Are Endomyocardial Ventricular Biopsies Useful for Assessing Myocardial Fibrosis?
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions and Future Perspectives
6. Study Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CM | chronic myocarditis |
| CVD | cardiovascular disease |
| EMB | endomyocardial biopsy |
| HCM | hypertrophic cardiomyopathy |
| HF | heart failure |
| HHD | hypertensive heart disease |
| IHD | ischemic heart disease |
| MRI | magnetic resonance imaging |
References
- Karev, V.; Starshinova, A.Y.; Glushkova, A.; Kudlay, D.; Starshinova, A. Features of Myocarditis: Morphological Differential Diagnosis in Post-COVID-19 Children. Diagnostics 2023, 13, 2499. [Google Scholar] [CrossRef] [PubMed]
- Karetnikova, V.N.; Kashtalap, V.V.; Kosareva, S.N.; Barbarash, O.L. Myocardial fibrosis: Current aspects of the problem. Ter. Arkhiv 2017, 89, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.C.; Piehler, K.; Meier, C.G.; Testa, S.M.; Klock, A.M.; Aneizi, A.A.; Shakesprere, J.; Kellman, P.; Shroff, S.G.; Schwartzman, D.S.; et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation 2012, 126, 1206–1216. [Google Scholar] [CrossRef]
- Yamada, T.; Fukunami, M.; Ohmori, M.; Iwakura, K.; Kumagai, K.; Kondoh, N.; Minamino, T.; Tsujimura, E.; Nagareda, T.; Kotoh, K.; et al. Which subgroup of patients with dilated cardiomyopathy would benefit from long-term beta-blocker therapy? A histologic viewpoint. J. Am. Coll. Cardiol. 1993, 21, 628–633. [Google Scholar] [CrossRef]
- Porcari, A.; Baggio, C.; Fabris, E.; Merlo, M.; Bussani, R.; Perkan, A.; Sinagra, G. Endomyocardial biopsy in the clinical context: Current indications and challenging scenarios. Heart Fail. Rev. 2023, 28, 123–135. [Google Scholar] [CrossRef]
- Grasso, M.; Bondavalli, D.; Vilardo, V.; Cavaliere, C.; Gatti, I.; Di Toro, A.; Giuliani, L.; Urtis, M.; Ferrari, M.; Cattadori, B.; et al. The new 2023 ESC guidelines for the management of cardiomyopathies: A guiding path for cardiologist decisions. Eur. Heart J. 2024, 26 (Suppl. S1), i1–i5. [Google Scholar] [CrossRef]
- Iorio, A.; Lucà, F.; Pozzi, A.; Rao, C.M.; Chimenti, C.; Di Fusco, S.A.; Rossini, R.; Caretta, G.; Cornara, S.; Giubilato, S.; et al. Anderson–Fabry Disease: Red Flags for Early Diagnosis of Cardiac Involvement. Diagnostics 2024, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Cundari, G.; Galea, N.; Mergen, V.; Alkadhi, H.; Eberhard, M. Myocardial extracellular volume quantification with computed tomography-current status and future outlook. Insights Imaging 2023, 14, 156. [Google Scholar] [CrossRef]
- Hinderer, S.; Schenke-Layland, K. Cardiac fibrosis—A short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 77–82. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Mitrofanova, L.B.; Rybakova, M.G. Causes and mechanisms of sudden cardiac death in children. Sud.-Meditsinskaya Ekspertisa 2021, 64, 43–49. (In Russian) [Google Scholar] [CrossRef]
- Oken, D.E.; Boucek, R.J. Quantitation of collagen in human myocardium. Circ Res. 1957, 5, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.B.; Ferro, M.; Rodrigues, B.; Souza, M.R.; Araujo, R.C.; Souza, R.R. Quantification of left ventricular myocardial collagen system in children, young adults, and the elderly. Medicina 2012, 72, 216–220. [Google Scholar] [PubMed]
- Miles, C.; Westaby, J.; Ster, I.C.; Asimaki, A.; Boardman, P.; Joshi, A.; Papadakis, M.; Sharma, S.; Behr, E.R.; Sheppard, M.N. Morphometric characterization of collagen and fat in normal ventricular myocardium. Cardiovasc. Pathol. 2020, 48, 107224. [Google Scholar] [CrossRef] [PubMed]
- Meckel, C.R.; Wilson, J.E.; Sears, T.D.; Rogers, J.G.; Goaley, T.J.; McManus, B.M. Myocardial fibrosis in endomyocardial biopsy specimens: Do different bioptomes affect estimation? Am. J. Cardiovasc. Pathol. 1989, 2, 309–313. [Google Scholar] [PubMed]
- Hahn, V.S.; Yanek, L.R.; Vaishnav, J.; Ying, W.; Vaidya, D.; Lee, Y.Z.J.; Riley, S.J.; Subramanya, V.; Brown, E.E.; Hopkins, C.D.; et al. Endomyocardial Biopsy Characterization of Heart Failure With Preserved Ejection Fraction and Prevalence of Cardiac Amyloidosis. JACC Heart Fail. 2020, 8, 712–724. [Google Scholar] [CrossRef] [PubMed]
- López, B.; Querejeta, R.; González, A.; Larman, M.; Díez, J. Collagen cross-linking but not collagen amount associates with elevated filling pressures in hypertensive patients with stage C heart failure: Potential role of lysyl oxidase. Hypertension 2012, 60, 677–683. [Google Scholar] [CrossRef] [PubMed]
- López, B.; González, A.; Querejeta, R.; Larman, M.; Rábago, G.; Díez, J. Association of cardiotrophin-1 with myocardial fibrosis in hypertensive patients with heart failure. Hypertension 2014, 63, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Díez, J.; González, A.; Kovacic, J.C. Myocardial Interstitial Fibrosis in Nonischemic Heart Disease, Part 3/4: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 2204–2218. [Google Scholar] [CrossRef]
- Aoki, T.; Fukumoto, Y.; Sugimura, K.; Oikawa, M.; Satoh, K.; Nakano, M.; Nakayama, M.; Shimokawa, H. Prognostic impact of myocardial interstitial fibrosis in non-ischemic heart failure.—Comparison between preserved and reduced ejection fraction heart failure. Circ. J. 2011, 75, 2605–2613. [Google Scholar] [CrossRef]
- Liu, B.; Neil, D.A.H.; Premchand, M.; Bhabra, M.; Patel, R.; Barker, T.; Nikolaidis, N.; Billing, J.S.; Treibel, T.A.; Moon, J.C.; et al. Myocardial fibrosis in asymptomatic and symptomatic chronic severe primary mitral regurgitation and relationship to tissue characterisation and left ventricular function on cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 86. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanova, L.B.; Orshanskaya, V.; Ho, S.Y.; Platonov, P.G. Histological evidence of inflammatory reaction associated with fibrosis in the atrial and ventricular walls in a case-control study of patients with history of atrial fibrillation. Europace 2016, 18 (Suppl. S4), iv156–iv162. [Google Scholar] [CrossRef] [PubMed]
- Diao, K.-Y.; Yang, Z.-G.; Xu, H.-Y.; Liu, X.; Zhang, Q.; Shi, K.; Jiang, L.; Xie, L.-J.; Wen, L.-Y.; Guo, Y.-K. Histologic validation of myocardial fibrosis measured by T1 mapping: A systematic review and meta-analysis. J. Cardiovasc. Magn. Reson. 2016, 18, 92. [Google Scholar] [CrossRef]
- Kasner, M.; Westermann, D.; Lopez, B.; Gaub, R.; Escher, F.; Kühl, U.; Schultheiss, H.-P.; Tschöpe, C. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J. Am. Coll. Cardiol. 2011, 57, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanova, L.B.; Gorshkov, A.N.; Lebedev, D.S.; Mikhaylov, E.N. Evidence of specialized tissue in human interatrial septum: Histological, immunohistochemical and ultrastructural findings. PLoS ONE 2014, 9, e113343. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Brilla, C.G. Factors associated with reactive and reparative fibrosis of the myocardium. Basic. Res. Cardiol. 1992, 87 (Suppl. S1), 291–301. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef]
- Bonavida, V.; Ghassemi, K.; Ung, G.; Inouye, K.; Thankam, F.G.; Agrawal, D.K. Novel Approaches to Program Cells to Differentiate into Cardiomyocytes in Myocardial Regeneration. Rev. Cardiovasc. Med. 2022, 23, 392. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef]
- Schimmel, K.; Ichimura, K.; Reddy, S.; Haddad, F.; Spiekerkoetter, E. Cardiac Fibrosis in the Pressure Overloaded Left and Right Ventricle as a Therapeutic Target. Front. Cardiovasc. Med. 2022, 9, 886553. [Google Scholar] [CrossRef]
- He, X.; Du, T.; Long, T.; Liao, X.; Dong, Y.; Huang, Z.P. Signaling cascades in the failing heart and emerging therapeutic strategies. Signal Transduct. Target. Ther. 2022, 7, 134. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, H.; Yao, Y.; Tao, Z.; Chen, W.; Huang, F.; Chen, X. Macrophage, a potential targeted therapeutic immune cell for cardiomyopathy. Front. Cell Dev. Biol. 2022, 10, 908790. [Google Scholar] [CrossRef]
- Nasyrov, R.A.; Ivanov, D.O.; Krasnogorskaya, O.L.; Timchenko, V.N.; Fedotova, E.P.; Chepelev, A.S.; Galichina, V.A.; Sidorova, N.A.; Anichkov, N.M. COVID-19 in Children: Molecular Profile and Pathological Features. Int. J. Mol. Sci. 2023, 24, 16750. [Google Scholar] [CrossRef]
- Vasichkina, E.; Kofeynikova, O.; Fetisova, S.; Starshinova, A.Y.; Sheyanova, E.; Vershinina, T.; Ryzhkov, A.; Skripnik, A.; Alekseeva, D.; Nechaeva, E.; et al. Severe Course of COVID-19 and Long-COVID-19 in Children: Difficulties in Diagnosis. Life 2023, 13, 781. [Google Scholar] [CrossRef]
- Yu, B.; Wu, Y.; Song, X.; Liu, G.; Wang, F.; Zhang, F.; Liang, B. Possible Mechanisms of SARS-CoV2-Mediated Myocardial Injury. Cardiovasc. Innov. Appl. 2023, 8, e981. [Google Scholar] [CrossRef]
- Vasichkina, E.; Alekseeva, D.; Kudryavtsev, I.; Glushkova, A.; Starshinova, A.Y.; Malkova, A.; Kudlay, D.; Starshinova, A. COVID-19 Heart Lesions in Children: Clinical, Diagnostic and Immunological Changes. Int. J. Mol. Sci. 2023, 24, 1147. [Google Scholar] [CrossRef]
- Mitrofanova, L.B.; Makarov, I.A.; Gorshkov, A.N.; Runov, A.L.; Vonsky, M.S.; Pisareva, M.M.; Komissarov, A.B.; Makarova, T.A.; Li, Q.; Karonova, T.L.; et al. Comparative Comparative Study of the Myocardium of Patients from Four COVID-19 Waves. Diagnostics 2023, 13, 1645. [Google Scholar] [CrossRef]
- Jones, E.A.V. Mechanism of COVID-19-Induced Cardiac Damage from Patient, In Vitro and Animal Studies. Curr. Heart Fail. Rep. 2023, 20, 451–460. [Google Scholar] [CrossRef]
- Makarov, I.; Mayrina, S.; Makarova, T.; Karonova, T.; Starshinova, A.; Kudlay, D.; Mitrofanova, L. Morphological Changes in the Myocardium of Patients with Post-Acute Coronavirus Syndrome: A Study of Endomyocardial Biopsies. Diagnostics 2023, 13, 2212. [Google Scholar] [CrossRef]
- Kudlay, D.; Kofiadi, I.; Khaitov, M. Peculiarities of the T Cell Immune Response in COVID-19. Vaccines 2022, 10, 242. [Google Scholar] [CrossRef]
- Westmeier, J.; Paniskaki, K.; Karaköse, Z.; Werner, T.; Sutter, K.; Dolff, S.; Overbeck, M.; Limmer, A.; Liu, J.; Zheng, X.; et al. Impaired cytotoxic CD8+ T cell response in elderly COVID-19 patients. mBio 2020, 11, 11, Erratum in mBio 2020, 11, e02243-20. [Google Scholar] [CrossRef]
- Mitrofanova, L.; Makarov, I.; Goncharova, E.; Makarova, T.; Starshinova, A.; Kudlay, D.; Shlaykhto, E. High Risk of Heart Tumors after COVID-19. Life 2023, 13, 2087. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S. T Cells in Fibrosis and Fibrotic Diseases. Front. Immunol. 2020, 11, 1142. [Google Scholar] [CrossRef]


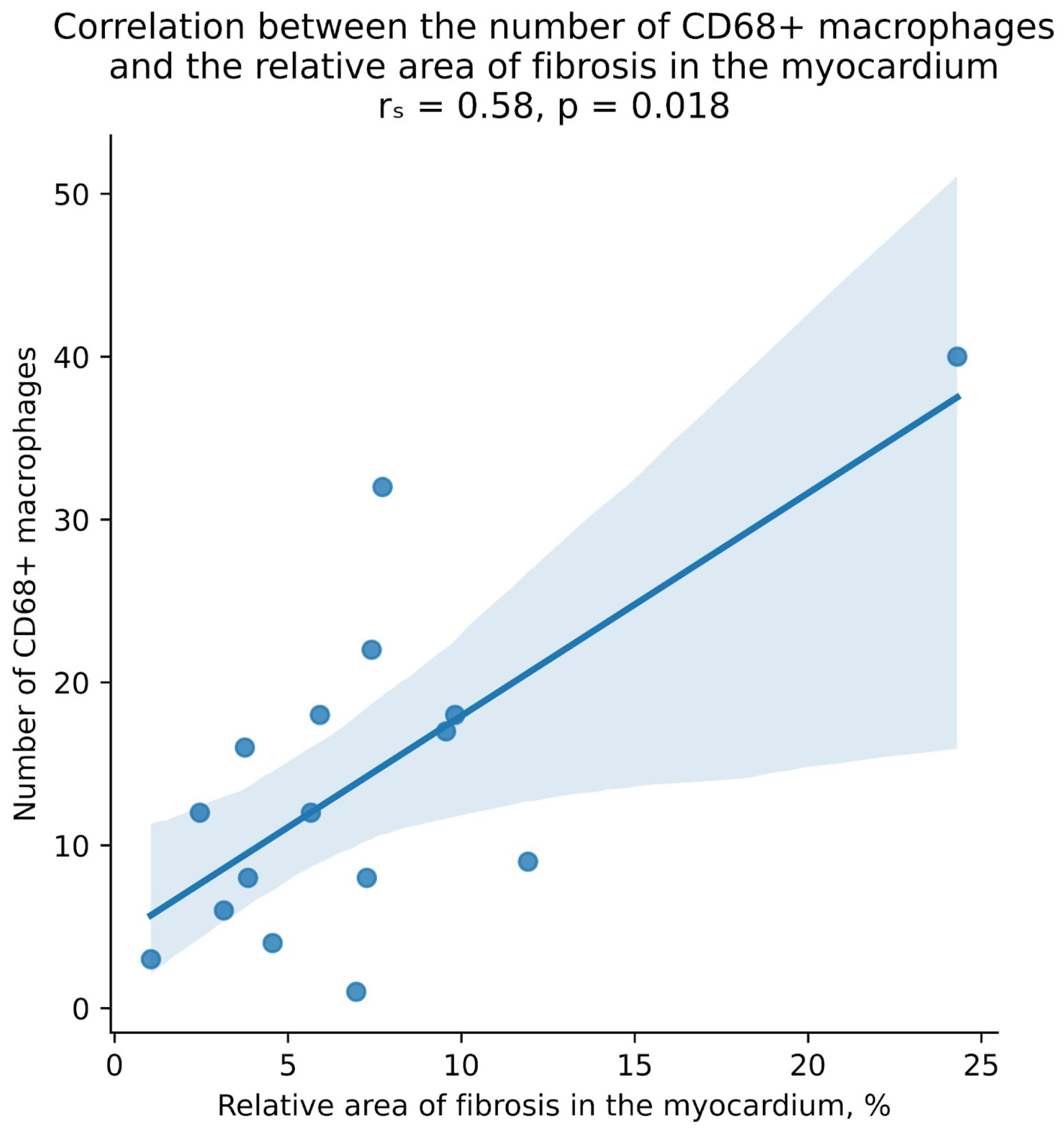
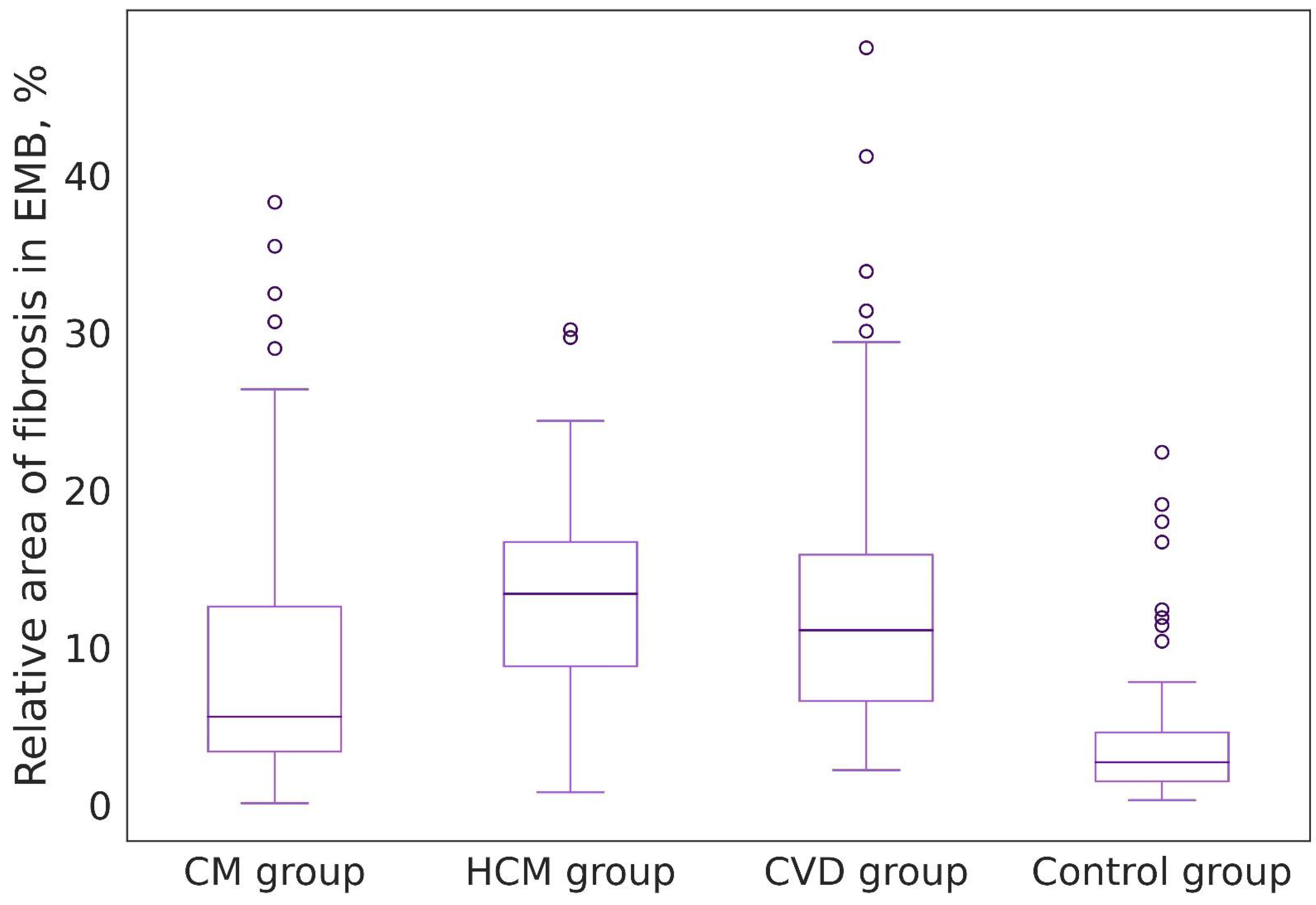

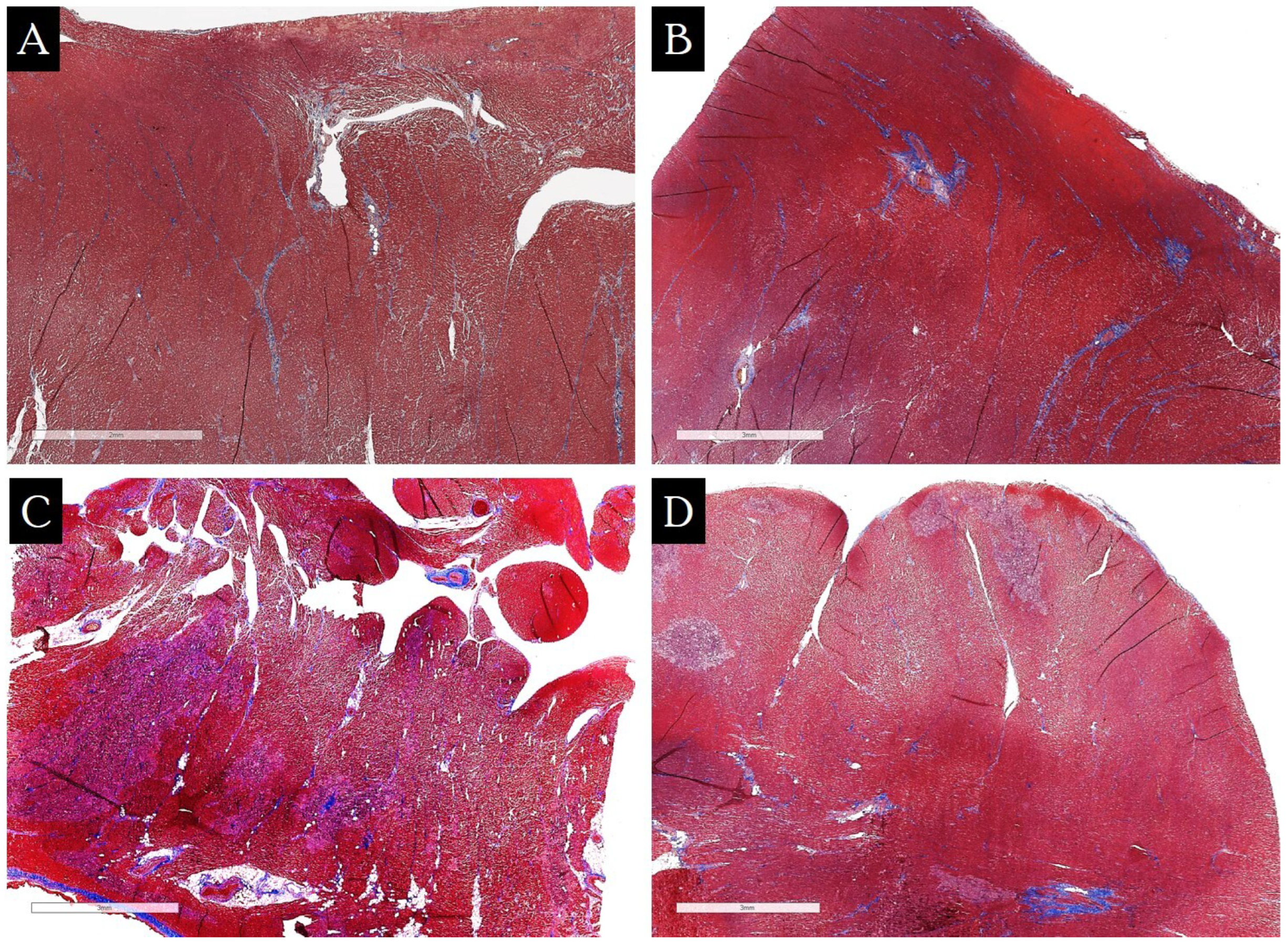

| Groups of Patients | Value Range (%) | Median, 25%, and 75% Percentiles (%) | Foci of Replacement Cardiosclerosis in Biopsy Specimens (n/%) |
|---|---|---|---|
| Group I: EMBs of patients with chronic lymphocytic myocarditis (n = 20) | 0.1–38.3 | 5.6 [3.3; 12.6] | 14 (15.2) |
| Group II: Endomyocardial fragments obtained during septal reduction of patients with a long history of coronary heart disease combined with hypertension (n = 36) | 2.2–48.1 | 11.1 [6.6; 15.9] | 7 (10.7) |
| Group III: Endomyocardial fragments obtained during septal reduction of patients with hypertrophic cardiomyopathy (n = 12) | 0.8–30.2 | 13.4 [8.8; 16.7] | 3 (14.3) |
| Control group: EMB of patients on 12–14 days after heart transplantation (n = 28) | 0.3–22.4 | 2.7 [1.5; 4.6] | 1 (1.6) |
| Patient | Age | Gender | Time between EMB, Days | Dynamics of LVEF, % | Dynamics of TAPSE, in mm | Dynamics of CD3+ Cells in the Inflammatory Infiltrate | Dynamics of CD68+ Cells in the Inflammatory Infiltrate | Dynamics of VP1-EntV Expression in Cardiomyocytes, % | Dynamics of the Relative Area of Fibrosis in the Myocardium, % | Specific Therapy | Treatment of HF and Concomitant Pathology |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 | F | 133 | 49 vs. 61 | 16 vs. 20 | 46 vs. 15 | 22 vs. 8 | 80 vs. 0 | 7.41 vs. 3.45 | Prednisolone: starting dose 30 mg with dose reduction to 15 mg Human immunoglobulin normal 0.4 g/kg | β-adrenoblocker ACE inhibitor Rivaroxaban Torasemide Spironolactone |
| 2 | 32 | M | 130 | 38 vs. 48 | 17 vs. 19 | 19 vs. 14 | 40 vs. 6 | 80 vs. 20 | 24.30 vs. 3.15 | Prednisolone: starting dose 30 mg with dose reduction to 15 mg | β-adrenoblocker Digoxin Rivaroxaban Amiodarone Torsemide Molsidomine |
| 3 | 31 | F | 89 | 42 vs. 51 | 14 vs. 15 | 18 vs. 8 | 12 vs. 16 | 0 vs. 0 | 2.46 vs. 3.76 | Prednisolone: starting dose 30 mg with dose reduction to 15 mg | β-adrenoblocker ACE inhibitor Spironolactone |
| 4 | 26 | F | 115 | 12 vs. 15 | 13 vs. 17 | 18 vs. 3 | 32 vs. 8 | 0 vs. 0 | 7.73 vs. 7.27 | Prednisolone: starting dose 1 mg/kg with dose reduction to 15 mg Mycophenolate mofetil 2 g/day | β-adrenoblocker Valsartan + Sacubitril Apixaban Amiodarone Torsemide Spironolactone |
| 5 | 41 | M | 209 | 19 vs. 33 | 13 vs. 17 | 26 vs. 4 | 3 vs. 18 | 90 vs. 20 | 1.05 vs. 5.92 | Prednisolone: starting dose 30 mg with dose reduction to 15 mg | β-adrenoblocker ACE inhibitor Spironolactone Warfarin Levosimendan three times |
| 6 | 33 | M | 358 | 58 vs. 64 | 14 vs. 15 | 25 vs. 5 | 9 vs. 4 | 100 vs. 50 | 11.92 vs. 4.56 | None | β-adrenoblocker ACE inhibitor Acetylsalicylic acid |
| 7 | 59 | F | 531 | 16 vs. 18 | 11 vs. 17 | 50 vs. 20 | 17 vs. 18 | 80 vs. 50 | 9.56 vs. 9.82 | Prednisolone: starting dose 30 mg with dose reduction to 15 mg Human immunoglobulin normal 0.4 g/kg | β-adrenoblocker ACE inhibitor Spironolactone Rivaroxaban Amiodarone Torsemide |
| 8 | 30 | M | 504 | 60 vs. 48 | 16 vs. 17 | 15 vs. 1 | 12 vs. 1 | 0 vs. 0 | 5.66 vs. 6.97 | None | β-adrenoblocker ACE inhibitor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarov, I.; Voronkina, D.; Gurshchenkov, A.; Ryzhkov, A.; Starshinova, A.; Kudlay, D.; Mitrofanova, L. Are Endomyocardial Ventricular Biopsies Useful for Assessing Myocardial Fibrosis? J. Clin. Med. 2024, 13, 3275. https://doi.org/10.3390/jcm13113275
Makarov I, Voronkina D, Gurshchenkov A, Ryzhkov A, Starshinova A, Kudlay D, Mitrofanova L. Are Endomyocardial Ventricular Biopsies Useful for Assessing Myocardial Fibrosis? Journal of Clinical Medicine. 2024; 13(11):3275. https://doi.org/10.3390/jcm13113275
Chicago/Turabian StyleMakarov, Igor, Daria Voronkina, Alexander Gurshchenkov, Anton Ryzhkov, Anna Starshinova, Dmitry Kudlay, and Lubov Mitrofanova. 2024. "Are Endomyocardial Ventricular Biopsies Useful for Assessing Myocardial Fibrosis?" Journal of Clinical Medicine 13, no. 11: 3275. https://doi.org/10.3390/jcm13113275
APA StyleMakarov, I., Voronkina, D., Gurshchenkov, A., Ryzhkov, A., Starshinova, A., Kudlay, D., & Mitrofanova, L. (2024). Are Endomyocardial Ventricular Biopsies Useful for Assessing Myocardial Fibrosis? Journal of Clinical Medicine, 13(11), 3275. https://doi.org/10.3390/jcm13113275







