Advancements in Neuroendocrine Neoplasms: Imaging and Future Frontiers
Abstract
1. Introduction
2. Country-Related Trends in Epidemiology
3. Pathology and Radiology: A Symbiotic Partnership
4. Tools and Pitfalls in Radiology and Nuclear Medicine
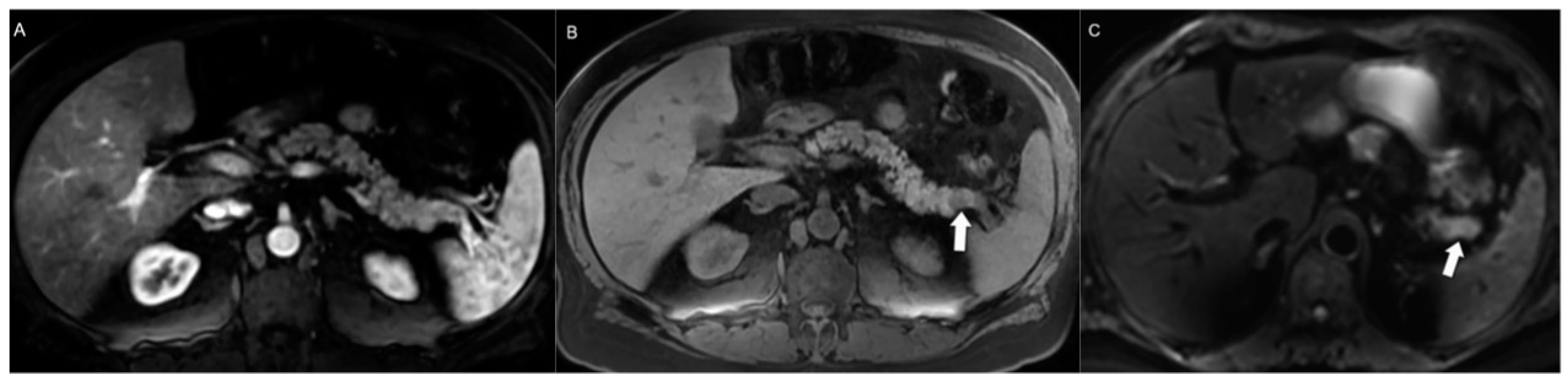
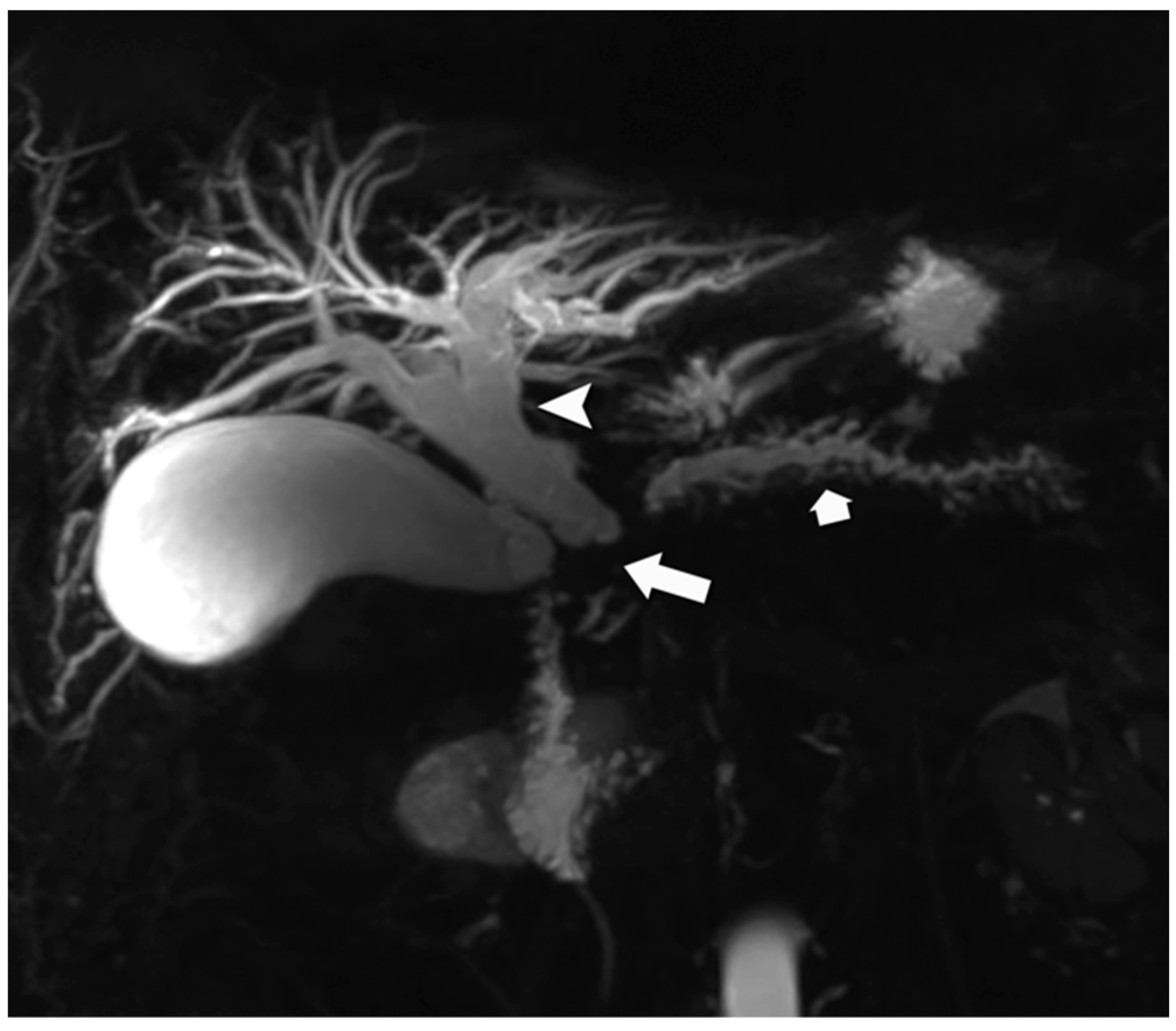
4.1. Morphologic Imaging of NENs
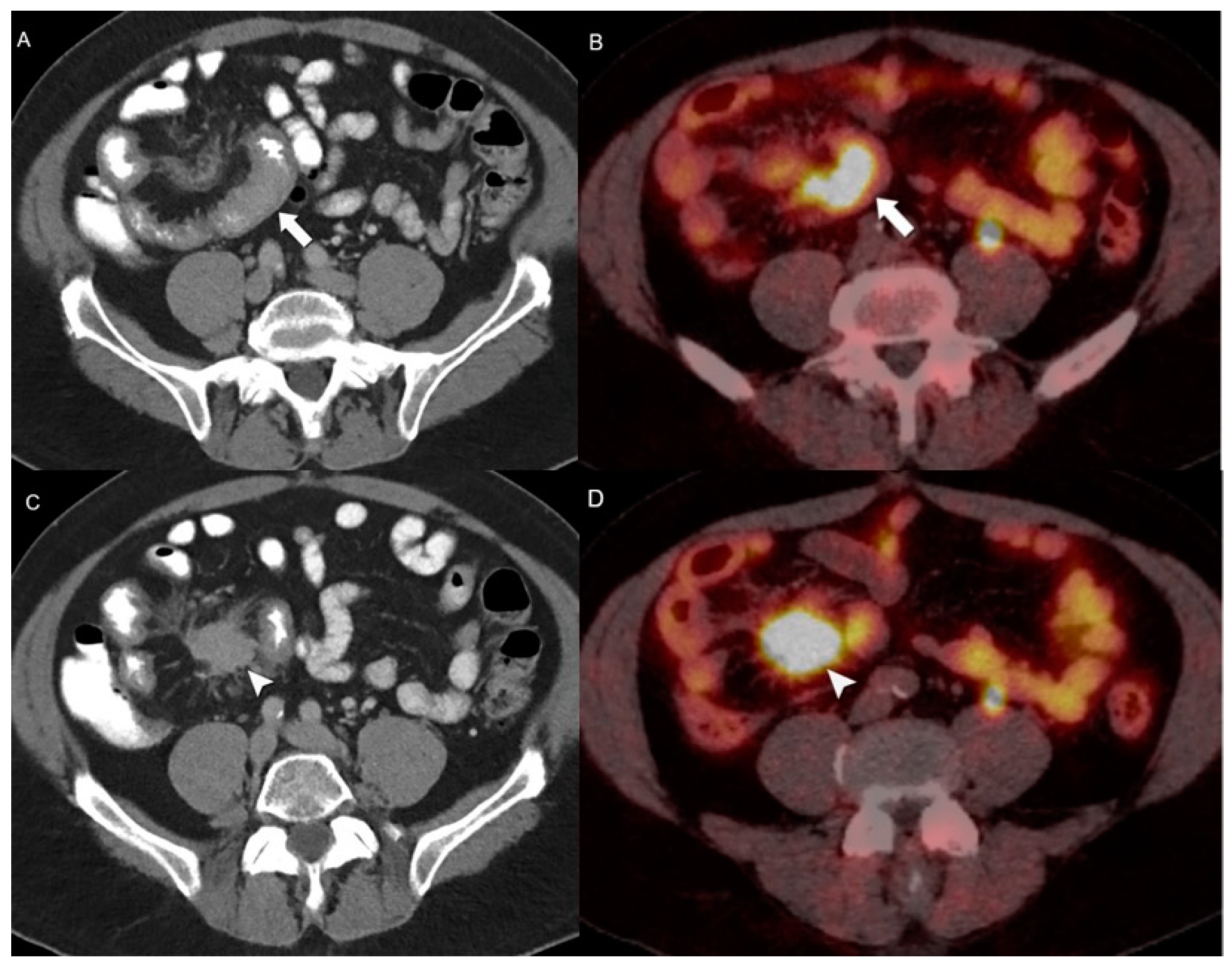
4.2. Hybrid Imaging of NENs
5. Spectrum of Therapeutic Options
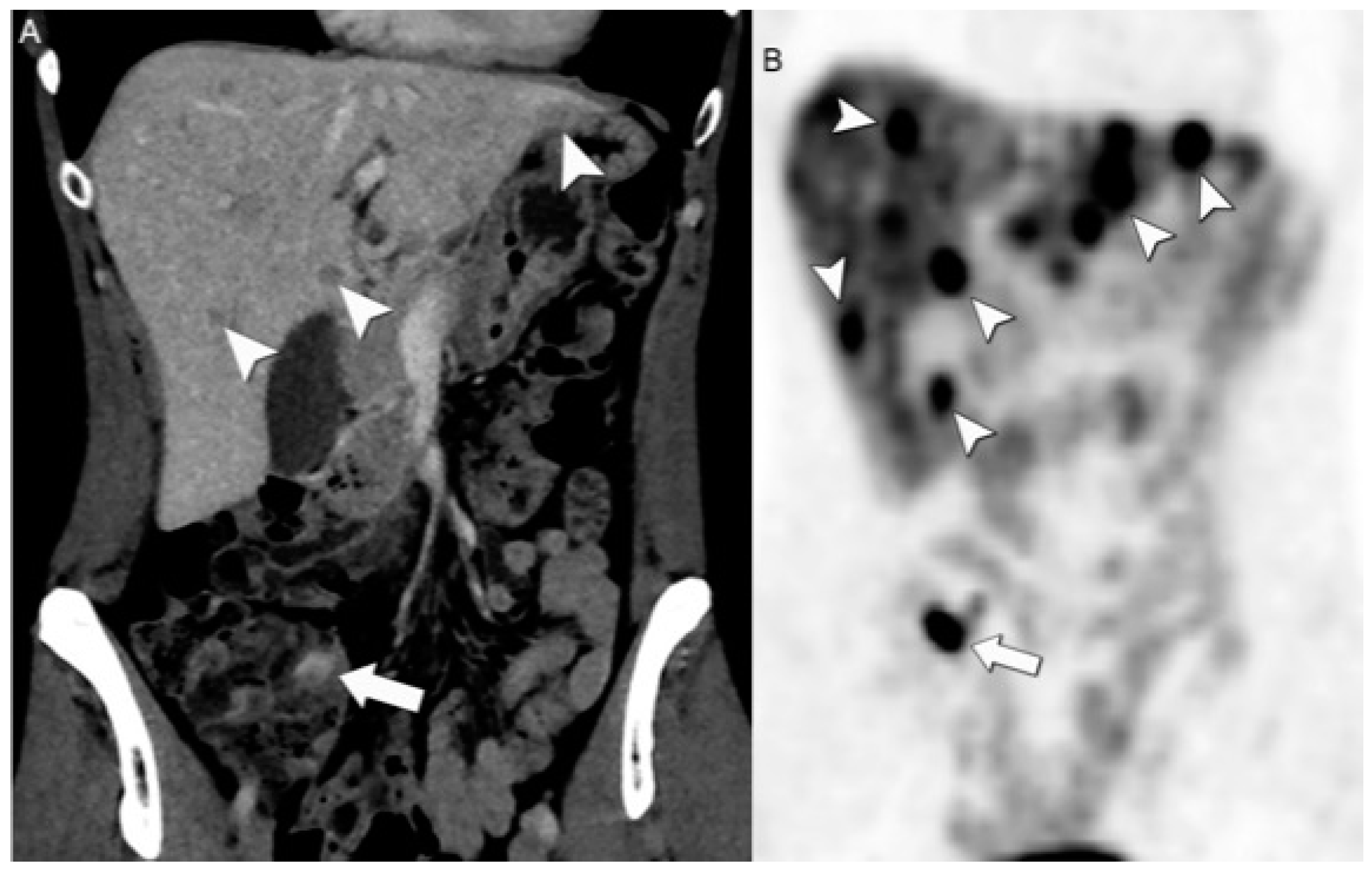
6. Follow-Up Imaging: Strengths and Challenges
7. Future Frontiers in Radiomics
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Assarzadegan, N.; Montgomery, E. What Is New in the 2019 World Health Organization (WHO) Classification of Tumors of the Digestive System Review of Selected Updates on Neuroendocrine Neoplasms, Appendiceal Tumors, and Molecular Testing. Arch. Pathol. Lab. Med. 2021, 145, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Gosain, R.; Groman, A.; Gosain, R.; Dasari, A.; Halfdanarson, T.R.; Mukherjee, S. Incidence and Survival Outcomes in Patients with Lung Neuroendocrine Neoplasms in the United States. Cancers 2021, 13, 1753. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic Neuroendocrine Neoplasms: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Bodei, L.; Sundin, A.; Kidd, M.; Prasad, V.; Modlin, I.M. The Status of Neuroendocrine Tumor Imaging: From Darkness to Light? Neuroendocrinology 2015, 101, 1–17. [Google Scholar] [CrossRef]
- Treglia, G.; Castaldi, P.; Rindi, G.; Giordano, A.; Rufini, V. Diagnostic Performance of Gallium-68 Somatostatin Receptor PET and PET/CT in Patients with Thoracic and Gastroenteropancreatic Neuroendocrine Tumours: A Meta-Analysis. Endocrine 2012, 42, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, L.; Dai, S.; Chen, M.; Li, F.; Sun, J.; Luo, F. Epidemiologic Trends of and Factors Associated With Overall Survival for Patients With Gastroenteropancreatic Neuroendocrine Tumors in the United States. JAMA Netw. Open 2021, 4, e2124750. [Google Scholar] [CrossRef] [PubMed]
- Gastrointestinal Pathology Study Group of Korean Society of Pathologists; Cho, M.-Y.; Kim, J.M.; Sohn, J.H.; Kim, M.-J.; Kim, K.-M.; Kim, W.H.; Kim, H.; Kook, M.-C.; Park, D.Y.; et al. Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000-2009: Multicenter Study. Cancer Res. Treat. 2012, 44, 157–165. [Google Scholar] [CrossRef]
- Chang, J.S.; Chen, L.-T.; Shan, Y.-S.; Chu, P.-Y.; Tsai, C.-R.; Tsai, H.-J. An Updated Analysis of the Epidemiologic Trends of Neuroendocrine Tumors in Taiwan. Sci. Rep. 2021, 11, 7881. [Google Scholar] [CrossRef]
- Wyld, D.; Wan, M.H.; Moore, J.; Dunn, N.; Youl, P. Epidemiological Trends of Neuroendocrine Tumours over Three Decades in Queensland, Australia. Cancer Epidemiol. 2019, 63, 101598. [Google Scholar] [CrossRef]
- Kaltsas, G.A.; Besser, G.M.; Grossman, A.B. The Diagnosis and Medical Management of Advanced Neuroendocrine Tumors. Endocr. Rev. 2004, 25, 458–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-E.; Su, C.-H.; Kuo, Y.-J.; Shyr, Y.-M.; Li, A.F.-Y.; Chen, T.-H.; Wu, C.-W.; Lee, C.-H. Comparison of Functional and Nonfunctional Neuroendocrine Tumors in the Pancreas and Peripancreatic Region. Pancreas 2011, 40, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Sundin, A.; Arnold, R.; Baudin, E.; Cwikla, J.B.; Eriksson, B.; Fanti, S.; Fazio, N.; Giammarile, F.; Hicks, R.J.; Kjaer, A.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine and Hybrid Imaging. Neuroendocrinology 2017, 105, 212–244. [Google Scholar] [CrossRef] [PubMed]
- Morse, B.; Al-Toubah, T.; Montilla-Soler, J. Anatomic and Functional Imaging of Neuroendocrine Tumors. Curr. Treat. Options Oncol. 2020, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Anaye, A.; Mathieu, A.; Closset, J.; Bali, M.A.; Metens, T.; Matos, C. Successful Preoperative Localization of a Small Pancreatic Insulinoma by Diffusion-Weighted MRI. JOP 2009, 10, 528–531. [Google Scholar] [PubMed]
- Keck, K.J.; Maxwell, J.E.; Menda, Y.; Bellizzi, A.; Dillon, J.; O’Dorisio, T.M.; Howe, J.R. Identification of Primary Tumors in Patients Presenting with Metastatic Gastroenteropancreatic Neuroendocrine Tumors. Surgery 2017, 161, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Tamm, E.P.; Bhosale, P.; Lee, J.H.; Rohren, E.M. State-of-the-art Imaging of Pancreatic Neuroendocrine Tumors. Surg. Oncol. Clin. N. Am. 2016, 25, 375–400. [Google Scholar] [CrossRef]
- Fidler, J.L.; Fletcher, J.G.; Reading, C.C.; Andrews, J.C.; Thompson, G.B.; Grant, C.S.; Service, F.J. Preoperative Detection of Pancreatic Insulinomas on Multiphasic Helical CT. Am. J. Roentgenol. 2003, 181, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Procacci, C.; Carbognin, G.; Accordini, S.; Biasiutti, C.; Bicego, E.; Romano, L.; Guarise, A.; Minniti, S.; Pagnotta, N.; Falconi, M. Nonfunctioning Endocrine Tumors of the Pancreas: Possibilities of Spiral CT Characterization. Eur. Radiol. 2001, 11, 1175–1183. [Google Scholar] [CrossRef]
- Sundin, A.; Vullierme, M.-P.; Kaltsas, G.; Plöckinger, U. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological Examinations. Neuroendocrinology 2009, 90, 167–183. [Google Scholar] [CrossRef]
- Kim, J.H.; Eun, H.W.; Kim, Y.J.; Lee, J.M.; Han, J.K.; Choi, B.-I. Pancreatic Neuroendocrine Tumour (PNET): Staging Accuracy of MDCT and Its Diagnostic Performance for the Differentiation of PNET with Uncommon CT Findings from Pancreatic Adenocarcinoma. Eur. Radiol. 2016, 26, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Dromain, C.; Déandréis, D.; Scoazec, J.-Y.; Goere, D.; Ducreux, M.; Baudin, E.; Tselikas, L. Imaging of Neuroendocrine Tumors of the Pancreas. Diagn. Interv. Imaging 2016, 97, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Foti, G.; Boninsegna, L.; Falconi, M.; Mucelli, R.P. Preoperative Assessment of Nonfunctioning Pancreatic Endocrine Tumours: Role of MDCT and MRI. Radiol. Medica 2013, 118, 1082–1101. [Google Scholar] [CrossRef]
- Lee, L.; Ito, T.; Jensen, R.T. Imaging of Pancreatic Neuroendocrine Tumors: Recent Advances, Current Status, and Controversies. Expert Rev. Anticancer. Ther. 2018, 18, 837–860. [Google Scholar] [CrossRef] [PubMed]
- Hardie, A.D.; Picard, M.M.; Camp, E.R.; Perry, J.D.; Suranyi, P.; De Cecco, C.N.; Schoepf, U.J.; Wichmann, J.L. Application of an Advanced Image-Based Virtual Monoenergetic Reconstruction of Dual Source Dual-Energy CT Data at Low keV Increases Image Quality for Routine Pancreas Imaging. J. Comput. Assist. Tomogr. 2015, 39, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Johanssen, S.; Boivin, M.; Lochs, H.; Voderholzer, W. The Yield of Wireless Capsule Endoscopy in the Detection of Neuroendocrine Tumors in Comparison with CT Enteroclysis. Gastrointest. Endosc. 2006, 63, 660–665. [Google Scholar] [CrossRef]
- Pilleul, F.; Penigaud, M.; Milot, L.; Saurin, J.-C.; Chayvialle, J.-A.; Valette, P.-J. Possible Small-Bowel Neoplasms: Contrast-Enhanced and Water-Enhanced Multidetector CT Enteroclysis. Radiology 2006, 241, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Malla, S.; Kumar, P.; Madhusudhan, K.S. Radiology of the Neuroendocrine Neoplasms of the Gastrointestinal Tract: A Comprehensive Review. Abdom. Radiol. 2021, 46, 919–935. [Google Scholar] [CrossRef]
- Chang, S.; Choi, D.; Lee, S.J.; Lee, W.J.; Park, M.-H.; Kim, S.W.; Lee, D.K.; Jang, K.-T. Neuroendocrine Neoplasms of the Gastrointestinal Tract: Classification, Pathologic Basis, and Imaging Features. RadioGraphics 2007, 27, 1667–1679. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.H.; Kim, M.-A.; Shin, C.-I.; Han, J.K.; Choi, B.I. CT Differentiation of Poorly-Differentiated Gastric Neuroendocrine Tumours from Well-Differentiated Neuroendocrine Tumours and Gastric Adenocarcinomas. Eur. Radiol. 2015, 25, 1946–1957. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, S.H.; Han, J.K. Poorly-Differentiated Colorectal Neuroendocrine Tumour: CT Differentiation from Well-Differentiated Neuroendocrine Tumour and poorly-Differentiated Adenocarcinomas. Eur. Radiol. 2017, 27, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.M.; Vummidi, D.; Benditt, J.O.; Godwin, J.D.; Pipavath, S. What Is DIPNECH? Clin. Imaging 2012, 36, 647–649. [Google Scholar] [CrossRef]
- Chassagnon, G.; Favelle, O.; Marchand-Adam, S.; De Muret, A.; Revel, M.P. DIPNECH: When to Suggest This Diagnosis on CT. Clin. Radiol. 2015, 70, 317–325. [Google Scholar] [CrossRef]
- Foran, P.J.; Hayes, S.A.; Blair, D.J.; Zakowski, M.F.; Ginsberg, M.S. Imaging Appearances of Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia. Clin. Imaging 2015, 39, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Dromain, C.; de Baere, T.; Lumbroso, J.; Caillet, H.; Laplanche, A.; Boige, V.; Ducreux, M.; Duvillard, P.; Elias, D.; Schlumberger, M.; et al. Detection of Liver Metastases From Endocrine Tumors: A Prospective Comparison of Somatostatin Receptor Scintigraphy, Computed Tomography, and Magnetic Resonance Imaging. J. Clin. Oncol. 2005, 23, 70–78. [Google Scholar] [CrossRef]
- Tirumani, S.H.; Jagannathan, J.P.; Braschi-Amirfarzan, M.; Qin, L.; Balthazar, P.; Ramaiya, N.H.; Shinagare, A.B. Value of Hepatocellular Phase Imaging after Intravenous Gadoxetate Disodium for Assessing Hepatic Metastases from Gastroenteropancreatic Neuroendocrine Tumors: Comparison with Other MRI Pulse Sequences and with Extracellular Agent. Abdom. Radiol. 2018, 43, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- d’Assignies, G.; Fina, P.; Bruno, O.; Vullierme, M.-P.; Tubach, F.; Paradis, V.; Sauvanet, A.; Ruszniewski, P.; Vilgrain, V. High Sensitivity of Diffusion-Weighted MR Imaging for the Detection of Liver Metastases from Neuroendocrine Tumors: Comparison with T2-Weighted and Dynamic Gadolinium-enhanced MR Imaging. Radiology 2013, 268, 390–399. [Google Scholar] [CrossRef]
- Putzer, D.; Gabriel, M.; Henninger, B.; Kendler, D.; Uprimny, C.; Dobrozemsky, G.; Decristoforo, C.; Bale, R.J.; Jaschke, W.; Virgolini, I.J. Bone Metastases in Patients with Neuroendocrine Tumor: 68Ga-DOTA-Tyr3-Octreotide PET in Comparison to CT and Bone Scintigraphy. J. Nucl. Med. 2009, 50, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Di Dato, C.; Martini, C.; Sciammarella, C.; Di Sarno, A.; Colao, A.; Faggiano, A. Bone Metastases in Neuroendocrine Neoplasms: From Pathogenesis to Clinical Management. Cancers 2019, 11, 1332. [Google Scholar] [CrossRef]
- Meijer, W.G.; Van Der Veer, E.; Jager, P.L.; Van Der Jagt, E.J.; Piers, B.A.; Kema, I.P.; De Vries, E.G.E.; Willemse, P.H.B. Bone Metastases in Carcinoid Tumors: Clinical Features, Imaging Characteristics, and Markers of Bone Metabolism. J. Nucl. Med. 2003, 44, 184–191. [Google Scholar]
- Hwang, E.J.; Lee, J.M.; Yoon, J.H.; Kim, J.H.; Han, J.K.; Choi, B.I.; Lee, K.-B.; Jang, J.-Y.; Kim, S.-W.; Nickel, M.D.; et al. Intravoxel Incoherent Motion Diffusion-Weighted Imaging of Pancreatic Neuroendocrine Tumors. Investig. Radiol. 2014, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Mebis, W.; Snoeckx, A.; Corthouts, B.; El Addouli, H.; Nicolay, S.; Van Hoyweghen, A.; Spinhoven, M.; de Beeck, B.O. Correlation Between Apparent Diffusion Coefficient Value on MRI and Histopathologic WHO Grades of Neuroendocrine Tumors. J. Belg. Soc. Radiol. 2020, 104, 7. [Google Scholar] [CrossRef] [PubMed]
- Zong, R.L.; Geng, L.; Wang, X.; Xie, D. Diagnostic Performance of Apparent Diffusion Coefficient for Prediction of Grading of Pancreatic Neuroendocrine Tumors. Pancreas 2019, 48, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, M.F.; Virgolini, I.; Balogova, S.; Beheshti, M.; Rubello, D.; Decristoforo, C.; Ambrosini, V.; Kjaer, A.; Delgado-Bolton, R.; Kunikowska, J.; et al. Guideline for PET/CT Imaging of Neuroendocrine Neoplasms with 68Ga-DOTA-Conjugated Somatostatin Receptor Targeting Peptides and 18F-DOPA. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1588–1601. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Sadeghi, R.; Giovinazzo, F.; Galiandro, F.; Annunziata, S.; Muoio, B.; Kroiss, A.S. PET with Different Radiopharmaceuticals in Neuroendocrine Neoplasms: An Umbrella Review of Published Meta-Analyses. Cancers 2021, 13, 5172. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.J.; Kwekkeboom, D.J.; Krenning, E.; Bodei, L.; Grozinsky-Glasberg, S.; Arnold, R.; Borbath, I.; Cwikla, J.; Toumpanakis, C.; Kaltsas, G.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms: Peptide Receptor Radionuclide Therapy with Radiolabelled Somatostatin Analogues. Neuroendocrinology 2017, 105, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Kayani, I.; Conry, B.G.; Groves, A.M.; Win, T.; Dickson, J.; Caplin, M.; Bomanji, J.B. A Comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in Pulmonary Neuroendocrine Tumors. J. Nucl. Med. 2009, 50, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
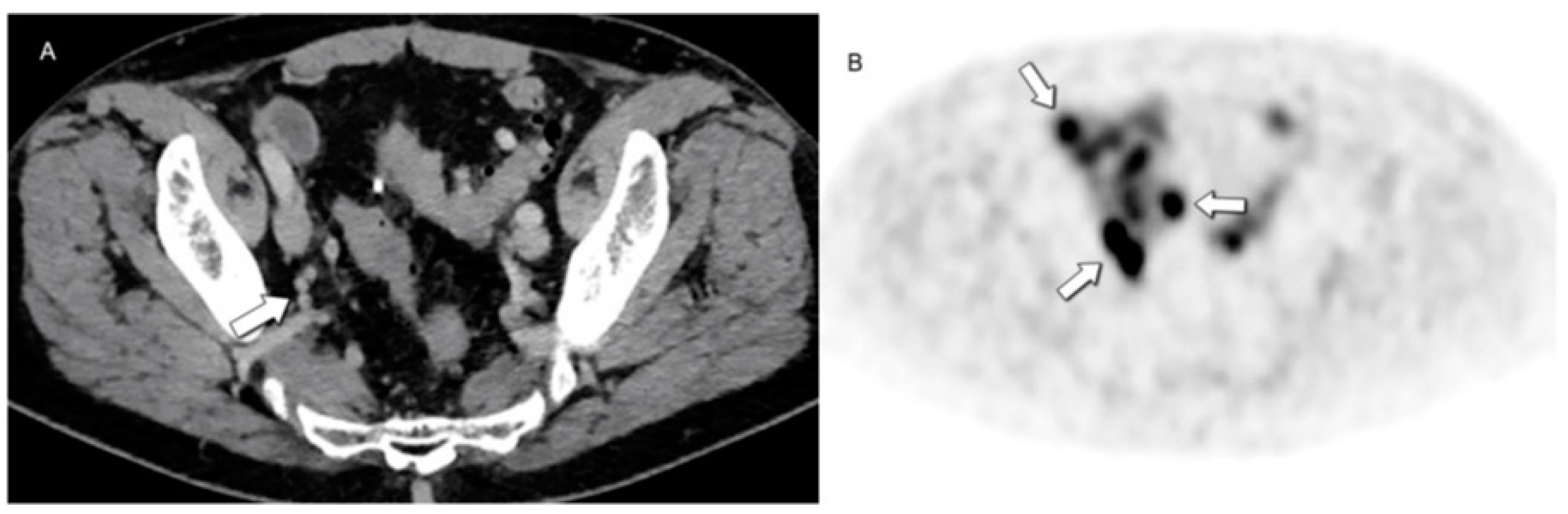
- Tabacchi, E.; Fortunati, E.; Argalia, G.; Zanoni, L.; Calabrò, D.; Telo, S.; Campana, D.; Lamberti, G.; Ricci, C.; Casadei, R.; et al. [68Ga]Ga-DOTANOC Uptake at Pancreatic Head/Uncinate Process: Is It a Persistent Diagnostic Pitfall Over Time? Cancers 2022, 14, 3541. [Google Scholar] [CrossRef]
- Taieb, D.; Hicks, R.J.; Hindie, E.; Guillet, B.A.; Avram, A.; Ghedini, P.; Timmers, H.J.; Scott, A.T.; Elojeimy, S.; Rubello, D.; et al. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for Radionuclide Imaging of Phaeochromocytoma and Paraganglioma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2112–2137. [Google Scholar] [CrossRef]
- Bar-Sever, Z.; Biassoni, L.; Shulkin, B.; Kong, G.; Hofman, M.S.; Lopci, E.; Manea, I.; Koziorowski, J.; Castellani, R.; Boubaker, A.; et al. Guidelines on Nuclear Medicine Imaging in Neuroblastoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2009–2024. [Google Scholar] [CrossRef]
- Ambrosini, V.; Kunikowska, J.; Baudin, E.; Bodei, L.; Bouvier, C.; Capdevila, J.; Cremonesi, M.; de Herder, W.W.; Dromain, C.; Falconi, M.; et al. Consensus on Molecular Imaging and Theranostics in Neuroendocrine Neoplasms. Eur. J. Cancer 2021, 146, 56–73. [Google Scholar] [CrossRef]
- Karfis, I.; Marin, G.; Levillain, H.; Drisis, S.; Muteganya, R.; Critchi, G.; Taraji-Schiltz, L.; Guix, C.A.; Shaza, L.; Elbachiri, M.; et al. Prognostic Value of a Three-Scale Grading System Based on Combining Molecular Imaging with 68Ga-DOTATATE and 18F-FDG PET/CT in Patients with Metastatic Gastroenteropancreatic Neuroendocrine Neoplasias. Oncotarget 2020, 11, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Graf, J.; Pape, U.-F.; Jann, H.; Denecke, T.; Arsenic, R.; Brenner, W.; Pavel, M.; Prasad, V. Prognostic Significance of Somatostatin Receptor Heterogeneity in Progressive Neuroendocrine Tumor Treated with Lu-177 DOTATOC or Lu-177 DOTATATE. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 881–894. [Google Scholar] [CrossRef]
- Binderup, T.; Knigge, U.; Johnbeck, C.B.; Loft, A.; Berthelsen, A.K.; Oturai, P.; Mortensen, J.; Federspiel, B.; Langer, S.W.; Kjaer, A. 18F-FDG PET Is Superior to WHO Grading as a Prognostic Tool in Neuroendocrine Neoplasms and Useful in Guiding PRRT: A Prospective 10-Year Follow-up Study. J. Nucl. Med. 2021, 62, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.C.; Bressem, K.K.; Brangsch, J.; Reimann, C.; Nowak, K.J.; Brenner, W.; Makowski, M.R. Quantitative 3D Assessment of 68Ga-DOTATOC PET/MRI with Diffusion-Weighted Imaging to Assess Imaging Markers for Gastroenteropancreatic Neuroendocrine Tumors: Preliminary Results. J. Nucl. Med. 2020, 61, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Stefanova, M.; Mavriopoulou, E.; Holland-Letz, T.; Dimitrakopoulou-Strauss, A.; Afshar-Oromieh, A.; Mier, W.; Haberkorn, U.; Giesel, F.L. SUV of [68Ga]DOTATOC-PET/CT Predicts Response Probability of PRRT in Neuroendocrine Tumors. Mol. Imaging Biol. 2015, 17, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Öksüz, M.; Winter, L.; Pfannenberg, C.; Reischl, G.; Müssig, K.; Bares, R.; Dittmann, H. Peptide Receptor Radionuclide Therapy of Neuroendocrine Tumors with 90Y-DOTATOC: Is Treatment Response Predictable by Pre-Therapeutic Uptake of 68Ga-DOTATOC? Diagn. Interv. Imaging 2014, 95, 289–300. [Google Scholar] [CrossRef]
- Sharma, R.; Wang, W.M.; Yusuf, S.; Evans, J.; Ramaswami, R.; Wernig, F.; Frilling, A.; Mauri, F.; Al-Nahhas, A.; Aboagye, E.O.; et al. 68Ga-DOTATATE PET/CT Parameters Predict Response to Peptide Receptor Radionuclide Therapy in Neuroendocrine Tumours. Radiother. Oncol. 2019, 141, 108–115. [Google Scholar] [CrossRef]
- Chan, D.L.; Pavlakis, N.; Schembri, G.P.; Bernard, E.J.; Hsiao, E.; Hayes, A.; Barnes, T.; Diakos, C.; Khasraw, M.; Samra, J.; et al. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics 2017, 7, 1149–1158. [Google Scholar] [CrossRef]
- Hindié, E. The NETPET Score: Combining FDG and Somatostatin Receptor Imaging for Optimal Management of Patients with Metastatic Well-Differentiated Neuroendocrine Tumors. Theranostics 2017, 7, 1159–1163. [Google Scholar] [CrossRef]
- Tacher, V.; Le Deley, M.-C.; Hollebecque, A.; Deschamps, F.; Vielh, P.; Hakime, A.; Ileana, E.; Abedi-Ardekani, B.; Charpy, C.; Massard, C.; et al. Factors Associated with Success of Image-Guided Tumour Biopsies: Results from a Prospective Molecular Triage Study (MOSCATO-01). Eur. J. Cancer 2016, 59, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Gilson, P.; Merlin, J.-L.; Harlé, A. Deciphering Tumour Heterogeneity: From Tissue to Liquid Biopsy. Cancers 2022, 14, 1384. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.; Cleeren, F.; Tshibangu, T.; Koole, M.; Serdons, K.; Dekervel, J.; Van Cutsem, E.; Verslype, C.; Van Laere, K.; Bormans, G.; et al. [18F]AlF-NOTA-Octreotide PET Imaging: Biodistribution, Dosimetry and First Comparison with [68Ga]Ga-DOTATATE in Neuroendocrine Tumour Patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 3033–3046. [Google Scholar] [CrossRef]
- Hicks, R.J.; Jackson, P.; Kong, G.; Ware, R.E.; Hofman, M.S.; Pattison, D.A.; Akhurst, T.A.; Drummond, E.; Roselt, P.; Callahan, J.; et al. 64Cu-SARTATE PET Imaging of Patients with Neuroendocrine Tumors Demonstrates High Tumor Uptake and Retention, Potentially Allowing Prospective Dosimetry for Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2019, 60, 777–785. [Google Scholar] [CrossRef]
- Nicolas, G.P.; Beykan, S.; Bouterfa, H.; Kaufmann, J.; Bauman, A.; Lassmann, M.; Reubi, J.C.; Rivier, J.E.; Maecke, H.R.; Fani, M.; et al. Safety, Biodistribution, and Radiation Dosimetry of 68Ga-OPS202 in Patients with Gastroenteropancreatic Neuroendocrine Tumors: A Prospective Phase I Imaging Study. J. Nucl. Med. 2018, 59, 909–914. [Google Scholar] [CrossRef]
- Zhu, W.; Jia, R.; Yang, Q.; Cheng, Y.; Zhao, H.; Bai, C.; Xu, J.; Yao, S.; Huo, L. A Prospective Randomized, Double-Blind Study to Evaluate the Diagnostic Efficacy of 68Ga-NODAGA-LM3 and 68Ga-DOTA-LM3 in Patients with Well-Differentiated Neuroendocrine Tumors: Compared with 68Ga-DOTATATE. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1613–1622. [Google Scholar] [CrossRef]
- Wang, P.; Li, T.; Liu, Z.; Jin, M.; Su, Y.; Zhang, J.; Jing, H.; Zhuang, H.; Li, F. [18F]MFBG PET/CT Outperforming [123I]MIBG SPECT/CT in the Evaluation of Neuroblastoma. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3097–3106. [Google Scholar] [CrossRef] [PubMed]
- Schraml, C.; Schwenzer, N.F.; Sperling, O.; Aschoff, P.; Lichy, M.P.; Muller, M.; Brendle, C.; Werner, M.K.; Claussen, C.D.; Pfannenberg, C. Staging of Neuroendocrine Tumours: Comparison of [68Ga]DOTATOC Multiphase PET/CT and Whole-Body MRI. Cancer Imaging 2013, 13, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Fuin, N.; Catalano, O.A.; Scipioni, M.; Canjels, L.P.; Izquierdo-Garcia, D.; Pedemonte, S.; Catana, C. Concurrent Respiratory Motion Correction of Abdominal PET and Dynamic Contrast-Enhanced–MRI Using a Compressed Sensing Approach. J. Nucl. Med. 2018, 59, 1474–1479. [Google Scholar] [CrossRef]
- Beiderwellen, K.J.; Poeppel, T.D.; Hartung-Knemeyer, V.; Buchbender, C.; Kuehl, H.; Bockisch, A.; Lauenstein, T.C. Simultaneous 68Ga-DOTATOC PET/MRI in Patients With Gastroenteropancreatic Neuroendocrine Tumors. Investig. Radiol. 2013, 48, 273–279. [Google Scholar] [CrossRef]
- Jawlakh, H.; Velikyan, I.; Welin, S.; Sundin, A. 68Ga-DOTATOC-PET/MRI and 11C-5-HTP-PET/MRI Are Superior to 68Ga-DOTATOC-PET/CT for Neuroendocrine Tumour Imaging. J. Neuroendocr. 2021, 33, e12981. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, L.M.; Deuschl, C.; Beiderwellen, K.; Ruhlmann, V.; Poeppel, T.D.; Heusch, P.; Lahner, H.; Führer, D.; Bockisch, A.; Herrmann, K.; et al. Evaluation of 68Ga-DOTATOC PET/MRI for Whole-Body Staging of Neuroendocrine Tumours in Comparison with 68Ga-DOTATOC PET/CT. Eur. Radiol. 2017, 27, 4091–4099. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Kessler, L.; Schaarschmidt, B.; Fendler, W.P.; Lahner, H.; Antoch, G.; Umutlu, L.; Herrmann, K.; Rischpler, C. Treatment-Related Changes in Neuroendocrine Tumors as Assessed by Textural Features Derived from 68Ga-DOTATOC PET/MRI with Simultaneous Acquisition of Apparent Diffusion Coefficient. BMC Cancer 2020, 20, 326. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.J.; Leung, G.; Eads, J.R.; Katona, B.W. Gastroenteropancreatic Neuroendocrine Tumors. Gastroenterol. Clin. North Am. 2022, 51, 625–647. [Google Scholar] [CrossRef] [PubMed]
- Öberg, K. Future Aspects of Somatostatin- Receptor-Mediated Therapy. Neuroendocrinology 2004, 80, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarson, T.R.; Strosberg, J.R.; Tang, L.; Bellizzi, A.M.; Bergsland, E.K.; O’Dorisio, T.M.; Halperin, D.M.; Fishbein, L.; Eads, J.; Hope, T.A.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 863–881. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, S.; De Braud, F.; Russo, G.L.; Concas, L.; Femia, D.; Vernieri, C.; Indini, A.; Formisano, B.; Buzzoni, R. How Do the Results of the RADIANT Trials Impact on the Management of NET Patients? A Systematic Review of Published Studies. Oncotarget 2016, 7, 44841–44847. [Google Scholar] [CrossRef]
- Bajetta, E.; Catena, L.; Procopio, G.; De Dosso, S.; Bichisao, E.; Ferrari, L.; Martinetti, A.; Platania, M.; Verzoni, E.; Formisano, B.; et al. Are Capecitabine and Oxaliplatin (XELOX) Suitable Treatments for Progressing Low-Grade and High-Grade Neuroendocrine Tumours? Cancer Chemother. Pharmacol. 2007, 59, 637–642. [Google Scholar] [CrossRef]
- Triponez, F.; Sadowski, S.M.; Pattou, F.; Cardot-Bauters, C.; Mirallié, E.; Le Bras, M.; Sebag, F.; Niccoli, P.; Deguelte, S.; Cadiot, G.; et al. Long-Term Follow-Up of MEN1 Patients Who Do Not Have Initial Surgery for Small ≤2 cm Nonfunctioning Pancreatic Neuroendocrine Tumors, an AFCE and GTE Study. Ann. Surg. 2018, 268, 158–164. [Google Scholar] [CrossRef]
- de Baere, T.; Deschamps, F.; Tselikas, L.; Ducreux, M.; Planchard, D.; Pearson, E.; Berdelou, A.; Leboulleux, S.; Elias, D.; Baudin, E. GEP-NETS UPDATE: Interventional Radiology: Role in the Treatment of Liver Metastases from GEP-NETs. Eur. J. Endocrinol. 2015, 172, R151–R166. [Google Scholar] [CrossRef]
- Lou, F.; Sarkaria, I.; Pietanza, C.; Travis, W.; Roh, M.S.; Sica, G.; Healy, D.; Rusch, V.; Huang, J. Recurrence of Pulmonary Carcinoid Tumors after Resection: Implications for Postoperative Surveillance. Ann. Thorac. Surg. 2013, 96, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Gosain, R.; Groman, A.; Yendamuri, S.S.; Iyer, R.; Mukherjee, S. Role of Adjuvant Chemotherapy in Pulmonary Carcinoids: An NCDB Analysis. Anticancer. Res. 2019, 39, 6835–6842. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Wachsman, A. Imaging of Neuroendocrine Tumors. Endocrinol. Metab. Clin. North Am. 2017, 46, 795–814. [Google Scholar] [CrossRef] [PubMed]
- Chapiro, J.; Duran, R.; Lin, M.; Schernthaner, R.; Lesage, D.; Wang, Z.; Savic, L.J.; Geschwind, J.-F. Early Survival Prediction after Intra-Arterial Therapies: A 3D Quantitative MRI Assessment of Tumour Response after TACE or Radioembolization of Colorectal Cancer Metastases to the Liver. Eur. Radiol. 2015, 25, 1993–2003. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (MRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 052–060. [Google Scholar] [CrossRef] [PubMed]
- Gowdra Halappa, V.; Corona-Villalobos, C.P.; Bonekamp, S.; Li, Z.; Reyes, D.; Cosgrove, D.; Pawlik, T.M.; Diaz, L.A.; Bhagat, N.; Eng, J.; et al. Neuroendocrine Liver Metastasis Treated by Using Intraarterial Therapy: Volumetric Functional Imaging Biomarkers of Early Tumor Response and Survival. Radiology 2013, 266, 502–513. [Google Scholar] [CrossRef]
- de Mestier, L.; Dromain, C.; d’Assignies, G.; Scoazec, J.-Y.; Lassau, N.; Lebtahi, R.; Brixi, H.; Mitry, E.; Guimbaud, R.; Courbon, F.; et al. Evaluating Digestive Neuroendocrine Tumor Progression and Therapeutic Responses in the Era of Targeted Therapies: State of the Art. Endocr.-Relat. Cancer 2014, 21, R105–R120. [Google Scholar] [CrossRef] [PubMed]
- Lecouvet, F.E.; Larbi, A.; Pasoglou, V.; Omoumi, P.; Tombal, B.; Michoux, N.; Malghem, J.; Lhommel, R.; Vande Berg, B.C. MRI for Response Assessment in Metastatic Bone Disease. Eur. Radiol. 2013, 23, 1986–1997. [Google Scholar] [CrossRef]
- Frilling, A.; Modlin, I.M.; Kidd, M.; Russell, C.; Breitenstein, S.; Salem, R.; Kwekkeboom, D.; Lau, W.-Y.; Klersy, C.; Vilgrain, V.; et al. Recommendations for Management of Patients with Neuroendocrine Liver Metastases. Lancet Oncol. 2014, 15, e8–e21. [Google Scholar] [CrossRef]
- Sahu, S.; Schernthaner, R.; Ardon, R.; Chapiro, J.; Zhao, Y.; Sohn, J.H.; Fleckenstein, F.N.; Lin, M.; Geschwind, J.-F.; Duran, R. Imaging Biomarkers of Tumor Response in Neuroendocrine Liver Metastases Treated with Transarterial Chemoembolization: Can Enhancing Tumor Burden of the Whole Liver Help Predict Patient Survival? Radiology 2017, 283, 883–894. [Google Scholar] [CrossRef]
- Dromain, C.; Sundin, A.; Najran, P.; Vidal Trueba, H.; Dioguardi Burgio, M.; Crona, J.; Opalinska, M.; Carvalho, L.; Franca, R.; Borg, P.; et al. Tumor Growth Rate to Predict the Outcome of Patients with Neuroendocrine Tumors: Performance and Sources of Variability. Neuroendocrinology 2021, 111, 831–839. [Google Scholar] [CrossRef]
- Spina, J.C.; Hume, I.; Pelaez, A.; Peralta, O.; Quadrelli, M.; Garcia Monaco, R. Expected and Unexpected Imaging Findings after 90Y Transarterial Radioembolization for Liver Tumors. RadioGraphics 2019, 39, 578–595. [Google Scholar] [CrossRef]
- Fairweather, M.; Swanson, R.; Wang, J.; Brais, L.K.; Dutton, T.; Kulke, M.H.; Clancy, T.E. Management of Neuroendocrine Tumor Liver Metastases: Long-Term Outcomes and Prognostic Factors from a Large Prospective Database. Ann. Surg. Oncol. 2017, 24, 2319–2325. [Google Scholar] [CrossRef] [PubMed]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; de Herder, W.W.; Feelders, R.A.; Krenning, E.P.; Kwekkeboom, D.J. Pitfalls in the Response Evaluation after Peptide Receptor Radionuclide Therapy with [177Lu-DOTA0,Tyr3]Octreotate. Endocrine-Related Cancer 2017, 24, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Galgano, S.J.; Iravani, A.; Bodei, L.; El-Haddad, G.; Hofman, M.S.; Kong, G. Imaging of Neuroendocrine Neoplasms: Monitoring Treatment Response—AJR Expert Panel Narrative Review. Am. J. Roentgenol. 2022, 218, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.H.; Keppke, A.L.; Reddy, D.; Huang, J.; Jin, J.; Mulcahy, M.F.; Salem, R. Response of Liver Metastases after Treatment with Yttrium-90 Microspheres: Role of Size, Necrosis, and PET. Am. J. Roentgenol. 2007, 188, 776–783. [Google Scholar] [CrossRef]
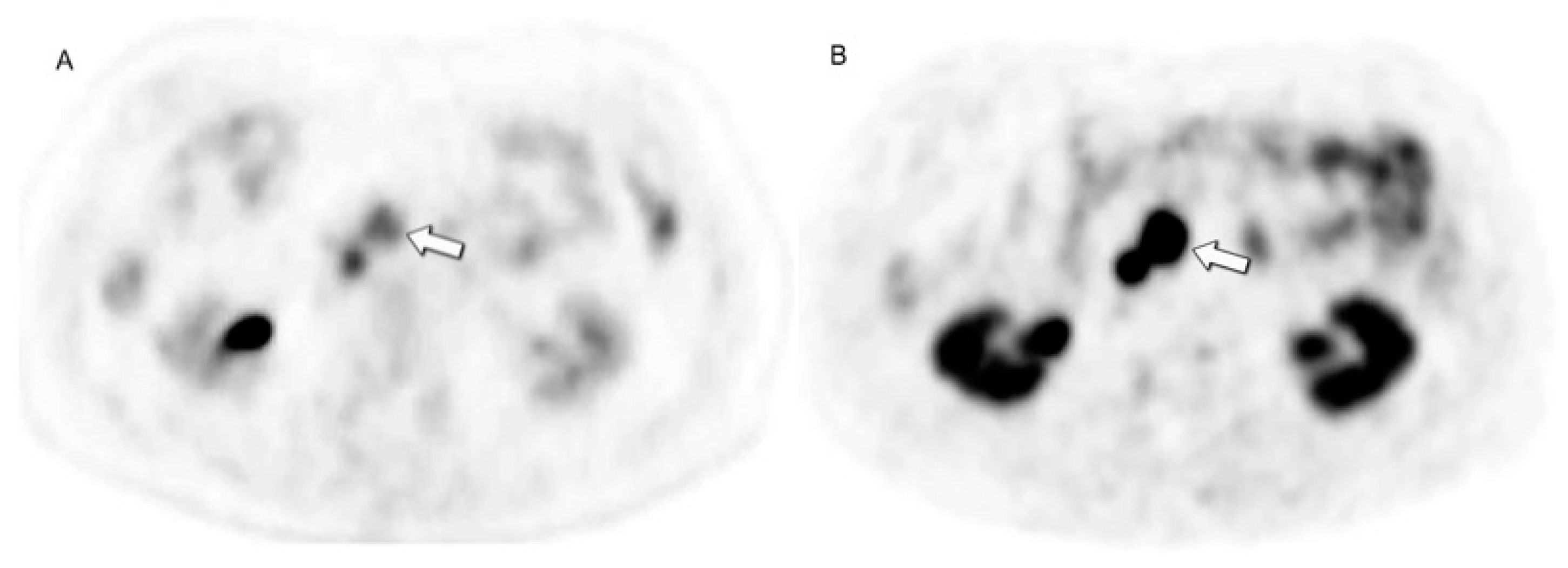
- Cherk, M.H.; Kong, G.; Hicks, R.J.; Hofman, M.S. Changes in Biodistribution on 68Ga-DOTA-Octreotate PET/CT after Long Acting Somatostatin Analogue Therapy in Neuroendocrine Tumour Patients May Result in Pseudoprogression. Cancer Imaging 2018, 18, 3. [Google Scholar] [CrossRef]
- Gålne, A.; Almquist, H.; Almquist, M.; Hindorf, C.; Ohlsson, T.; Nordenström, E.; Sundlöv, A.; Trägårdh, E. A Prospective Observational Study to Evaluate the Effects of Long-Acting Somatostatin Analogs on 68Ga-DOTATATE Uptake in Patients with Neuroendocrine Tumors. J. Nucl. Med. 2019, 60, 1717–1723. [Google Scholar] [CrossRef]
- Russo, A.; Gangi, A. The Evolving Landscape of Neuroendocrine Tumors. Surg. Oncol. Clin. N. Am. 2023, 32, 185–198. [Google Scholar] [CrossRef]
- Zhu, H.B.; Zhu, H.T.; Jiang, L.; Nie, P.; Hu, J.; Tang, W.; Zhang, X.Y.; Li, X.T.; Yao, Q.; Sun, Y.S. Radiomics analysis from magnetic resonance imaging in predicting the grade of nonfunctioning pancreatic neuroendocrine tumors: A multicenter study. Eur. Radiol. 2023, 34, 90–102. [Google Scholar] [CrossRef]
- Ye, J.Y.; Fang, P.; Peng, Z.P.; Huang, X.T.; Xie, J.Z.; Yin, X.Y. A radiomics-based interpretable model to predict the pathological grade of pancreatic neuroendocrine tumors. Eur. Radiol. 2024, 34, 1994–2005. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asmundo, L.; Rizzetto, F.; Blake, M.; Anderson, M.; Mojtahed, A.; Bradley, W.; Shenoy-Bhangle, A.; Fernandez-del Castillo, C.; Qadan, M.; Ferrone, C.; et al. Advancements in Neuroendocrine Neoplasms: Imaging and Future Frontiers. J. Clin. Med. 2024, 13, 3281. https://doi.org/10.3390/jcm13113281
Asmundo L, Rizzetto F, Blake M, Anderson M, Mojtahed A, Bradley W, Shenoy-Bhangle A, Fernandez-del Castillo C, Qadan M, Ferrone C, et al. Advancements in Neuroendocrine Neoplasms: Imaging and Future Frontiers. Journal of Clinical Medicine. 2024; 13(11):3281. https://doi.org/10.3390/jcm13113281
Chicago/Turabian StyleAsmundo, Luigi, Francesco Rizzetto, Michael Blake, Mark Anderson, Amirkasra Mojtahed, William Bradley, Anuradha Shenoy-Bhangle, Carlos Fernandez-del Castillo, Motaz Qadan, Cristina Ferrone, and et al. 2024. "Advancements in Neuroendocrine Neoplasms: Imaging and Future Frontiers" Journal of Clinical Medicine 13, no. 11: 3281. https://doi.org/10.3390/jcm13113281
APA StyleAsmundo, L., Rizzetto, F., Blake, M., Anderson, M., Mojtahed, A., Bradley, W., Shenoy-Bhangle, A., Fernandez-del Castillo, C., Qadan, M., Ferrone, C., Clark, J., Ambrosini, V., Picchio, M., Mapelli, P., Evangelista, L., Leithner, D., Nikolaou, K., Ursprung, S., Fanti, S., ... Catalano, O. A. (2024). Advancements in Neuroendocrine Neoplasms: Imaging and Future Frontiers. Journal of Clinical Medicine, 13(11), 3281. https://doi.org/10.3390/jcm13113281










