Axillary Brachial Plexus Block Compared with Other Regional Anesthesia Techniques in Distal Upper Limb Surgery: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
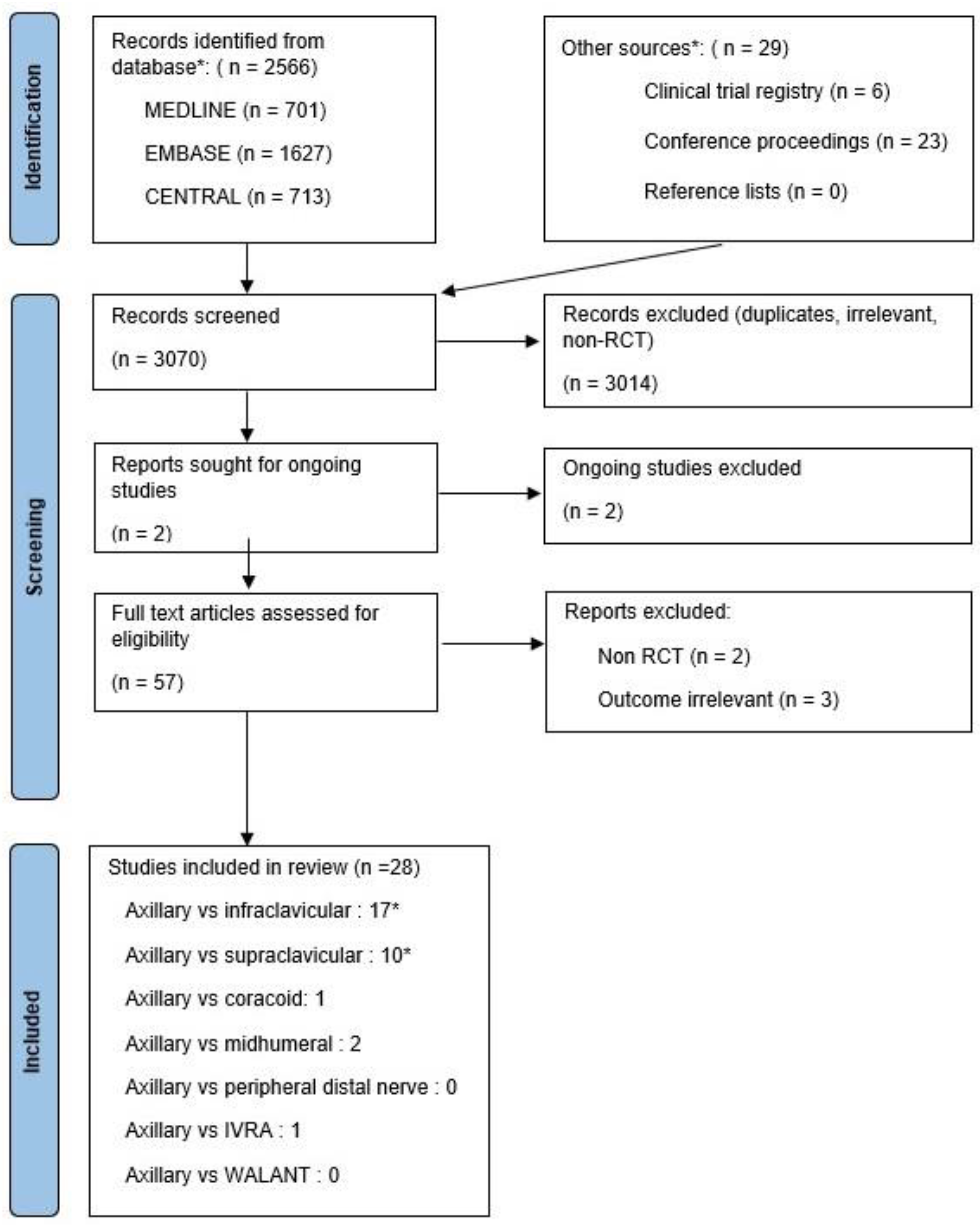
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection and Reliability
2.4. Data Extraction and Quality Assessment
2.5. Outcome Measures
- (1)
- Adequate surgical anesthesia 30 min after block completion and without needing supplemental local anesthesia (LA) injection, systemic analgesia (opioids), or general anesthesia (GA).
- (2)
- The need for supplemental LA infiltration, additional RA block or systemic analgesia, or a combination to achieve adequate surgical anesthesia.
- (3)
- The need for GA to achieve adequate surgical anesthesia.
- (4)
- Performance time of RA block placement in minutes. No strict definition or method was specified in advance.
- (5)
- Onset time of adequate surgical anesthesia. This was defined as the time in minutes from block completion to the absence or decrease of any sensation in the operative area where surgery would be conducted.
- (6)
- Pain associated with RA block placement. No strict definition or method of assessment was specified in advance.
- (7)
- Patient satisfaction. No strict definition or method of assessment was specified in advance.
- (8)
- Block-related complications. Five complications were assessed: pneumothorax, vascular puncture, Horner’s syndrome, local anesthetic systemic toxicity (LAST), and neurological deficits, including residual neuropraxias unrelated to the surgical site, lasting more than 24 h. No strict definition or method of assessment was specified in advance. Some studies use trans-arterial RA guiding; these studies were excluded from evaluating block-related complications.
- (9)
- Tourniquet pain. No strict definition or method of assessment was specified in advance.
2.6. Statistics
3. Results
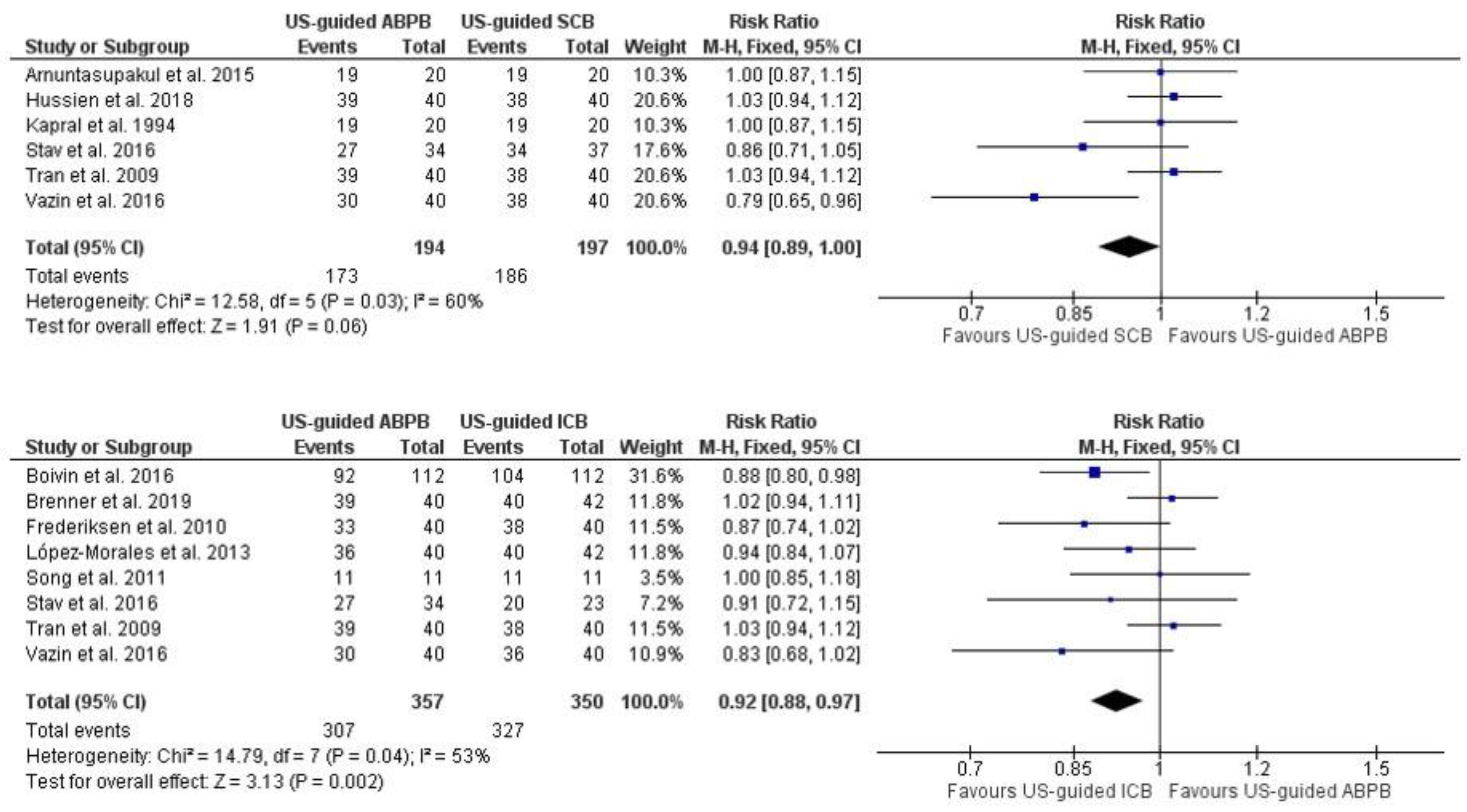
3.1. Primary Outcome: Adequate Surgical Anesthesia within 30 min of Block Completion
3.2. The Need for Supplemental LA Infiltration, Additional RA Block or Systemic Analgesia, or a Combination to Achieve Adequate Surgical Anesthesia
3.3. The Need for GA to Achieve Adequate Surgical Anesthesia
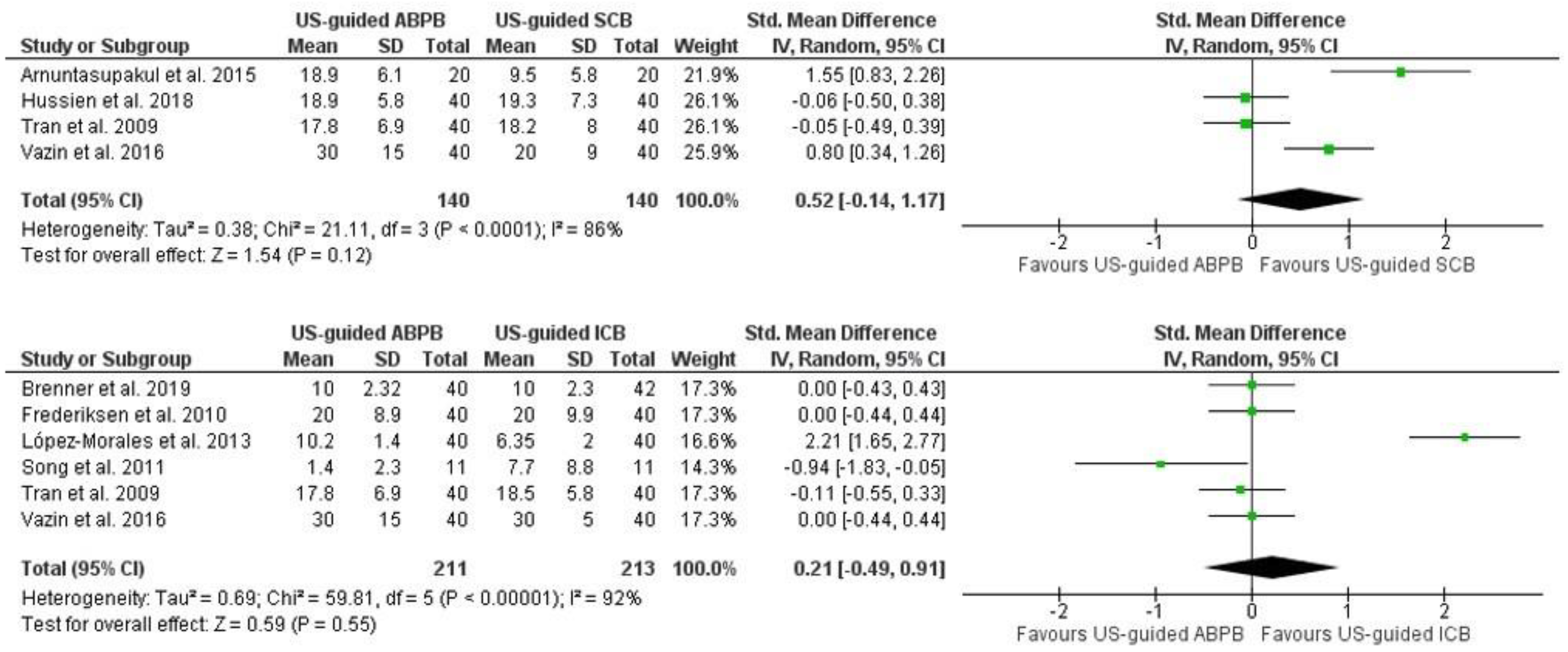
3.4. Performance Time of RA Block Placement in Minutes
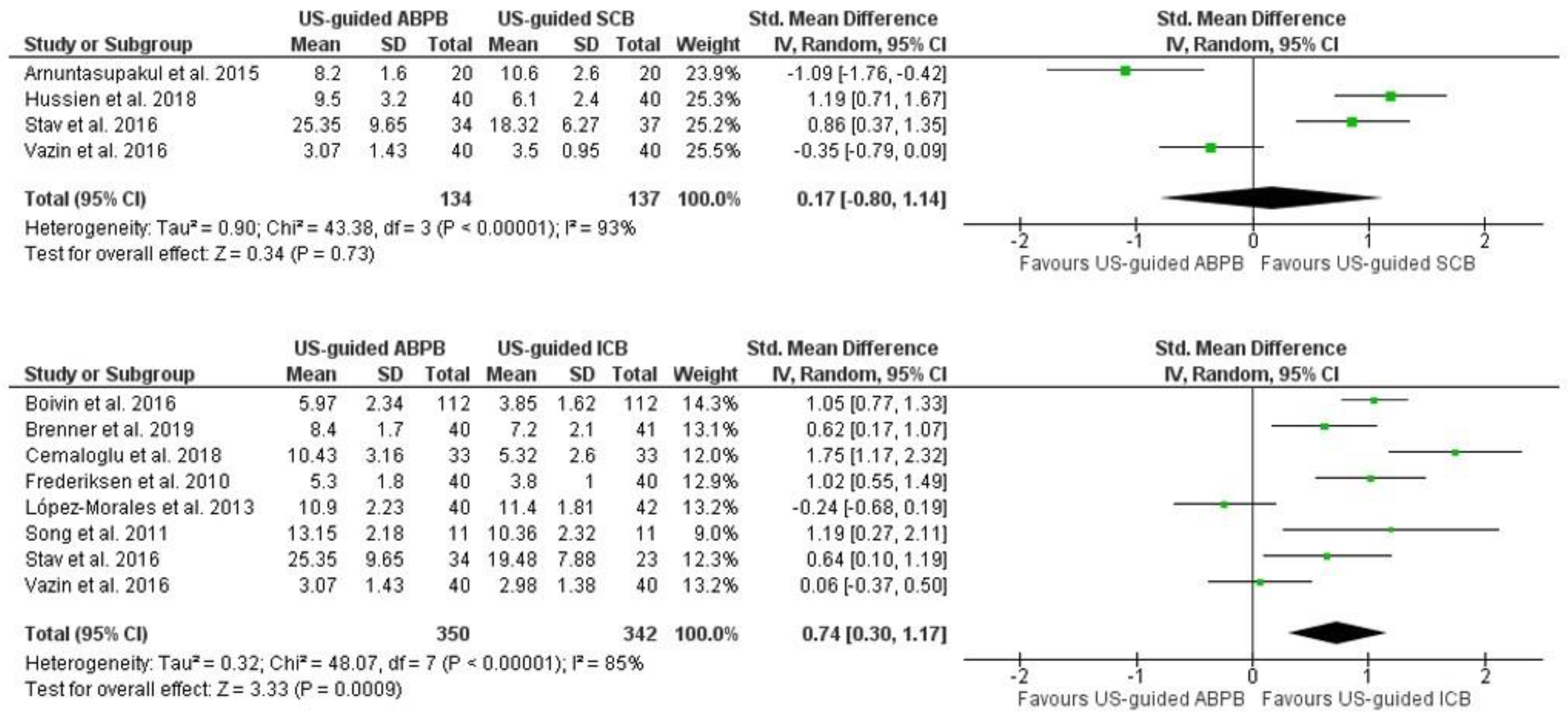
3.5. Onset Time of Adequate Surgical Anesthesia
3.6. Pain Associated with RA Block Placement
3.7. Patient Satisfaction
3.8. Block-Related Complications
3.9. Tourniquet Pain
4. Discussion
4.1. Limitations
4.2. Interpretation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teunkens, A.; Vermeulen, K.; Belmans, A.; Degreef, I.; Van de Velde, M.; Rex, S. Patient satisfaction with intravenous regional anaesthesia or an axillary block for minor ambulatory hand surgery: A randomised controlled study. Eur. J. Anaesthesiol. 2020, 37, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Nijs, K.; Ruette, J.; Van de Velde, M.; Stessel, B. Regional anaesthesia for ambulatory surgery. Best. Pract. Res. Clin. Anaesthesiol. 2023, 37, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Polshin, V.; Petro, J.; Wachtendorf, L.J.; Hammer, M.; Simopoulos, T.; Eikermann, M.; Santer, P. Effect of peripheral nerve blocks on postanesthesia care unit length of stay in patients undergoing ambulatory surgery: A retrospective cohort study. Reg. Anesth. Pain Med. 2021, 46, 233. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, K.P.J.; Cherry, R.A. Brachial plexus anesthesia compared to general anesthesia when a block room is available. Can. J. Anaesth. J. Can. Anesth. 2004, 51, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Nijs, K.; Jalil, H.; Callebaut, I.; Van de Velde, M.; Stessel, B. Patient knowledge and preference in regional anaesthesia: A single-centre cross-sectional survey. Eur. J. Anaesthesiol. 2022, 39, 633–635. [Google Scholar] [CrossRef] [PubMed]
- Streb, T.; Schneider, A.; Wiesmann, T.; Riecke, J.; Schubert, A.-K.; Dinges, H.-C.; Volberg, C. Rebound pain-From definition to treatment. Anaesthesiologie 2022, 71, 638–645. [Google Scholar] [CrossRef]
- Schubert, A.-K.; Wiesmann, T.; Volberg, C.; Riecke, J.; Schneider, A.; Wulf, H.; Dinges, H.-C. Rebound pain and postoperative pain profile following brachial plexus block compared to general anaesthesia-An observational study. Acta Anaesthesiol. Scand. 2023, 67, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Jalil, H.; Polfliet, F.; Nijs, K.; Bruckers, L.; De Wachter, G.; Callebaut, I.; Salimans, L.; Van de Velde, M.; Stessel, B. Efficacy of ultrasound-guided forearm nerve block versus forearm intravenous regional anaesthesia in patients undergoing carpal tunnel release: A randomized controlled trial. PLoS ONE 2021, 16, e0246863. [Google Scholar] [CrossRef] [PubMed]
- Nijs, K.; Lismont, A.; De Wachter, G.; Broux, V.; Callebaut, I.; Ory, J.-P.; Jalil, H.; Poelaert, J.; Van de Velde, M.; Stessel, B. The analgesic efficacy of forearm versus upper arm intravenous regional anesthesia (Bier’s block): A randomized controlled non-inferiority trial. J. Clin. Anesth. 2021, 73, 110329. [Google Scholar] [CrossRef]
- Lopera-Velásquez, L.M.; Restrepo-Garcés, C. Ultrasound and nerve stimulation-guided axillary block. Colomb. J. Anesthesiol. 2016, 44, 30–35. [Google Scholar]
- Satapathy, A.R.; Coventry, D.M. Axillary brachial plexus block. Anesthesiol. Res. Pract. 2011, 2011, 173796. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Smith, A.F.; Carlisle, J. Reviews, systematic reviews and Anaesthesia. Anaesthesia 2015, 70, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.Q.H.; Russo, G.; Muñoz, L.; Zaouter, C.; Finlayson, R.J. A prospective, randomized comparison between ultrasound-guided supraclavicular, infraclavicular, and axillary brachial plexus blocks. Reg. Anesth. Pain Med. 2009, 34, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Stav, A.; Reytman, L.; Stav, M.-Y.; Portnoy, I.; Kantarovsky, A.; Galili, O.; Luboshitz, S.; Sevi, R.; Sternberg, A. Comparison of the Supraclavicular, Infraclavicular and Axillary Approaches for Ultrasound-Guided Brachial Plexus Block for Surgical Anesthesia. Rambam Maimonides Med. J. 2016, 7, e0013. [Google Scholar] [CrossRef] [PubMed]
- Vazin, M.; Jensen, K.; Kristensen, D.L.; Hjort, M.; Tanggaard, K.; Karmakar, M.K.; Bendtsen, T.F.; Børglum, J. Low-Volume Brachial Plexus Block Providing Surgical Anesthesia for Distal Arm Surgery Comparing Supraclavicular, Infraclavicular, and Axillary Approach: A Randomized Observer Blind Trial. BioMed Res. Int. 2016, 2016, 7094121. [Google Scholar] [CrossRef] [PubMed]
- Song, I.A.; Gil, N.-S.; Choi, E.-Y.; Sim, S.-E.; Min, S.-W.; Ro, Y.-J.; Kim, C.S. Axillary approach versus the infraclavicular approach in ultrasound-guided brachial plexus block: Comparison of anesthetic time. Korean J. Anesthesiol. 2011, 61, 12–18. [Google Scholar] [CrossRef]
- Brenner, D.; Iohom, G.; Mahon, P.; Shorten, G. Efficacy of axillary versus infraclavicular brachial plexus block in preventing tourniquet pain: A randomised trial. Eur. J. Anaesthesiol. 2019, 36, 48–54. [Google Scholar] [CrossRef]
- Arnuntasupakul, V.; Leurcharusmee, P.; Chora De La Garza, D.; Ah-Kye, S.; Finlayson, R.J.; Tran, D.Q.H. A randomized trial comparing axillary block versus targeted intracluster injection supraclavicular block for upper limb surgery. Can. J. Anaesth. J. Can. Anesth. 2015, 62, 1287–1294. [Google Scholar] [CrossRef]
- Singh, A.; Gupta, R.; Vashisth, M.; Singh, S.; Kumari, A.; Aujla, K.S. Comparison of Effectiveness of Brachial Plexus Block by Supraclavicular and Axillary Approach Alone or in Combination. J. Anaesthesiol. Clin. Pharmacol. 2010, 26, 31–34. [Google Scholar]
- Bouaziz, H.; Narchi, P.; Mercier, F.J.; Labaille, T.; Zerrouk, N.; Girod, J.; Benhamou, D. Comparison between conventional axillary block and a new approach at the midhumeral level. Anesth. Analg. 1997, 84, 1058–1062. [Google Scholar] [CrossRef]
- Boivin, A.; Nadeau, M.J.; Dion, N.; Lévesque, S.; Nicole, P.C.; Turgeon, A.F. Ultrasound-Guided Single-Injection Infraclavicular Block Versus Ultrasound-Guided Double-Injection Axillary Block: A Noninferiority Randomized Controlled Trial. Anesth. Analg. 2016, 122, 273–278. [Google Scholar] [CrossRef]
- Tedore, T.R.; YaDeau, J.T.; Maalouf, D.B.; Weiland, A.J.; Tong-Ngork, S.; Wukovits, B.; Paroli, L.; Urban, M.K.; Zayas, V.M.; Wu, A.; et al. Comparison of the transarterial axillary block and the ultrasound-guided infraclavicular block for upper extremity surgery: A prospective randomized trial. Reg. Anesth. Pain Med. 2009, 34, 361–365. [Google Scholar] [CrossRef]
- Kapral, S.; Jandrasits, O.; Schabernig, C.; Likar, R.; Reddy, B.; Mayer, N.; Weinstabl, C. Lateral infraclavicular plexus block vs. axillary block for hand and forearm surgery. Acta Anaesthesiol. Scand. 1999, 43, 1047–1052. [Google Scholar] [CrossRef]
- Fleck, J.W.; Moorthy, S.S.; Daniel, J.; Dierdorf, S.F. Brachial plexus block. A comparison of the supraclavicular lateral paravascular and axillary approaches. Reg. Anesth. 1994, 19, 14–17. [Google Scholar] [PubMed]
- López-Morales, S.; Moreno-Martín, A.; Leal del Ojo, J.D.; Rodriguez-Huertas, F. Ultrasound-guided axillary block versus ultrasound-guided infraclavicular block for upper extremity surgery. Rev. Esp. Anestesiol. Reanim. 2013, 60, 313–319. [Google Scholar] [CrossRef]
- Ertug, Z.; Yegin, A.; Ertem, S.; Sahin, N.; Hadimioglu, N.; Dösemeci, L.; Erman, M. Comparison of two different techniques for brachial plexus block: Infraclavicular versus axillary technique. Acta Anaesthesiol. Scand. 2005, 49, 1035–1039. [Google Scholar] [CrossRef]
- Kapral, S.; Krafft, P.; Eibenberger, K.; Fitzgerald, R.; Gosch, M.; Weinstabl, C. Ultrasound-guided supraclavicular approach for regional anesthesia of the brachial plexus. Anesth. Analg. 1994, 78, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Hussien, R.M.; Ibrahim, D. Ultrasound Guided Axillary Brachial Plexus Block Versus Supraclavicular Block In Emergency Crushed Hand Patients: A Comparative Study. Open Anesth. J. 2018, 12, 34–41. [Google Scholar] [CrossRef]
- Deleuze, A.; Gentili, M.E.; Marret, E.; Lamonerie, L.; Bonnet, F. A comparison of a single-stimulation lateral infraclavicular plexus block with a triple-stimulation axillary block. Reg. Anesth. Pain Med. 2003, 28, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Rettig, H.C.; Gielen, M.J.M.; Boersma, E.; Klein, J. A comparison of the vertical infraclavicular and axillary approaches for brachial plexus anaesthesia. Acta Anaesthesiol. Scand. 2005, 49, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, M.; Winnie, S.; Wing-Hong, K.; Li, M. Ultrasound guided brachial plexus block: The onset of sensory blockade is faster after an axillary approach than with the supraclavicular approach. Reg. Anesth. Pain Med. 2012, 37, 1–3. [Google Scholar]
- Fuzier, R.; Fourcade, O.; Pianezza, A.; Gilbert, M.L.; Bounes, V.; Olivier, M. A comparison between double-injection axillary brachial plexus block and midhumeral block for emergency upper limb surgery. Anesth. Analg. 2006, 102, 1856–1858. [Google Scholar] [CrossRef] [PubMed]
- Koscielniak-Nielsen, Z.J.; Rotbøll Nielsen, P.; Risby Mortensen, C. A comparison of coracoid and axillary approaches to the brachial plexus. Acta Anaesthesiol. Scand. 2000, 44, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, B.S.; Koscielniak-Nielsen, Z.J.; Jacobsen, R.B.; Rasmussen, H.; Hesselbjerg, L. Procedural pain of an ultrasound-guided brachial plexus block: A comparison of axillary and infraclavicular approaches. Acta Anaesthesiol. Scand. 2010, 54, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Koscielniak-Nielsen, Z.J.; Rasmussen, H.; Hesselbjerg, L.; Nielsen, T.P.; Gürkan, Y. Infraclavicular block causes less discomfort than axillary block in ambulatory patients. Acta Anaesthesiol. Scand. 2005, 49, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Cemaloglu, S.; Ozkan, D.; Sayin, M.M. Injection optimization of infraclavicular and axillary brachial plexus block: Comparison of number of attempts and performance time. Reg. Anesth. Pain Med. 2018, ESRA8-0258, e113. [Google Scholar]
- Tran, D.Q.H.; Clemente, A.; Tran, D.Q.; Finlayson, R.J. A comparison between ultrasound-guided infraclavicular block using the ‘double bubble’ sign and neurostimulation-guided axillary block. Anesth. Analg. 2008, 107, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Lahori, V.U.; Raina, A.; Gulati, S.; Kumar, D.; Gupta, S.D. A randomized comparative study of efficacy of axillary and infraclavicular approaches for brachial plexus block for upper limb surgery using peripheral nerve stimulator. Indian J. Anaesth. 2011, 55, 253–259. [Google Scholar] [CrossRef]
- Dardon, J.; Castro, L.; Perez, A. A prospective and comparative study of three anesthetic techniques for the brachial plexus block. Rev. Mex. Anestesiol. 2000, 23, 7–10. [Google Scholar]
- Jones, C.P.; Chuan, A.; Sun, A.X. Anatomical variability of the musculocutaneous nerve as observed during ultrasound-guided axillary plexus nerve blocks. Anaesth. Intensive Care 2020, 48, 246–248. [Google Scholar] [CrossRef]
- Macfarlane, A.; Anderson, K. Infraclavicular brachial plexus blocks. Contin. Educ. Anaesth. Crit. Care Pain 2009, 9, 139–143. [Google Scholar] [CrossRef]
- Gauss, A.; Tugtekin, I.; Georgieff, M.; Dinse-Lambracht, A.; Keipke, D.; Gorsewski, G. Incidence of clinically symptomatic pneumothorax in ultrasound-guided infraclavicular and supraclavicular brachial plexus block. Anaesthesia 2014, 69, 327–336. [Google Scholar] [CrossRef]
- Walid, T.; Mondher, B.A.; Mohamed Anis, L.; Mustapha, F. A Case of Horner’s Syndrome following Ultrasound-Guided Infraclavicular Brachial Plexus Block. Case Rep. Anesthesiol. 2012, 2012, 125346. [Google Scholar] [CrossRef][Green Version]
- Chin, K.J.; Alakkad, H.; Adhikary, S.D.; Singh, M. Infraclavicular brachial plexus block for regional anaesthesia of the lower arm. Cochrane Database Syst. Rev. 2013, 8, 1–70. [Google Scholar] [CrossRef]
- Guay, J. Adverse events associated with intravenous regional anesthesia (Bier block): A systematic review of complications. J. Clin. Anesth. 2009, 21, 585–594. [Google Scholar] [CrossRef]
- Dekoninck, V.; Hoydonckx, Y.; Van de Velde, M.; Ory, J.P.; Dubois, J.; Jamaer, L.; Jalil, H.; Stessel, B. The analgesic efficacy of intravenous regional anesthesia with a forearm versus conventional upper arm tourniquet: A systematic review. BMC Anesthesiol. 2018, 18, 86. [Google Scholar] [CrossRef]
- Sauter, A.R.; Smith, H.J.; Stubhaug, A.; Dodgson, M.S.; Klaastad, Ø. Use of magnetic resonance imaging to define the anatomical location closest to all three cords of the infraclavicular brachial plexus. Anesth. Analg. 2006, 103, 1574–1576. [Google Scholar] [CrossRef]
- Tschaikowsky, K.; Hemmerling, T. Comparison of the effect of EMLA and semicircular subcutaneous anaesthesia in the prevention of tourniquet pain during plexus block anaesthesia of the arm. Anaesthesia 1998, 53, 390–393. [Google Scholar] [CrossRef]
- Boselli, E.; Hopkins, P.; Lamperti, M.; Estèbe, J.-P.; Fuzier, R.; Biasucci, D.G.; Disma, N.; Pittiruti, M.; Traskaite, V.; Macas, A.; et al. European Society of Anaesthesiology and Intensive Care Guidelines on peri-operative use of ultrasound for regional anaesthesia (PERSEUS regional anesthesia): Peripheral nerves blocks and neuraxial anaesthesia. Eur. J. Anaesthesiol. 2021, 38, 219–250. [Google Scholar] [CrossRef]
- Neal, J.M.; Brull, R.; Horn, J.L.; Liu, S.S.; McCartney, C.J.L.; Perlas, A.; Salinas, F.V.; Tsui, B.C. The Second American Society of Regional Anesthesia and Pain Medicine Evidence-Based Medicine Assessment of Ultrasound-Guided Regional Anesthesia: Executive Summary. Reg. Anesth. Pain Med. 2016, 41, 181–194. [Google Scholar] [CrossRef]
- Dohlman, L.E.; Kwikiriza, A.; Ehie, O. Benefits and Barriers to Increasing Regional Anesthesia in Resource-Limited Settings. Local Reg. Anesth. 2020, 13, 147–158. [Google Scholar] [CrossRef]
- Thomas, K.; Sajjad, H.; Bordoni, B. Anatomy, Shoulder and Upper Limb, Medial Brachial Cutaneous Nerve. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Herekar, R.; Bordoni, B.; Daly, D.T. Anatomy, Shoulder and Upper Limb, Intercostobrachial Nerves. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
| Author | Type of ABPB | First Intervention | Second Intervention | Group Numbers | Mean Age (years) | Male/Female Ratio | Surgery (Elbow/Forearm/ Wrist–Hand) | |
|---|---|---|---|---|---|---|---|---|
| 1 | Tran et al., 2009 [15] | US-guided ABPB | US-guided ICB | US-guided SCB | 40/40/40 | 51/42/40 | 71/49 | 11/34/75 |
| 2 | Frederiksen et al., 2010 [36] | US-guided ABPB | US-guided ICB | 40/40 | 50/50 | 36/44 | 4/21/55 | |
| 3 | Song et al., 2011 [18] | US-guided ABPB | US-guided ICB | 11/11 | 49.5/37.9 | 16/6 | 0/22/0 | |
| 4 | López-Morales et al., 2013 [27] | US-guided ABPB | US-guided ICB | 40/42 | 58.7/52.9 | 32/50 | 15/11/56 | |
| 5 | Boivin et al., 2016 [23] | US-guided ABPB | US-guided ICB | 112/112 | 48/52 | 145/79 | 11/6/207 | |
| 6 | Stav et al., 2016 [16] | US-guided ABPB | US-guided ICB | US-guided SCB | 34/23/37 | 60/63/63 | 48/46 | NA |
| 7 | Vazin et al., 2016 [17] | US-guided ABPB | US-guided ICB | US-guided SCB | 40/40/40 | 60/52/59 | NA | 0/30/90 |
| 8 | Cemaloglu et al., 2018 [38] | US-guided ABPB | US-guided ICB | 33/33 | NA | NA | NA | |
| 9 | Brenner et al., 2019 [19] | US-guided ABPB | US-guided ICB | 40/42 | 51.9/54.5 | 33/49 | 0/70/11 | |
| 10 | Tran et al., 2008 [39] | NS-guided ABPB | US-guided ICB | 35/35 | 46/50 | 47/23 | 1/27/42 | |
| 11 | Tedore et al., 2009 [24] | Trans-arterial ABPB | US-guided ICB | 109/111 | 51/49 | 110/110 | NA | |
| 12 | Kapral et al. 1999 [25] | NS-guided ABPB | NS-guided ICB | 20/20 | 48/46 | 22/18 | NA | |
| 13 | Deleuze et al., 2003 [31] | NS-guided ABPB | NS-guided ICB | 50/50 | 45/47 | 56/44 | NA | |
| 14 | Ertug et al., 2005 [28] | NS-guided ABPB | NS-guided ICB | 15/15 | 38.1/27 | NA | NA | |
| 15 | Koscielniak-N et al., 2005 [37] | NS-guided ABPB | NS-guided ICB | 40/40 | 45/49 | 48/32 | NA | |
| 16 | Rettig et al., 2005 [32] | NS-guided ABPB | NS-guided ICB | 30/30 | 45/59 | 26/34 | NA/NA/26 | |
| 17 | Lahori et al., 2011 [40] | NS-guided ABPB | NS-guided ICB | 30/30 | NA | NA | NA | |
| 18 | Kapral et al. 1994 [29] | US-guided ABPB | US-guided SCB | 20/20 | NA | NA | NA | |
| 19 | Karmakar et al., 2012 [33] | US-guided ABPB | US-guided SCB | 15/16 | NA | NA | -/-/31 | |
| 20 | Arnuntasupakul et al., 2015 [20] | US-guided ABPB | US-guided SCB | 20/20 | 45.6/42.6 | 20/20 | -/3/37 | |
| 21 | Hussien et al., 2018 [30] | US-guided ABPB | US-guided SCB | 40/40 | 42.7/45.5 | 41/39 | -/-/80 | |
| 22 | Singh et al., 2010 [21] | NS-guided ABPB | NS-guided SCB | ABPB + SCB | 25/25/25 | 33.5/35.9/30.8 | 60/15 | NA |
| 23 | Fleck et al. 1994 [26] | Paraesthesia ABPB | NS-guided SCB | 20/20 | 43.4/51.8 | 33/7 | NA | |
| 24 | Dardon et al., 2000 [41] | ABPB (NOS) | SCB (NOS) | CE | 26/20/30 | 29/30/28 | 44/32 | NA |
| 25 | Koscielniak-N. et al., 2000 [35] | NS-guided ABPB | NS-guided CB | 29/30 | 49/55 | 40/19 | NA | |
| 26 | Bouaziz et al. 1997 [22] | NS-guided ABPB | NS-guided MHB | 28/32 | 42/48 | - | 0/8/52 | |
| 27 | Fuzier et al., 2006 [34] | NS-guided ABPB | NS-guided MHB | 45/45 | 36/40 | 65/25 | 0/5/85 | |
| 28 | Teunkens et al., 2020 [1] | US-guided ABPB | IVRA | 60/60 | 50/53 | 57/63 | 0/0/120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nijs, K.; Hertogen, P.‘s.; Buelens, S.; Coppens, M.; Teunkens, A.; Jalil, H.; Van de Velde, M.; Al Tmimi, L.; Stessel, B. Axillary Brachial Plexus Block Compared with Other Regional Anesthesia Techniques in Distal Upper Limb Surgery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 3185. https://doi.org/10.3390/jcm13113185
Nijs K, Hertogen P‘s, Buelens S, Coppens M, Teunkens A, Jalil H, Van de Velde M, Al Tmimi L, Stessel B. Axillary Brachial Plexus Block Compared with Other Regional Anesthesia Techniques in Distal Upper Limb Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(11):3185. https://doi.org/10.3390/jcm13113185
Chicago/Turabian StyleNijs, Kristof, Pieter ‘s Hertogen, Simon Buelens, Marc Coppens, An Teunkens, Hassanin Jalil, Marc Van de Velde, Layth Al Tmimi, and Björn Stessel. 2024. "Axillary Brachial Plexus Block Compared with Other Regional Anesthesia Techniques in Distal Upper Limb Surgery: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 11: 3185. https://doi.org/10.3390/jcm13113185
APA StyleNijs, K., Hertogen, P. ‘s., Buelens, S., Coppens, M., Teunkens, A., Jalil, H., Van de Velde, M., Al Tmimi, L., & Stessel, B. (2024). Axillary Brachial Plexus Block Compared with Other Regional Anesthesia Techniques in Distal Upper Limb Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(11), 3185. https://doi.org/10.3390/jcm13113185






