Piezoelectric Bone Conduction Hearing Implant: A Case Series of Audiological, Surgical and Patient-Reported Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Outcome Measurements
2.2. Subjects
2.3. Surgical Technique and Processor Fitting
2.4. Statistics
3. Results
3.1. Peri- and Postoperative Complications
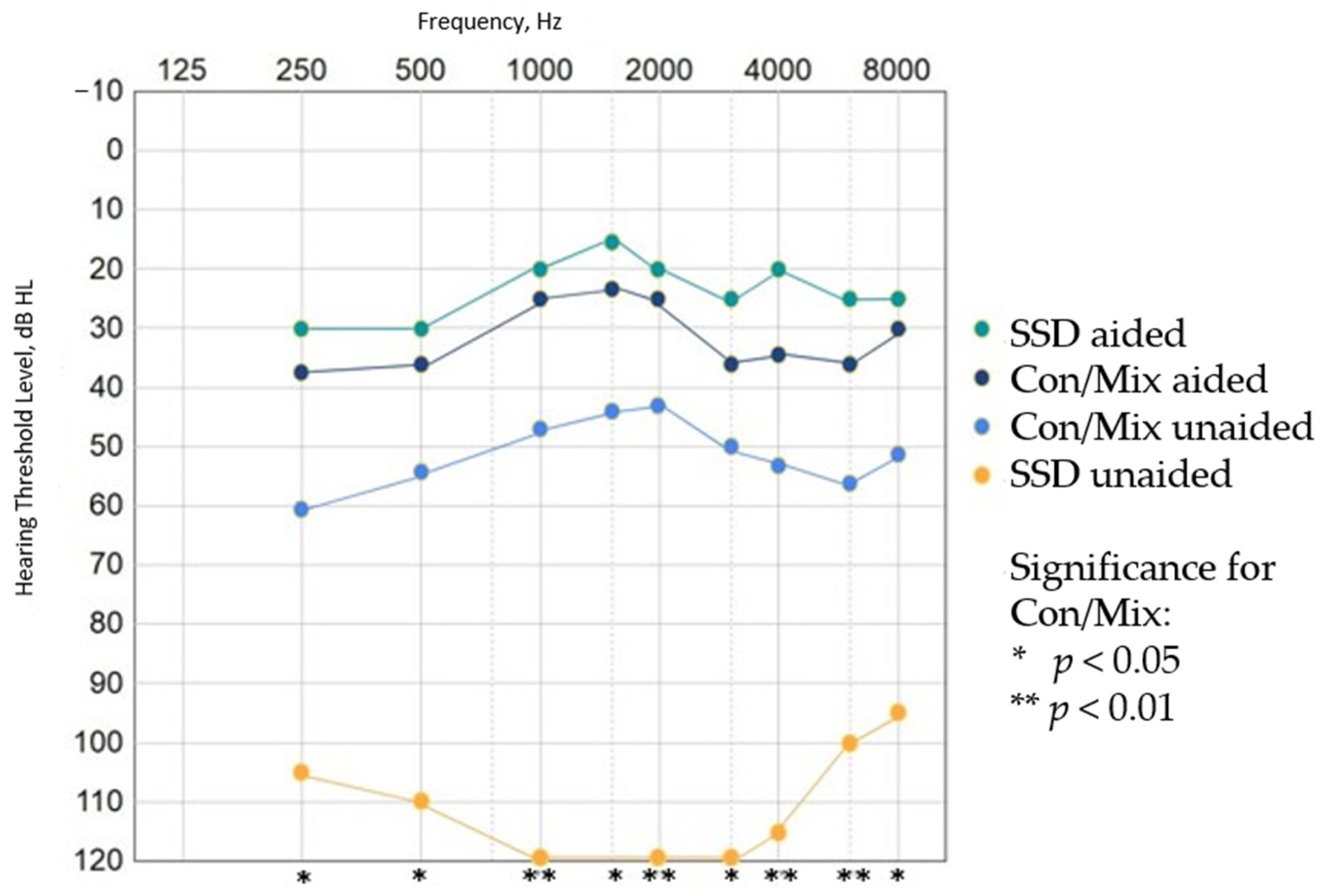
3.2. Audiological Results
3.3. Verification and Variability
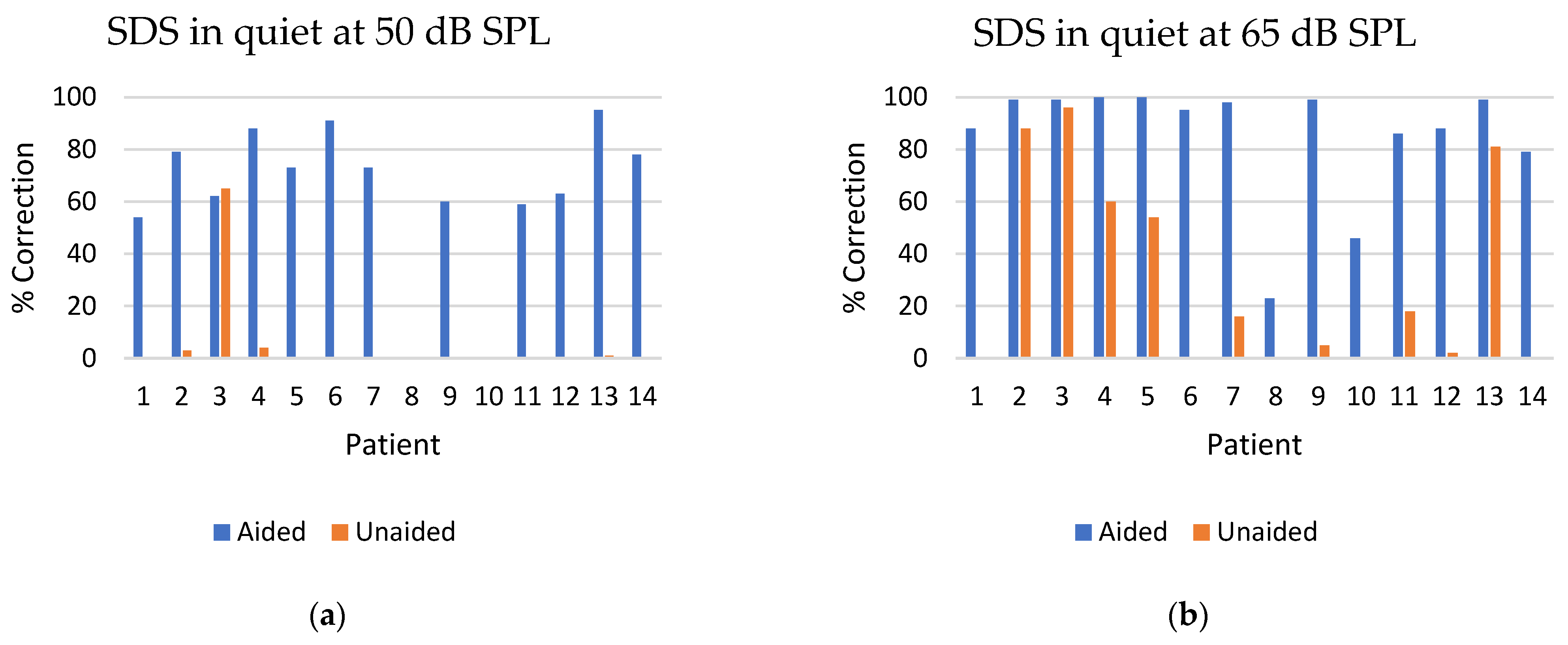
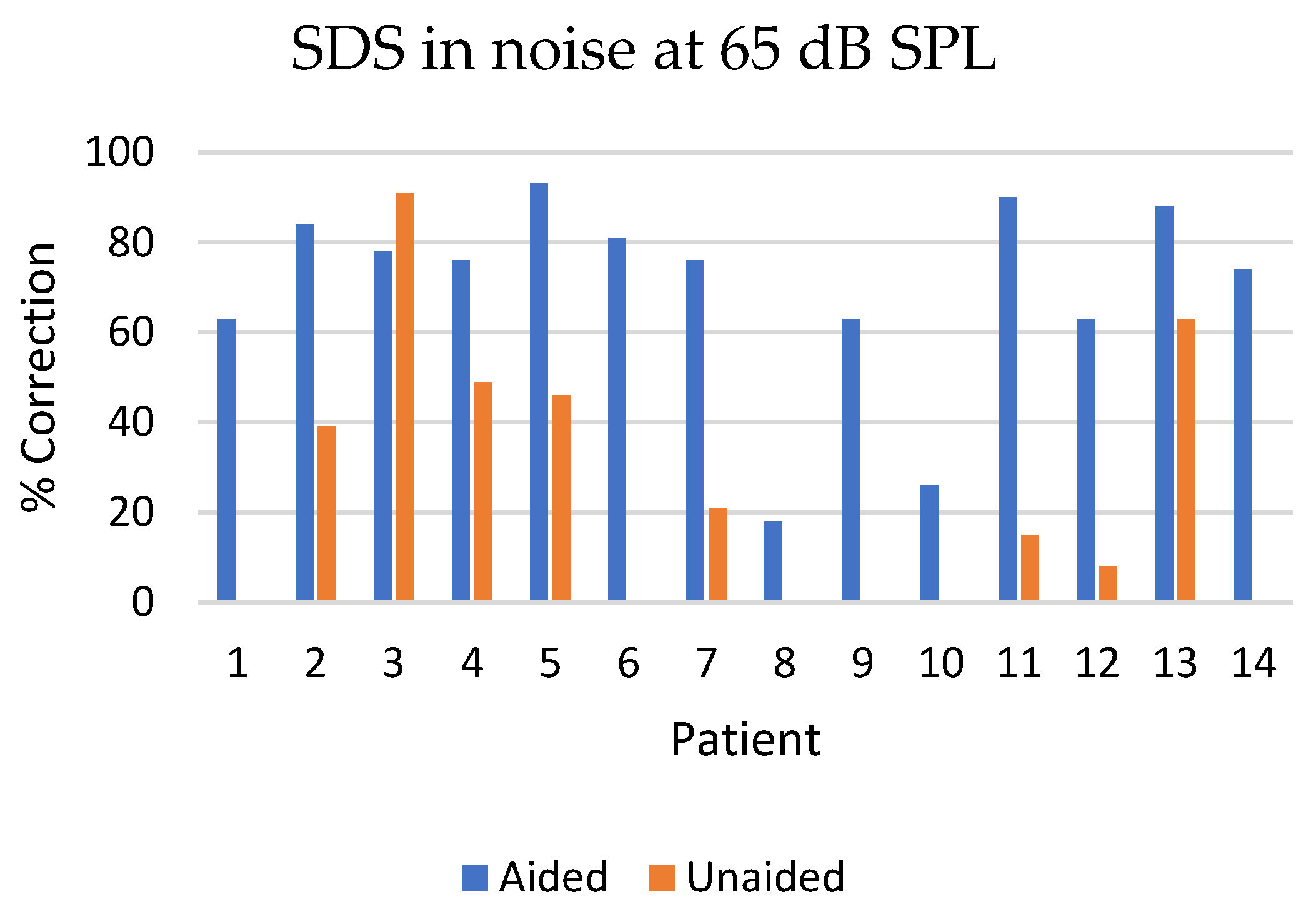
3.4. Speech Discrimination Score
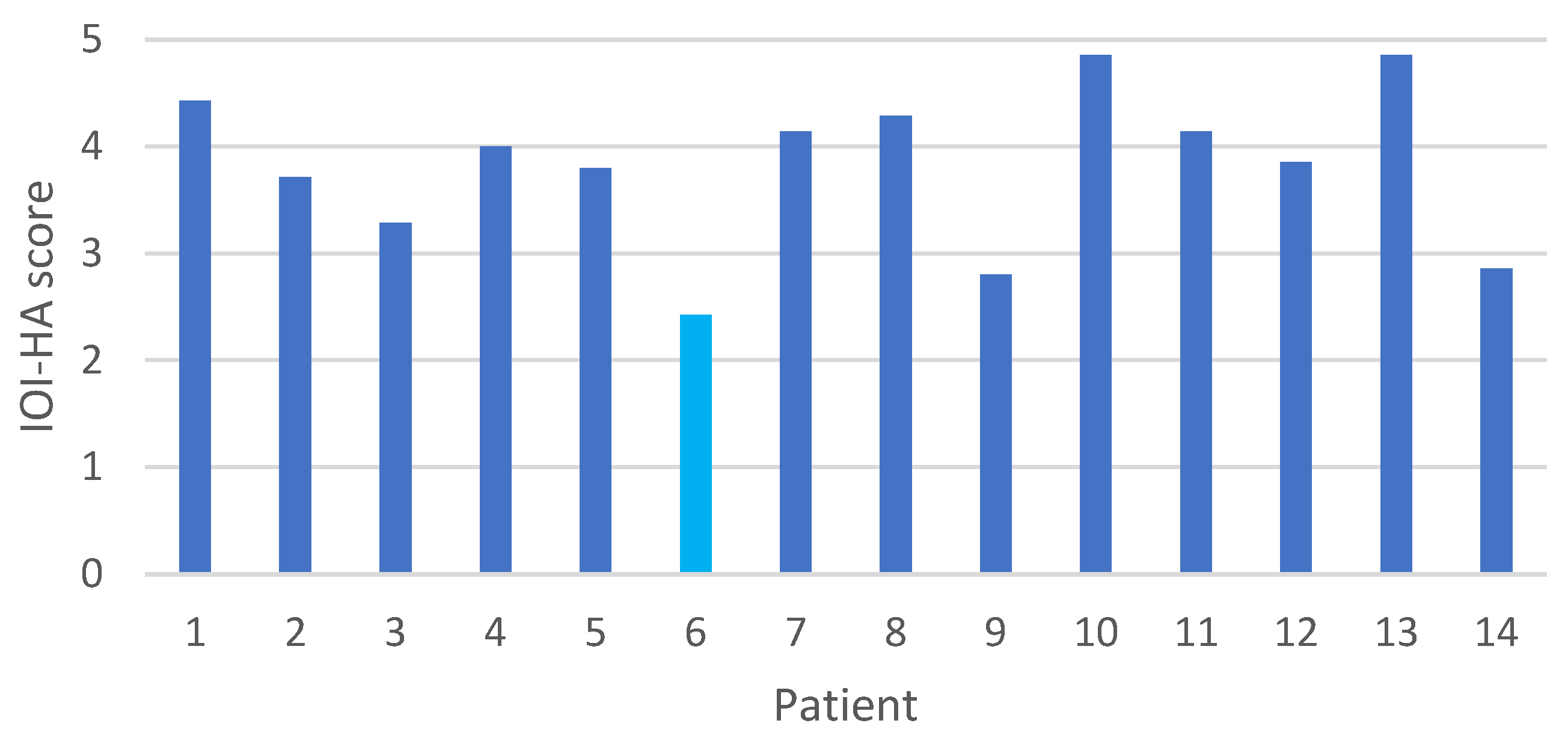
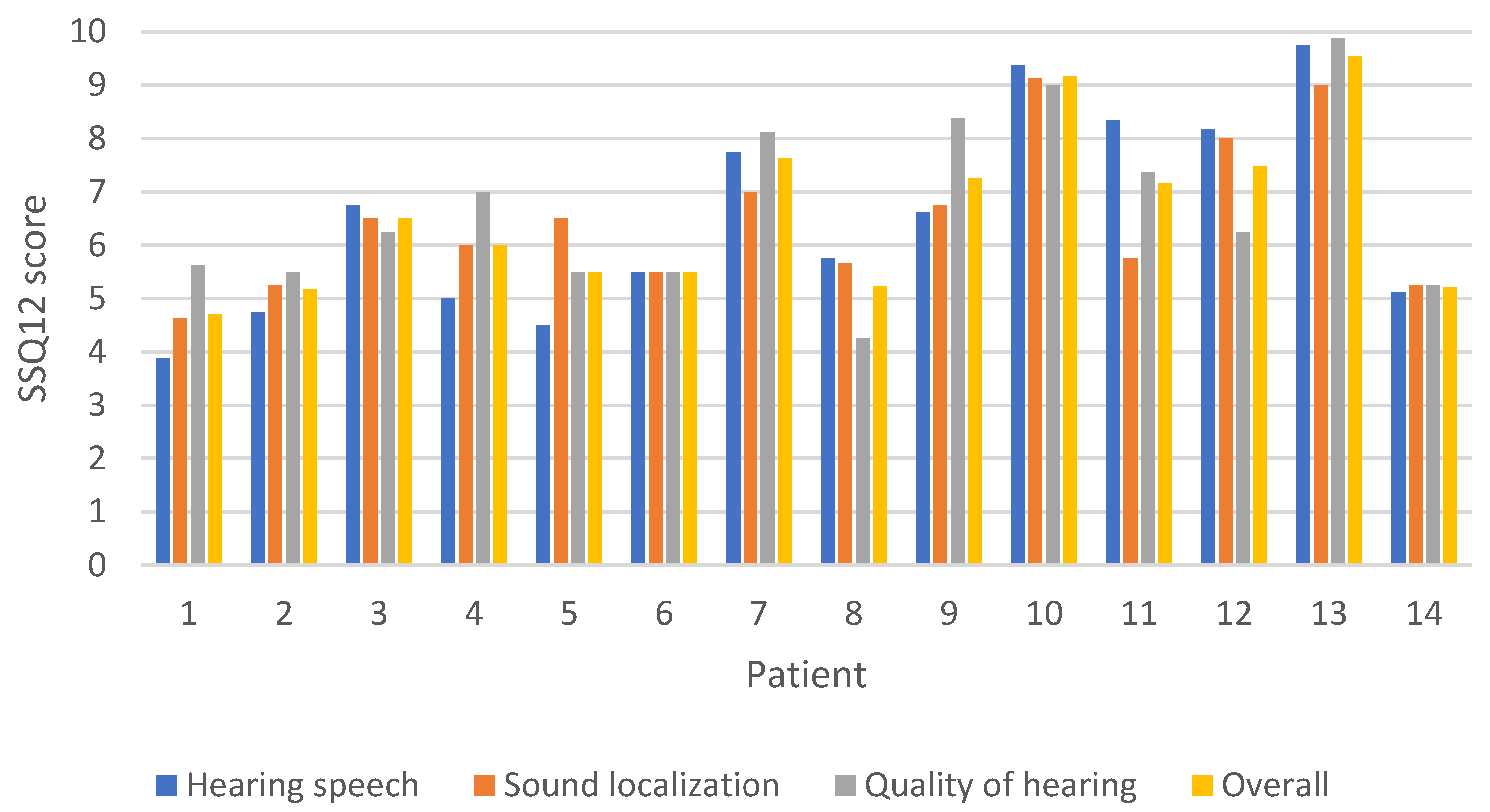
3.5. Questionnaire Results
4. Discussion
4.1. Surgical Efficacy
4.2. Audiological Benefit
4.3. Patient-Reported Outcome Measures
4.4. Strengths and Limitations
4.5. Music Perception and Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goycoolea, M.; Ribalta, G.; Tocornal, F.; Levy, R.; Alarcón, P.; Bryman, M.; Cagnacci, B.; Catenacci, C.; Oyanguren, V.; Vilches, I.; et al. Clinical Performance of the OsiaTM System, a New Active Osseointegrated Implant System. Results from a Prospective Clinical Investigation. Acta Otolaryngol. 2020, 140, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Sprinzl, G.; Toner, J.; Koitschev, A.; Berger, N.; Keintzel, T.; Rasse, T.; Baumgartner, W.-D.; Honeder, C.; Magele, A.; Plontke, S.; et al. Multicentric Study on Surgical Information and Early Safety and Performance Results with the Bonebridge BCI 602: An Active Transcutaneous Bone Conduction Hearing Implant. Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. EUFOS Affil. Ger. Soc. Oto-Rhino-Laryngol.—Head Neck Surg. 2023, 280, 1565–1579. [Google Scholar] [CrossRef] [PubMed]
- Key, S.; Mohamed, N.; Da Cruz, M.; Kong, K.; Hasan, Z. Systematic Review and Meta-Analysis of a New Active Transcutaneous Bone Conduction Implant. Laryngoscope 2023, 134, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Marszał, J.; Gibasiewicz, R.; Błaszczyk, M.; Gawłowska, M.; Gawęcki, W. Piezoelectric Bone Conduction Hearing Implant Osia®—Audiological and Quality of Life Benefits. Otolaryngol. Pol. Pol. Otolaryngol. 2021, 75, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.M.; Sim, J.H.; Xie, Y.Z.; Chatzimichalis, M.; Ullrich, O.; Röösli, C. The Bonebridge: Preclinical Evaluation of a New Transcutaneously-Activated Bone Anchored Hearing Device. Hear. Res. 2013, 301, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Gawęcki, W.; Gibasiewicz, R.; Marszał, J.; Błaszczyk, M.; Gawłowska, M.; Wierzbicka, M. The Evaluation of a Surgery and the Short-Term Benefits of a New Active Bone Conduction Hearing Implant—The Osia®. Braz. J. Otorhinolaryngol. 2022, 88, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Alzhrani, F. Objective and Subjective Results of the Bonebridge Transcutaneous Active Direct-Drive Bone Conduction Hearing Implant. Saudi Med. J. 2019, 40, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Cywka, K.B.; Skarzynski, P.H.; Krol, B.; Hatzopoulos, S.; Skarzynski, H. Evaluation of the Bonebridge BCI 602 Active Bone Conductive Implant in Adults: Efficacy and Stability of Audiological, Surgical, and Functional Outcomes. Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. EUFOS Affil. Ger. Soc. Oto-Rhino-Laryngol.—Head Neck Surg. 2022, 279, 3525–3534. [Google Scholar] [CrossRef] [PubMed]
- Rauch, A.-K.; Wesarg, T.; Aschendorff, A.; Speck, I.; Arndt, S. Long-Term Data of the New Transcutaneous Partially Implantable Bone Conduction Hearing System Osia®. Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. EUFOS Affil. Ger. Soc. Oto-Rhino-Laryngol.—Head Neck Surg. 2022, 279, 4279–4288. [Google Scholar] [CrossRef]
- Eberhard, K.E.; Olsen, S.Ø.; Miyazaki, H.; Bille, M.; Caye-Thomasen, P. Objective and Subjective Outcome of a New Transcutaneous Bone Conduction Hearing Device: Half-Year Follow-up of the First 12 Nordic Implantations. Otol. Neurotol. 2016. Publish Ahead of Print. [Google Scholar] [CrossRef]
- Elberling, C.; Ludvigsen, C.; Lyregaard, P.E. Dantale: A New Danish Speech Material. Scand. Audiol. 1989, 18, 169–175. [Google Scholar] [CrossRef]
- Cox, R.M.; Alexander, G.C. The International Outcome Inventory for Hearing Aids (IOI-HA): Psychometric Properties of the English Version: El Inventario International de Resultados Para Auxiliares Auditivos (IOI-HA): Propiedades Psicometricas de La Version En Ingles. Int. J. Audiol. 2002, 41, 30–35. [Google Scholar] [CrossRef]
- Thunberg Jespersen, C.; Bille, M.; Legarth, J.V. Psychometric Properties of a Revised Danish Translation of the International Outcome Inventory for Hearing Aids (IOI-HA). Int. J. Audiol. 2014, 53, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Lassaletta, L.; Calvino, M.; Zernotti, M.; Gavilán, J. Postoperative Pain in Patients Undergoing a Transcutaneous Active Bone Conduction Implant (Bonebridge). Eur. Arch. Otorhinolaryngol. 2016, 273, 4103–4110. [Google Scholar] [CrossRef] [PubMed]
- Szabo, L.; Nagy, R.; Posta, B.; Kiss, J.G.; Rovo, L.; Bere, Z. BAHA Attract to Osia Conversion Patients: Comparison of the Two Systems and Long-Term Outcomes. J. Laryngol. Otol. 2023, 137, 757–762. [Google Scholar] [CrossRef]
- Crowder, H.R.; Bestourous, D.E.; Reilly, B.K. Adverse Events Associated with Bonebridge and Osia Bone Conduction Implant Devices. Am. J. Otolaryngol. 2021, 42, 102968. [Google Scholar] [CrossRef] [PubMed]
- Cowan, R.; Lewis, A.T.; Hallberg, C.; Tong, M.C.F.; Birman, C.S.; Ng, I.H.-Y.; Briggs, R. Clinical Performance, Safety, and Patient-Reported Outcomes of an Active Osseointegrated Bone-Conduction Hearing Implant System at 24-Month Follow-Up. Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. EUFOS Affil. Ger. Soc. Oto-Rhino-Laryngol.—Head Neck Surg. 2023, 281, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.; Birman, C.S.; Baulderstone, N.; Lewis, A.T.; Ng, I.H.Y.; Östblom, A.; Rousset, A.; Tari, S.; Tong, M.C.F.; Cowan, R. Clinical Performance, Safety, and Patient-Reported Outcomes of an Active Osseointegrated Steady-State Implant System. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2022, 43, 827–834. [Google Scholar] [CrossRef]
- Florentine, M.M.; Virbalas, J.; Chan, D.K. Early Surgical and Audiologic Outcomes of Active, Transcutaneous, Osseointegrated Bone-Conduction Hearing Device (Osia 2® System) Placement. Int. J. Pediatr. Otorhinolaryngol. 2022, 156, 111114. [Google Scholar] [CrossRef]
- Lau, K.; Scotta, G.; Wright, K.; Proctor, V.; Greenwood, L.; Dawoud, M.; Ray, J. First United Kingdom Experience of the Novel Osia Active Transcutaneous Piezoelectric Bone Conduction Implant. Eur. Arch. Otorhinolaryngol. 2020, 277, 2995–3002. [Google Scholar] [CrossRef]
- Goldstein, M.R.; Bourn, S.; Jacob, A. Early Osia® 2 Bone Conduction Hearing Implant Experience: Nationwide Controlled-Market Release Data and Single-Center Outcomes. Am. J. Otolaryngol. 2021, 42, 102818. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choe, G.; Oh, H.; Choi, B.Y. A Comparative Study of Audiological Outcomes and Compliance between the Osia System and Other Bone Conduction Hearing Implants. Eur. Arch. Otorhinolaryngol. 2023, 280, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Frosolini, A.; Badin, G.; Sorrentino, F.; Brotto, D.; Pessot, N.; Fantin, F.; Ceschin, F.; Lovato, A.; Coppola, N.; Mancuso, A.; et al. Application of Patient Reported Outcome Measures in Cochlear Implant Patients: Implications for the Design of Specific Rehabilitation Programs. Sensors 2022, 22, 8770. [Google Scholar] [CrossRef] [PubMed]
- Jiam, N.T.; Formeister, E.J.; Chari, D.A.; David, A.P.; Alsoudi, A.F.; Purnell, S.; Jiradejvong, P.; Limb, C.J. Music Perception in Bone-Anchored Hearing Implant Users. Laryngoscope 2024, 134, 1381–1387. [Google Scholar] [CrossRef] [PubMed]

| Patient | Sex | Age at Surgery (Y) | Type of Hearing Loss on the Implanted Side | Etiology | Implanted Ear | Type of Hearing Loss on the Non-Implanted Side | Follow-Up (Mo) | AC, Implanted Ear, (PTA4) (dB HL) | BC, Implanted Ear, (PTA4) (dB HL) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 51 | Mixed | Otitis chronica | sin. | Bilateral mixed | 18 | 40, 20, 20, 45 (31) | 20, 20, 30, 45 (29) |
| 2 | M | 46 | Conductive | Cholesteatoma bilat. | sin. | Bilateral conductive | 16 | 30, 10, 20, 40 (25) | 10, 15, 15, 25 (16) |
| 3 | F | 52 | Conductive | Cholesteatoma | sin. | Normal hearing | 15 | 55, 25, 25, 35 (35) | 25, 10, 20, 20 (19) |
| 4 | M | 19 | Conductive | Cholesteatoma | sin. | Normal hearing | 15 | 35, 20, 25, 20 (25) | 10, −5, 0, 10 (4) |
| 5 | F | 42 | Conductive | Cholesteatoma | dxt. | Normal hearing | 15 | 30, 15, 10, 20 (19) | −10, 0, 0, 10 (0) |
| 6 | F | 29 | aSSD | Complication after tympanoplasty | dxt. | Normal hearing | 12 | Anacusis | Anacusis |
| 7 | M | 17 | Conductive | Microtia | dxt. | Normal hearing | 12 | 35, 30, 15, 15 (24) | 5, 15, 10, 5 (9) |
| 8 | F | 63 | Mixed | Otitis chronica | dxt. | Bilateral mixed | 12 | 50, 45, 60, 75 (58) | 30, 35, 55, 60 (45) |
| 9 | F | 48 | Conductive | Cholesteatoma | sin. | Normal hearing. | 8 | 45, 25, 20, 40 (33) | 20, 35, 20, 25 (25) |
| 10 | F | 65 | Mixed | Cholesteatoma | dxt. | Bilateral mixed | 10 | 55, 50, 50, 60 (54) | 25, 40, 55, 60 (45) |
| 11 | F | 31 | Conductive | Aural atresia | dxt. | Normal hearing | 7 | 35, 20, 25, 25 (26) | 15, 0, 10, 10 (9) |
| 12 | M | 49 | Conductive | Invasive invert papilloma | dxt. | Normal hearing | 7 | 25, 20, 20, 25 (23) | 5, 14, 10, 10 (10) |
| 13 | F | 51 | Conductive | Otosclerosis | sin. | Normal hearing | 3 | 35, 20, 15, 25 (24) | 0, 5, 0, −5 (0) |
| 14 | F | 21 | Conductive | Microtia | dxt. | Normal hearing | 1 (b16) | 30, 20, 20, 20 (22.5) | 10, 10, 10, 5 (9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagle, M.; Bille, M.; Gordon Jensen, R. Piezoelectric Bone Conduction Hearing Implant: A Case Series of Audiological, Surgical and Patient-Reported Outcomes. J. Clin. Med. 2024, 13, 3111. https://doi.org/10.3390/jcm13113111
Vagle M, Bille M, Gordon Jensen R. Piezoelectric Bone Conduction Hearing Implant: A Case Series of Audiological, Surgical and Patient-Reported Outcomes. Journal of Clinical Medicine. 2024; 13(11):3111. https://doi.org/10.3390/jcm13113111
Chicago/Turabian StyleVagle, Mai, Michael Bille, and Ramon Gordon Jensen. 2024. "Piezoelectric Bone Conduction Hearing Implant: A Case Series of Audiological, Surgical and Patient-Reported Outcomes" Journal of Clinical Medicine 13, no. 11: 3111. https://doi.org/10.3390/jcm13113111
APA StyleVagle, M., Bille, M., & Gordon Jensen, R. (2024). Piezoelectric Bone Conduction Hearing Implant: A Case Series of Audiological, Surgical and Patient-Reported Outcomes. Journal of Clinical Medicine, 13(11), 3111. https://doi.org/10.3390/jcm13113111





