margins in parotid malignancies: Institutional variation and survival association. Laryngoscope 2019, 129, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.B.; Dierks, E.J.; Homer, L.; Potter, B.E. Management and outcome of patients with malignant salivary gland tumors. J. Oral. Maxillofac. Surg. 2005, 63, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Koul, R.; Dubey, A.; Butler, J.; Cooke, A.L.; Abdoh, A.; Nason, R. Prognostic factors depicting disease-specific survival in parotid-gland tumors. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Terakedis, B.E.; Hunt, J.P.; Buchmann, L.O.; Avizonis, V.N.; Anker, C.J.; Hitchcock, Y.J. The prognostic significance of facial nerve involvement in carcinomas of the parotid gland. Am. J. Clin. Oncol. 2017, 40, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Koto, M.; Hasegawa, A.; Takagi, R.; Ikawa, H.; Naganawa, K.; Mizoe, J.E.; Jingu, K.; Tsujii, H.; Tsuji, H.; Kamada, T.; et al. Definitive carbon-ion radiotherapy for locally advanced parotid gland carcinomas. Head Neck 2017, 39, 724–729. [Google Scholar] [CrossRef]
- Azami, Y.; Hayashi, Y.; Nakamura, T.; Kimura, K.; Yamaguchi, H.; Ono, T.; Takayama, K.; Hirose, K.; Yabuuchi, T.; Suzuki, M.; et al. Proton beam therapy for locally recurrent parotid gland cancer. Indian J. Otolaryngol. Head Neck Surg. 2019, 71 (Suppl. S1), 49–54. [Google Scholar] [CrossRef]
- Hayashi, K.; Koto, M.; Demizu, Y.; Saitoh, J.I.; Suefuji, H.; Okimoto, T.; Ohno, T.; Shioyama, Y.; Takagi, R.; Ikawa, H.; et al. A retrospective multicenter study of carbon-ion radiotherapy for major salivary gland carcinomas: Subanalysis of J-CROS 1402 HN. Cancer Sci. 2018, 109, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, K.; Wang, H.; Kang, J.J.; Lee, A.; Romesser, P.; Mohamed, N.; Gelblum, D.; Sherman, E.; Dunn, L.; Boyle, J.; et al. Outcomes and prognostic factors of major salivary gland tumors treated with proton beam radiation therapy. Head Neck 2021, 43, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Margalit, D.N.; Sacco, A.G.; Cooper, J.S.; Ridge, J.A.; Bakst, R.L.; Beadle, B.M.; Beitler, J.J.; Chang, S.S.; Chen, A.M.; Galloway, T.J.; et al. Systematic review of postoperative therapy for resected squamous cell carcinoma of the head and neck: Executive summary of the American Radium Society Appropriate Use Criteria. Head Neck 2021, 43, 367–391. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Kato, T.; Nakamura, T.; Azami, Y.; Ono, T.; Suzuki, M.; Takada, A.; Yamaguchi, H.; Seto, I.; Nakasato, T.; et al. Proton Beam Therapy Combined with Intra-Arterial Infusion Chemotherapy for Stage IV Adenoid Cystic Carcinoma of the Base of the Tongue. Cancers 2019, 11, 1413. [Google Scholar] [CrossRef] [PubMed]
- Giridhar, P.; Venkatesulu, B.P.; Yoo, R.; V, P.; Rath, G.K.; Mallick, S.; Upadhyay, A.; Chan, D.P. Demography, patterns of care, and survival outcomes in patients with salivary duct carcinoma: An individual patient data analysis of 857 patients. Future Sci. OA 2022, 8, FSO791. [Google Scholar] [CrossRef]
- Masubuchi, T.; Tada, Y.; Maruya, S.; Osamura, Y.; Kamata, S.E.; Miura, K.; Fushimi, C.; Takahashi, H.; Kawakita, D.; Kishimoto, S.; et al. Clinicopathological significance of androgen receptor, HER2, Ki-67 and EGFR expressions in salivary duct carcinoma. Int. J. Clin. Oncol. 2015, 20, 35–44. [Google Scholar] [CrossRef]
- Boon, E.; Bel, M.; van Boxtel, W.; van der Graaf, W.T.A.; van Es, R.J.J.; Eerenstein, S.E.J.; Baatenburg de Jong, R.J.; van den Brekel, M.W.M.; van der Velden, L.A.; Witjes, M.J.H.; et al. A clinicopathological study and prognostic factor analysis of 177 salivary duct carcinoma patients from The Netherlands. Int. J. Cancer 2018, 143, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.D.; Perrone, F.; Cortelazzi, B.; Lo Vullo, S.; Bossi, P.; Dagrada, G.; Quattrone, P.; Bergamini, C.; Potepan, P.; Civelli, E.; et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck 2016, 38, 724–731. [Google Scholar] [CrossRef]
- Boon, E.; van Boxtel, W.; Buter, J.; Baatenburg de Jong, R.J.; van Es, R.J.J.; Bel, M.; Fiets, E.; Oosting, S.F.; Slingerland, M.; Hoeben, A.; et al. Androgen deprivation therapy for androgen receptor-positive advanced salivary duct carcinoma: A nationwide case series of 35 patients in The Netherlands. Head Neck 2018, 40, 605–613. [Google Scholar] [CrossRef]
- Takahashi, H.; Tada, Y.; Saotome, T.; Akazawa, K.; Ojiri, H.; Fushimi, C.; Masubuchi, T.; Matsuki, T.; Tani, K.; Osamura, R.Y.; et al. Phase II Trial of Trastuzumab and Docetaxel in Patients with Human Epidermal Growth Factor Receptor 2-Positive Salivary Duct Carcinoma. J. Clin. Oncol. 2019, 37, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, D.; Nagao, T.; Takahashi, H.; Kano, S.; Honma, Y.; Hirai, H.; Saigusa, N.; Akazawa, K.; Tani, K.; Ojiri, H.; et al. Survival benefit of HER2-targeted or androgen deprivation therapy in salivary duct carcinoma. Ther. Adv. Med. Oncol. 2022, 14, 17588359221119538. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network Guidelines, Head and Neck Cancers, Version 3. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 1 April 2024).
| Surgery (n = 26) | Particle Beam Therapy (n = 10) | p-Value | |
|---|---|---|---|
| Average age (years) | 67.3 | 61.3 | 0.02 |
| Sex (female/male) | 11/15 | 5/5 | 0.67 |
| Histopathology | |||
| Salivary duct carcinoma | 10 | 2 | |
| Adenoid cystic carcinoma | 2 | 3 | |
| Adenocarcinoma, not otherwise specified | 2 | 1 | |
| Mucoepidermoid carcinoma | 3 | 0 | |
| Acinic cell carcinoma | 0 | 3 | |
| Squamous cell carcinoma | 1 | 1 | |
| Epithelial–myoepithelial carcinoma | 2 | 0 | |
| Myoepithelial carcinoma | 1 | 0 | |
| Lymphoepithelial carcinoma | 1 | 0 | |
| Hybrid carcinoma | 1 | 0 | |
| Secretory carcinoma | 1 | 0 | |
| Carcinoma ex pleomorphic adenoma | 1 | 0 | |
| Angiosarcoma | 1 | 0 | |
| T classification | |||
| T1 | 0 | 1 | T1, T2 vs. T3, T4 |
| T2 | 7 | 2 | 0.85 |
| T3 | 7 | 1 | |
| T4a | 12 | 4 | |
| T4b | 0 | 2 | |
| N classification | |||
| N0 | 13 | 4 | N0 vs. N+ |
| N1 | 2 | 1 | 0.59 |
| N2b | 8 | 4 | |
| N3b | 3 | 1 | |
| Initial treatment | 26 | 5 | 0.0001 |
| Recurrent tumor | 0 | 5 |
| Patient No. | Age (Years) | Sex | Histopathology | TNM | Treatment (Type and Dose) | Outcome |
|---|---|---|---|---|---|---|
| 1 | 67 | Male | Acinic cell carcinoma | rT4bN1M0 | CIB, 64 Gy | AwoD, 15M |
| 2 | 56 | Female | Adenocarcinoma | rT2N2bM0 | PB, NS | DOD, 15M |
| 3 | 70 | Female | Squamous cell carcinoma | rT4aN2bM0 | PB, 70 Gy | AWD, 18M |
| 4 | 45 | Male | Adenoid cystic carcinoma | T4bN3bM0 | PB, 67.6 Gy | DOD, 18M |
| 5 | 49 | Female | Acinic cell carcinoma | rT2N0M0 | PB, 70.4 Gy | AwoD, 23M |
| 6 | 63 | Male | Salivary duct carcinoma | T4aN2bM0 | CIB, 64 Gy | DOD, 30M |
| 7 | 67 | Female | Acinic cell carcinoma | rT1N0M0 | PB, 70.4 Gy | AwoD, 39M |
| 8 | 81 | Male | Salivary duct carcinoma | T4aN0M0 | PB, 70.2 Gy | AwoD, 18M |
| 9 | 67 | Female | Adenoid cystic carcinoma | T4aN2bM0 | PB, 74.8 Gy | AwoD, 17M |
| 10 | 54 | Male | Adenoid cystic carcinoma | T3N0M0 | PB, 74.8 Gy | AwoD, 5M |
| No | Age | Sex | Histopathology | cTNM | Surgery | POCRT | Surgical Margin | Lymph Node Metastasis | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 77 | M | SDC | T4aN2b | TP + M + ND + ALT + NR | D/C + 60Gy | − | 18/33 II, III, IV, V | AwoD, 72M |
| 2 | 81 | M | angiosarcoma | T4aN2b | ETP + M + ND + ALT + NR | D/C/F | + | 4/12 II, III | DOD, 5M |
| 3 | 64 | M | SDC | T2N2b | SP + ND | D/C + 60Gy | - | 5/46 I, II, IV pN3b | AwoD, 45M |
| 4 | 78 | F | adenocarcinoma | T2N0 | ETP + M + ND + ALT + NR | - | - | - | AwoD, 67M |
| 5 | 65 | M | SDC | T4aN2b | ETP + M + ND + ALT + NR | D/C + 66Gy | - | 4/15 II pN3b | DOD, 68M |
| 6 | 72 | M | adenocarcinoma | T3N0 | SP + ND | - | + | 1/5 paraparotid pN1 | DOOD, 46M |
| 7 | 75 | M | SDC | T4aN2bM1 | TP + M + ND + NR | D/C | + | 11/23 II, III, IV pN3b | DOD, 9M |
| 8 | 64 | M | SDC | T2N0 | TR | 60Gy | - | - | AWD, 41M |
| 9 | 78 | M | SCC | T3N2b | ETP + M + ND + ALT + NR | - | - | 7/25 II, IV | AwoD, 55M |
| 10 | 73 | F | myoepithelial ca. | T4aN0 | TP | 66Gy | + | - | AwoD, 61M |
| 11 | 51 | F | MEC intermediate | T2N0 | TP + ND + NR | 60Gy | - | - | AwoD, 60M |
| 12 | 64 | M | SDC | T4aN0 | TP + ND + NR | - | + | - | DOD, 59M |
| 13 | 57 | F | MEC low | T4aN1 | ETP + M + ND + ALT + NR | - | - | - | AwoD, 56M |
| 14 | 86 | F | SDC | T4aN0 | TP + M + ND | - | - | - | N.D. |
| 15 | 69 | M | SDC | T4aN0 | ETP + SR + ND + ALT + NR | - | - | - | DOD, 26M |
| 16 | 79 | F | SDC | T4aN3b | TP + SR + ND + ALT + NR | - | - | 58/89 I, II, III, IV contra II pN3b | DOD, 11M |
| 17 | 39 | M | secretary ca. | T2N0 | TP + SR + ND + ALT + NR | - | - | 2/24 II, III pN2b | AwoD, 36M |
| 18 | 48 | F | ACC | T3N0 | ETP + M + ND + ALT + NR | 66Gy | - | - | AwoD, 44M |
| 19 | 65 | F | ACC | T2N0 | SP | - | - | - | AwoD, 13M |
| 20 | 60 | F | MEC low | T3N0 | ETP + M + ND + ALT + NR | - | + | - | AwoD, 39M |
| 21 | 60 | M | SDC | T3N3b | ETP + SR + M + ND + RAMC | C + 60Gy | + | 37/42 I, II, III, IV pN3b | AwoD, 26M |
| 22 | 82 | M | epith-myoepi ca. | T2N1 | TP + M + ND + NR | - | + | 1/48 paraparotid | AwoD, 22M |
| 23 | 62 | M | hybrid ca. | T4aN3b | SP + SR + RAMC | D/C + 66Gy | + | 8/47 I, II, IV pN3b | AWD, 10M |
| 24 | 82 | F | epith-myoepi ca | T4aN2b | TP + MR + ND | - | - | 3/33 I | N.D. |
| 25 | 69 | M | Ca. ex PMA | T3N0 | TP + ND | - | - | - | AwoD, 20M |
| 26 | 60 | F | lymphoepithelial ca. | T3N2b | TP + ND + NR | 66Gy | + | 26/67 I, II, III, V pN3b | AwoD, 10M |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katagiri, K.; Shiga, K.; Saito, D.; Oikawa, S.-I.; Ikeda, A.; Tsuchida, K.; Miyaguchi, J.; Kusaka, T.; Kusaka, I.; Ariga, H.; et al. Particle Beam Therapy and Surgery as Radical Treatments for Parotid Malignancies—A Single-Center Preliminary Case Study. J. Clin. Med. 2024, 13, 3314. https://doi.org/10.3390/jcm13113314
Katagiri K, Shiga K, Saito D, Oikawa S-I, Ikeda A, Tsuchida K, Miyaguchi J, Kusaka T, Kusaka I, Ariga H, et al. Particle Beam Therapy and Surgery as Radical Treatments for Parotid Malignancies—A Single-Center Preliminary Case Study. Journal of Clinical Medicine. 2024; 13(11):3314. https://doi.org/10.3390/jcm13113314
Chicago/Turabian StyleKatagiri, Katsunori, Kiyoto Shiga, Daisuke Saito, Shin-Ichi Oikawa, Aya Ikeda, Kodai Tsuchida, Jun Miyaguchi, Takahiro Kusaka, Iori Kusaka, Hisanori Ariga, and et al. 2024. "Particle Beam Therapy and Surgery as Radical Treatments for Parotid Malignancies—A Single-Center Preliminary Case Study" Journal of Clinical Medicine 13, no. 11: 3314. https://doi.org/10.3390/jcm13113314
APA StyleKatagiri, K., Shiga, K., Saito, D., Oikawa, S.-I., Ikeda, A., Tsuchida, K., Miyaguchi, J., Kusaka, T., Kusaka, I., Ariga, H., Seto, I., Nakasato, T., & Koto, M. (2024). Particle Beam Therapy and Surgery as Radical Treatments for Parotid Malignancies—A Single-Center Preliminary Case Study. Journal of Clinical Medicine, 13(11), 3314. https://doi.org/10.3390/jcm13113314






