Analysis of Patient Outcomes following Curative R0 Multiorgan Resections for Locally Advanced Gastric Cancer: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
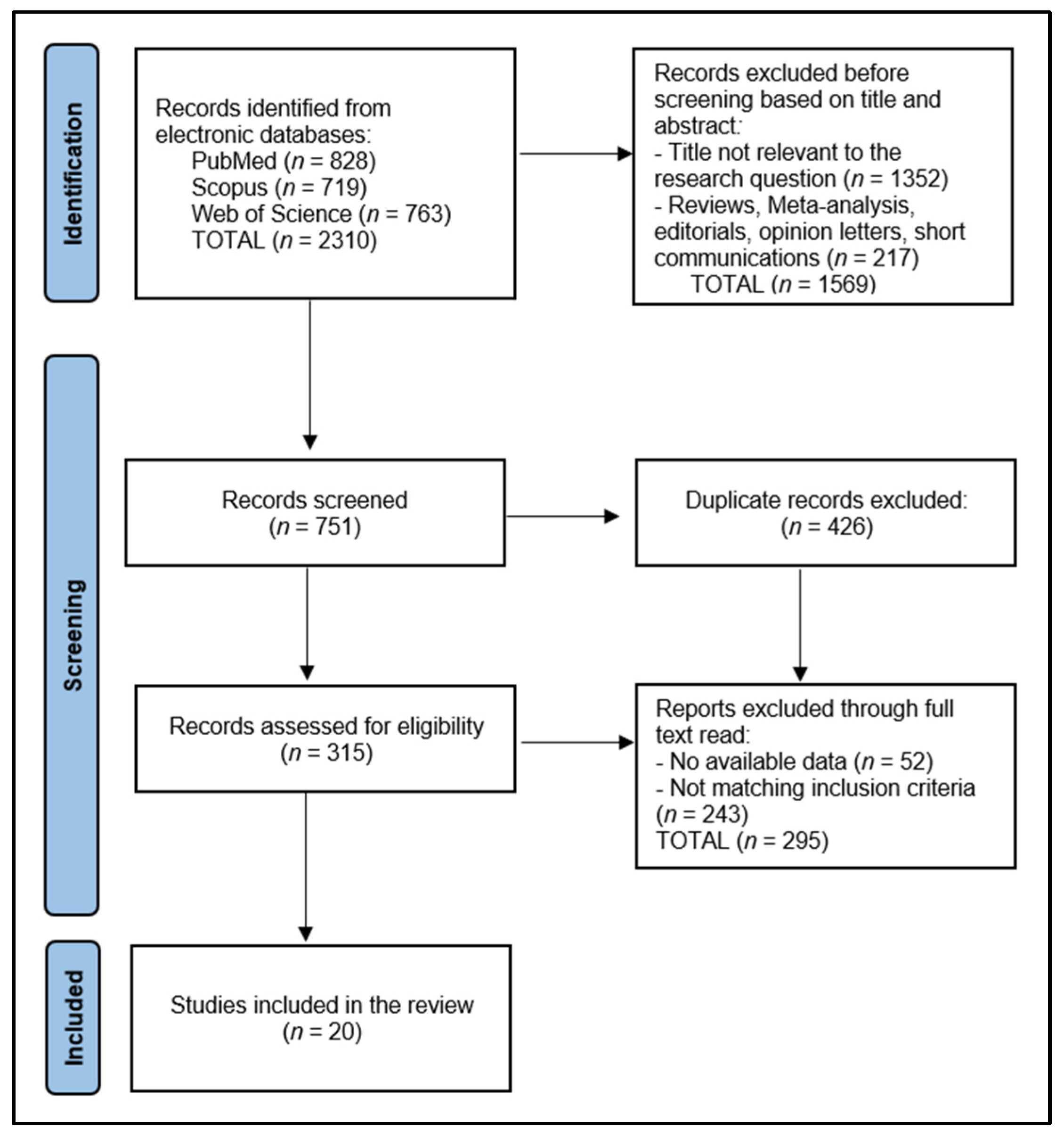
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Risk of Bias and Quality Assessment
2.8. Synthesis Methods
3. Results
3.1. Study Selection and Study Characteristics
3.2. Results of Individual Studies
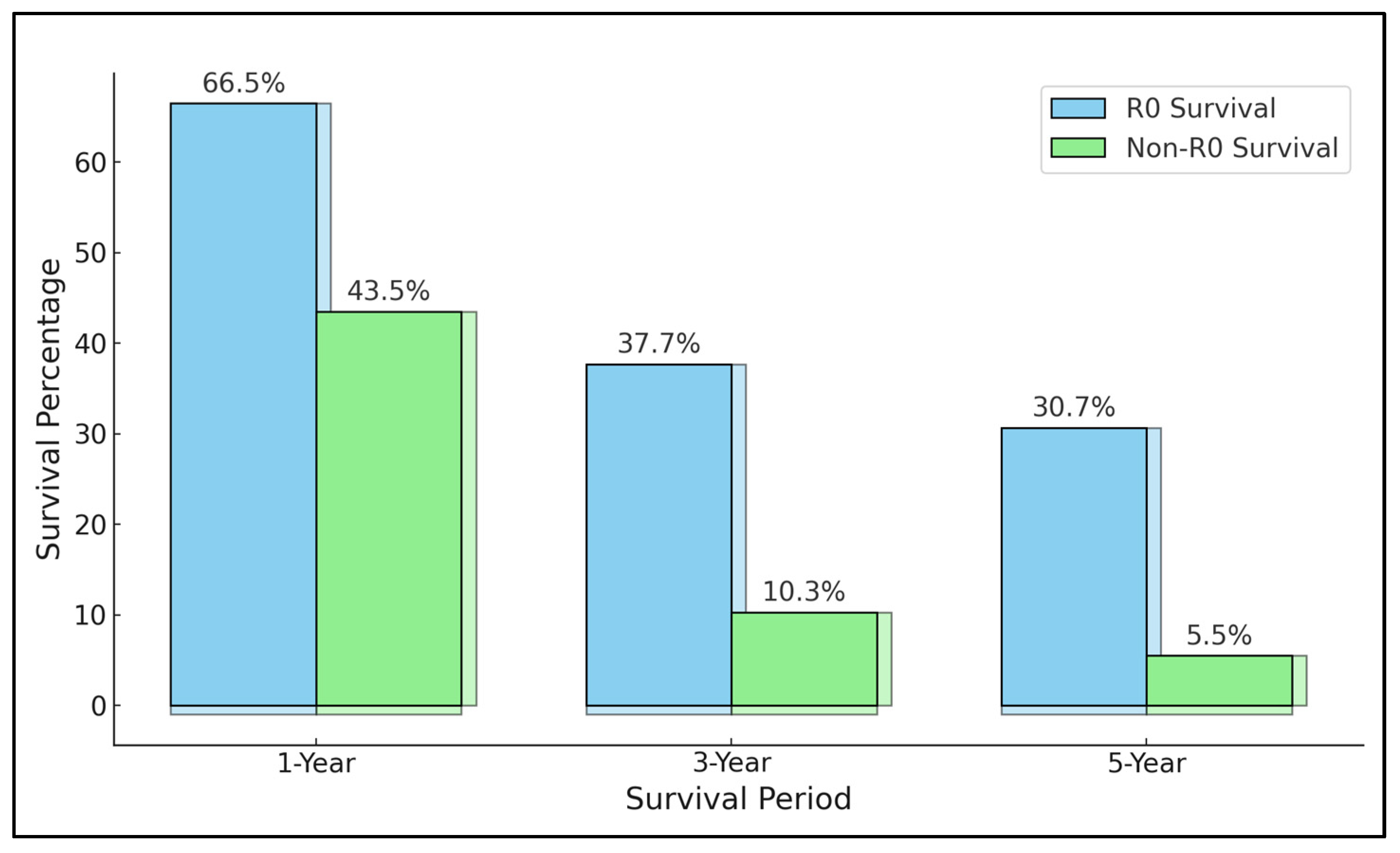
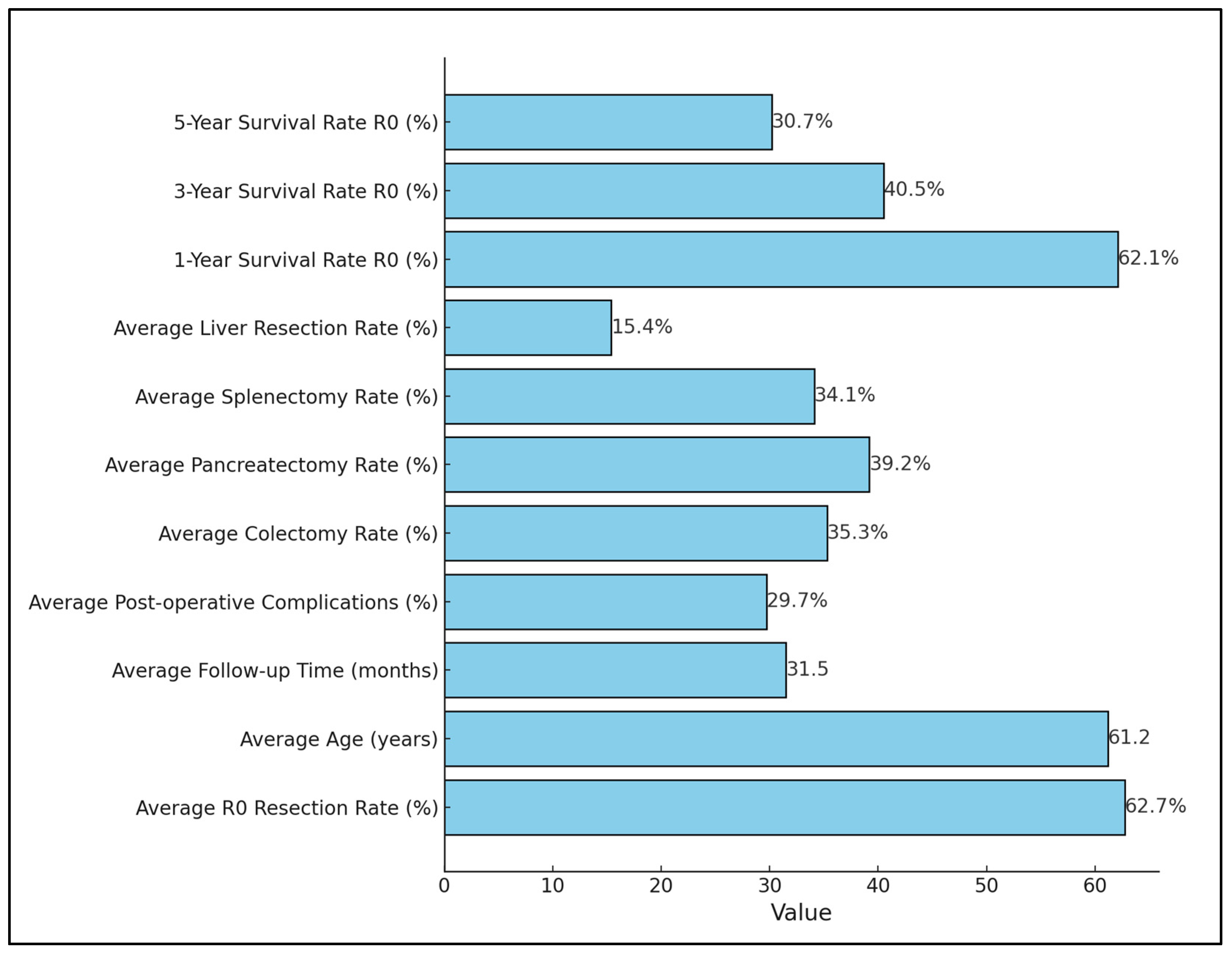
3.3. Results of Synthesis
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zurlo, I.V.; Basso, M.; Strippoli, A.; Calegari, M.A.; Orlandi, A.; Cassano, A.; Di Salvatore, M.; Garufi, G.; Bria, E.; Tortora, G.; et al. Treatment of Locally Advanced Gastric Cancer (LAGC): Back to Lauren’s Classification in Pan-Cancer Analysis Era? Cancers 2020, 12, 1749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yeh, J.H.; Yeh, Y.S.; Tsai, H.L.; Huang, C.W.; Chang, T.K.; Su, W.C.; Wang, J.Y. Neoadjuvant Chemoradiotherapy for Locally Advanced Gastric Cancer: Where Are We at? Cancers 2022, 14, 3026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, J.; Lu, X.; Liu, Q.; Fu, Y.; Liu, S.; Zhao, Y.; Zhou, J.; Chen, H.; Wang, M.; Li, L.; et al. Neoadjuvant sintilimab in combination with concurrent chemoradiotherapy for locally advanced gastric or gastroesophageal junction adenocarcinoma: A single-arm phase 2 trial. Nat. Commun. 2023, 14, 4904. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, A.; Zheng, S.; Chen, C.; Lyu, J. Recent Estimates and Predictions of 5-Year Survival in Patients with Gastric Cancer: A Model-Based Period Analysis. Cancer Control. 2022, 29, 10732748221099227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mukkamalla, S.K.R.; Recio-Boiles, A.; Babiker, H.M. Gastric Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459142/ (accessed on 13 November 2023).
- Biondi, A.; Persiani, R.; Cananzi, F.; Zoccali, M.; Vigorita, V.; Tufo, A.; D’Ugo, D. R0 resection in the treatment of gastric cancer: Room for improvement. World J. Gastroenterol. 2010, 16, 3358–3370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brar, S.S.; Seevaratnam, R.; Cardoso, R.; Yohanathan, L.; Law, C.; Helyer, L.; Coburn, N.G. Multivisceral resection for gastric cancer: A systematic review. Gastric Cancer 2012, 15 (Suppl. 1), S100–S107. [Google Scholar] [CrossRef] [PubMed]
- Arndt, M.; Lippert, H.; Croner, R.S.; Meyer, F.; Otto, R.; Ridwelski, K. Multivisceral resection of advanced colon and rectal cancer: A prospective multicenter observational study with propensity score analysis of the morbidity, mortality, and survival. Innov. Surg. Sci. 2023, 8, 61–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Purkayastha, J.; Singh, P.R.; Talukdar, A.; Das, G.; Yadav, J.; Bannoth, S. Feasibility and Outcomes of Multivisceral Resection in Locally Advanced Colorectal Cancer: Experience of a Tertiary Cancer Center in North-East India. Ann. Coloproctol. 2021, 37, 174–178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ilic, M.; Ilic, I. Epidemiology of stomach cancer. World J. Gastroenterol. 2022, 28, 1187–1203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, W.J.; Zhao, H.P.; Yu, Y.; Wang, J.H.; Guo, L.; Liu, J.Y.; Pu, J.; Lv, J. Updates on global epidemiology, risk and prognostic factors of gastric cancer. World J. Gastroenterol. 2023, 29, 2452–2468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, Q.; Shu, L.; Zhou, F.; Chen, L.P.; Feng, Y.L. Adherence to the Mediterranean diet and risk of gastric cancer: A systematic review and dose-response meta-analysis. Front. Nutr. 2023, 10, 1259453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duan, Y.; Xu, D. Microsatellite instability and immunotherapy in gastric cancer: A narrative review. Precis. Clin. Med. 2023, 10, pcm-22-48. [Google Scholar] [CrossRef]
- Radovanović, D.; Stevanović, D.; Pavlović, I.; Bajec, A.; Vekić, B.; Mitrović, N. Vrednost multiorganske resekcije kod bolesnika sa karcinomom zeluca [Multiorgan resection in patients with gastric cancer]. Med. Pregl. 2004, 57, 480–486. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gullo, I.; Grillo, F.; Mastracci, L.; Vanoli, A.; Carneiro, F.; Saragoni, L.; Limarzi, F.; Ferro, J.; Parente, P.; Fassan, M. Precancerous lesions of the stomach, gastric cancer and hereditary gastric cancer syndromes. Pathologica 2020, 112, 166–185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iyer, P.; Moslim, M.; Farma, J.M.; Denlinger, C.S. Diffuse gastric cancer: Histologic, molecular, and genetic basis of disease. Transl. Gastroenterol. Hepatol. 2020, 5, 52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhuang, A.; Chen, Y.; Wang, J.; Xu, J.; Tong, H.; Zhou, Y.; Zhang, Y.; Lu, W. Multivisceral resection of primary multifocal retroperitoneal sarcomas: A retrospective study from a high-volume sarcoma center. J. Clin. Transl. Res. 2023, 9, 101–109. [Google Scholar] [PubMed] [PubMed Central]
- Yamamoto, M.; Rashid, O.M.; Wong, J. Surgical management of gastric cancer: The East vs. West. perspective. J. Gastrointest. Oncol. 2015, 6, 79–88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gierisch, J.M.; Beadles, C.; Shapiro, A.; McDuffie, J.R.; Cunningham, N.; Bradford, D.; Strauss, J.; Callahan, M.; Chen, M.; Hemminger, A.; et al. Health Disparities in Quality Indicators of Healthcare Among Adults with Mental Illness [Internet]. In Appendix B, Newcastle-Ottawa Scale Coding Manual For Cohort Studies; Department of Veterans Affairs (US): Washington, DC, USA, 2014. Available online: https://www.ncbi.nlm.nih.gov/books/NBK299087/ (accessed on 13 November 2023).
- Dhar, D.K.; Kubota, H.; Tachibana, M.; Kinugasa, S.; Masunaga, R.; Shibakita, M.; Kohno, H.; Nagasue, N. Prognosis of T4 gastric carcinoma patients: An appraisal of aggressive surgical treatment. J. Surg. Oncol. 2001, 76, 278–282. [Google Scholar] [CrossRef]
- Kobayashi, A.; Nakagohri, T.; Konishi, M.; Inoue, K.; Takahashi, S.; Itou, M.; Sugitou, M.; Ono, M.; Saito, N.; Kinoshita, T. Aggressive surgical treatment for T4 gastric cancer. J. Gastrointest. Surg. 2004, 8, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, C.; Akiyama, H.; Nomura, M.; Matsuda, G.; Otsuka, Y.; Ono, H.A.; Nagahori, Y.; Takahashi, M.; Kito, F.; Shimada, H. Surgical outcomes in patients with T4 gastric carcinoma. J. Am. Coll. Surg. 2006, 202, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Carboni, F.; Lepiane, P.; Santoro, R.; Lorusso, R.; Mancini, P.; Sperduti, I.; Carlini, M.; Santoro, E. Extended multiorgan resection for T4 gastric carcinoma: 25-year experience. J. Surg. Oncol. 2005, 90, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Joo, J.K.; Seo, K.W.; Park, Y.K.; Ryu, S.Y.; Kim, H.R.; Kim, Y.J.; Kim, S.K. T4 gastric carcinoma: The benefit of non-curative resection. ANZ J. Surg. 2006, 76, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-B.; Yang, L.-T.; Zhang, Z.-W.; Guo, J.-M.; Cheng, X.-D. Pancreaticoduodenectomy for advanced gastric cancer with pancreaticoduodenal region involvement. World J. Gastroenterol. 2008, 14, 3425–3429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jeong, O.; Choi, W.Y.; Park, Y.K. Appropriate selection of patients for combined organ resection in cases of gastric carcinoma invading adjacent organs. J. Surg. Oncol. 2009, 100, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-T.; Tsai, C.-Y.; Hsu, J.-T.; Vinayak, R.; Liu, K.-H.; Yeh, C.-N.; Yeh, T.-S.; Hwang, T.-L.; Jan, Y.-Y. Aggressive surgical approach for patients with T4 gastric carcinoma: Promise or myth? Ann. Surg. Oncol. 2011, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Mita, K.; Ito, H.; Fukumoto, M.; Murabayashi, R.; Koizumi, K.; Hayashi, T.; Kikuchi, H. Surgical outcomes and survival after extended multiorgan resection for T4 gastric cancer. Am. J. Surg. 2012, 203, 107–111. [Google Scholar] [CrossRef]
- Pacelli, F.; Cusumano, G.; Rosa, F.; Marrelli, D.; Dicosmo, M.; Cipollari, C.; Marchet, A.; Scaringi, S.; Rausei, S.; di Leo, A.; et al. Multivisceral resection for locally advanced gastric cancer: An Italian multicenter observational study. JAMA Surg. 2013, 148, 353–360. [Google Scholar] [CrossRef]
- Xiao, L.; Li, M.; Xu, F.; Ye, H.; Wu, W.; Long, S.; Li, W.; He, Y. Extended multi-organ resection for cT4 gastric carcinoma: A retrospective analysis. Pak. J. Med. Sci. 2013, 29, 581–585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, M.-Z.; Deng, L.; Wang, J.-J.; Xiao, L.-B.; Wu, W.-H.; Yang, S.-B.; Li, W.-F. Surgical outcomes and prognostic factors of T4 gastric cancer patients without distant metastasis. PLoS ONE 2014, 9, e107061. [Google Scholar] [CrossRef]
- Mita, K.; Ito, H.; Katsube, T.; Tsuboi, A.; Yamazaki, N.; Asakawa, H.; Hayashi, T.; Fujino, K. Prognostic Factors Affecting Survival After Multivisceral Resection in Patients with Clinical T4b Gastric Cancer. J. Gastrointest. Surg. 2017, 21, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Ma, M.; Xiao, Y.; Ouyang, Y.; Tang, M.; Zhou, K.; Hong, Y.; Tang, B.; Zuo, C. Incomplete resection and linitis plastica are factors for poor survival after extended multiorgan resection in gastric cancer patients. Sci. Rep. 2017, 7, 15800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Y.; Hu, J.; Ma, Y.; Chen, G.; Liu, Y. Multivisceral resection for locally advanced gastric cancer: A retrospective study. Am. J. Surg. 2021, 221, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.R.; Pereira, M.A.; Ramos, M.F.K.P.; Oliveira, R.J.; Ribeiro, U.; Zilberstein, B.; Cecconello, I. Prediction scores for complication and recurrence after multivisceral resection in gastric cancer. Eur. J. Surg. Oncol. 2020, 46, 1097–1102. [Google Scholar] [CrossRef]
- Aversa, J.G.; Diggs, L.P.; Hagerty, B.L.; Dominguez, D.A.; Ituarte, P.H.G.; Hernandez, J.M.; Davis, J.L.; Blakely, A.M. Multivisceral Resection for Locally Advanced Gastric Cancer. J. Gastrointest. Surg. 2021, 25, 609–622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Wang, W.; Zhao, L.; Niu, P.; Guo, C.; Zhao, D.; Chen, Y. Short-term safety and Long-term efficacy of multivisceral resection in pT4b gastric cancer patients without distant metastasis: A 20-year experience in China National Cancer Center. J. Cancer 2022, 13, 3113–3120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bobrzyński, Ł.; Pach, R.; Szczepanik, A.; Kołodziejczyk, P.; Richter, P.; Sierzega, M. What determines complications and prognosis among patients subject to multivisceral resections for locally advanced gastric cancer? Langenbecks Arch. Surg. 2023, 408, 442. [Google Scholar] [CrossRef] [PubMed]
- Vladov, N.; Trichkov, T.; Mihaylov, V.; Takorov, I.; Kostadinov, R.; Lukanova, T. Are Multivisceral Resections for Gastric Cancer Acceptable: Experience from a High Volume Center and Extended Literature Review? Surg. J. 2023, 9, e28–e35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greenberg, C.C.; Weeks, J.C.; Stain, S.C. Disparities in oncologic surgery. World J. Surg. 2008, 32, 522–528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manekk, R.S.; Gharde, P.; Gattani, R.; Lamture, Y. Surgical Complications and Its Grading: A Literature Review. Cureus 2022, 14, e24963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narayan, R.R.; Poultsides, G.A. Advances in the surgical management of gastric and gastroesophageal junction cancer. Transl. Gastroenterol. Hepatol. 2021, 6, 16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Songun, I.; Putter, H.; Kranenbarg, E.M.; Sasako, M.; van de Velde, C.J. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Lorenzon, L.; Giudicissi, R.; Scatizzi, M.; Balducci, G.; Cantafio, S.; Biondi, A.; Persiani, R.; Mercantini, P.; D’Ugo, D. D1-plus vs D2 nodal dissection in gastric cancer: A propensity score matched comparison and review of published literature. BMC Surg. 2020, 20, 126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pareekutty, N.M.; Kadam, S.; Ankalkoti, B.; Balasubramanian, S.; Anilkumar, B. Gastrectomy with D2 Lymphadenectomy for Carcinoma of the Stomach in a Stand-alone Cancer Centre in Rural India. Indian. J. Surg. Oncol. 2020, 11, 256–262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deshpande, G.; Samarasam, I.; Chandran, B.S.; Abraham, V.; George, S.V.; Mathew, G. Extended multiorgan resection in locally advanced gastric cancer: A single centre experience from south India. Trop. Gastroenterol. 2013, 34, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Gezen, C.; Kement, M.; Altuntas, Y.E.; Okkabaz, N.; Seker, M.; Vural, S.; Gumus, M.; Oncel, M. Results after multivisceral resections of locally advanced colorectal cancers: An analysis on clinical and pathological t4 tumors. World J. Surg. Onc 2012, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Schizas, D.; Giannakodimos, I.; Mylonas, K.S.; Kapetanakis, E.I.; Papavgeri, A.; Lianos, G.D.; Dellaportas, D.; Mastoraki, A.; Alexandrou, A. Multivisceral Resection for Locally Advanced Gastric Cancer: A Systematic Review and Evidence Quality Assessment. J. Clin. Med. 2023, 12, 7360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, J.; Yang, Y.; Ma, Y.; Ning, Y.; Chen, G.; Liu, Y. Survival benefits from neoadjuvant treatment in gastric cancer: A systematic review and meta-analysis. Syst. Rev. 2022, 11, 136. [Google Scholar] [CrossRef] [PubMed]
| Study and Author | Country | Study Year | Study Design | Study Quality |
|---|---|---|---|---|
| 1 Dhar et al. [21] | Japan | 2001 | Retrospective cohort | Medium |
| 2 Kobayashi et al. [22] | Japan | 2004 | Retrospective cohort | Medium |
| 3 Kunisaki et al. [23] | Japan | 2005 | Retrospective cohort | Medium |
| 4 Carboni et al. [24] | Italy | 2005 | Prospective cohort | High |
| 5 Kim et al. [25] | South Korea | 2006 | Retrospective cohort | Low |
| 6 Wang et al. [26] | China | 2008 | Retrospective cohort | Low |
| 7 Jeong et al. [27] | South Korea | 2009 | Prospective cohort | High |
| 8 Cheng et al. [28] | Taiwan | 2011 | Prospective cohort | High |
| 9 Mita et al. [29] | Japan | 2012 | Retrospective cohort | Medium |
| 10 Pacelli et al. [30] | Italy | 2013 | Prospective cohort | Medium |
| 11 Xiao et al. [31] | China | 2013 | Retrospective cohort | Low |
| 12 Li et al. [32] | China | 2014 | Prospective cohort | Medium |
| 13 Mita et al. [33] | Japan | 2017 | Retrospective cohort | High |
| 14 Xiao et al. [34] | China | 2017 | Retrospective cohort | Medium |
| 15 Yang et al. [35] | China | 2020 | Retrospective cohort | High |
| 16 Dias et al. [36] | Brazil | 2020 | Retrospective cohort | Medium |
| 17 Aversa et al. [37] | Italy | 2021 | Retrospective cohort | High |
| 18 Zhang et al. [38] | China | 2022 | Retrospective cohort | High |
| 19 Bobrzyński et al. [39] | Poland | 2023 | Retrospective cohort | Medium |
| 20 Vladov et al. [40] | Bulgaria | 2023 | Retrospective cohort | Medium |
| Study Number | Sample Size | Gastric Tumor Location and Features | Follow-Up Time/Mean Survival | Age (years) | Gender Distribution | R0 Resection (%) | Staging, Grading, Histology | Surgery (Excluding Gastrectomy) | Complications | Adjuvant Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Dhar et al. [21] | 150 | Tumor location: NR Gastrectomy type: NR | 1 to 3 years | Mean: 62.5 Range: 28–87 | Men: 92 (61.3%) Women: 58 (38.7%) | 42.1% | T4 gastric carcinoma: 100% | NR | Post-operative complications: 31.3% Post-operative death: 2.0% | Adjuvant: 92.7% |
| 2 Kobayashi et al. [22] | 82 | Tumor location: NR Gastrectomy type: total 60.9%, subtotal (39.1%) | Median 23.1 months Range 1–93 months | Mean: 64.0 Range: 26–84 | Men: 58 (70.7%) Women: 24 (29.3%) | 60.9% | T3: 51.2% size 9.0 cm (mean) T4: 48.8% size 10.8 cm (mean) | Pancreatectomy + Splenectomy 43.9% Transverse colectomy 42.7% Liver resection 12.2% Adrenalectomy 8.5% | Post-operative complications: 28.0% Post-operative death: 1.2% | NR |
| 3 Kunisaki et al. [23] | 117 | Tumor location: lower 37.6%, middle 18.8%, upper 25.6%, entire 18.0% Gastrectomy type: distal 28.2%, total 71.8% | Mean 15.6 months | Mean: 64.7 | Men: 77 (65.8%) Women: 40 (34.2%) | 32.5% | T4 gastric carcinoma: 100% size 9.1 cm (mean) | Pancreatectomy 27.3% Liver resection 8.5% Transverse colectomy 29.1% | Post-operative complications: 22.2% | No adjuvant therapy |
| 4 Carboni et al. [24] | 65 | Tumor location: proximal 27.7%, middle 47.7%, distal 21.6%, diffuse 3.0% Gastrectomy type: proximal 1.5%, subtotal 18.5%, total 80.0% | Median 13 months Range 1–163 months | Median: 63 Range: 27–82 | Men: 39 (60.0%) Women: 26 (40.0%) | 61.5% | T3: 20.0% T4: 80.0% | Splenectomy 47.6% Pancreatectomy 43.1% Colectomy 24.6% Liver resection 18.4% | Post-operative complications: 27.7% Post-operative death: 12.3% | NR |
| 5 Kim et al. [25] | 288 | Tumor location: upper 12.2%, middle 26.0%, lower 53.8%, diffuse 8.0% Gastrectomy type: total 32.3%, subtotal 62.5%, other 5.2% | 1 to 3 years | Mean: 58.0 | Men: 198 (68.8%) Women: 90 (31.2%) | 32.9% | T4 gastric carcinoma: 100% | Colectomy 58.3% Pancreatectomy 64.9% Liver resection 8.7% | NR | NR |
| 6 Wang et al. [26] | 17 | Tumor location: NR Gastrectomy type: total 64.7%, subtotal 35.3% | Median 38 months Range 2–72 months | Mean: 56 Range: 38–71 | Men: 11 (68.8%) Women: 6 (31.2%) | 32.1% | T4 gastric carcinoma: 100% size 4.0 cm (mean) | Pancreaticoduodenectomy 100% | Post-operative complications: 75.0% Post-operative death: 0.0% | Adjuvant: 100% (etoposide + leucovorin +fluorouracil) |
| 7 Jeong et al. [27] | 71 | Tumor location: distal 36.6%, middle 21.1%, proximal 31.0%, diffuse 11.3% Gastrectomy type: subtotal 33.8%, total 66.2% | Median 17.6 months Range 2.6–44.6 months | Mean: 59.0 | Men: 50 (70.4%) Women: 21 (29.6%) | 66.2% | T2–3: 36.6% T4: 63.4% size 7.9 cm (mean) | Multiorgan resection: 85.9% Colectomy: 23.9% Pancreatectomy + Splenectomy 46.5% Liver resection: 7.0% | Post-operative complications: 26.8% Post-operative death: 3.3% | Adjuvant: 100% |
| 8 Cheng et al. [28] | 91 | Tumor location: upper 39.6%, middle 13.2%, lower 38.5%, diffuse 8.8% Gastrectomy type: subtotal 38.5%, total 61.5% | Mean 31.6 months Range 21.9–41.2 months | Mean: 64.2 | Men: 62 (68.1%) Women: 29 (31.9%) | 81.3% | T4 gastric carcinoma: 100% | Pancreatectomy 59.3% Splenectomy 50.5% Colectomy 26.4% Liver resection 17.6% | Post-operative complications: 28.6% Post-operative death: 4.4% | NR |
| 9 Mita et al. [29] | 41 | Tumor location: upper 29.3%, 24.4%, 36.6%, diffuse 9.7% Gastrectomy type: proximal 4.9%, subtotal 12.2%, total 82.9% | Median 23.9 months | Mean: 60.0 Range: 43–90 | Men: 32 (78.0%) Women: 9 (22.0%) | 70.7% | T3: 46.3% T4: 53.7% | Pancreatectomy + Splenectomy 31.7% Pancreaticoduodenectomy 12.2% | Post-operative complications: 17.1% | Adjuvant: 85.4% |
| 10 Pacelli et al. [30] | 112 | Tumor location: antrum 19.6%, body 40.2%, fundus 18.8%, cardias 8.0%, plastic lynitis 11.6%, gastric stump 1.8% Gastrectomy type: total 67.9%, subtotal 29.5%, degastrogastrectromy 2.7% | Mean 24.9 months | Mean: 63.5 | Men: 71 (63.4%) Women: 41 (36.6%) | 38.4% | pT4a: 12.5% pT4b: 87.5% Size 7.9 cm (mean) | Colectomy 38.4% Pancreatectomy 41.1% Liver resection 15.2% Splenectomy 3.9% | Post-operative complications: 33.9% Post-operative death: 3.6% | Adjuvant: 100% (epirubicin, cisplatin, fluorouracil) |
| 11 Xiao et al. [31] | 63 | Tumor location: upper 38.1%, middle 27.0%, lower 23.8%, diffuse 11.1% Gastrectomy type: subtotal 25.4%, total 74.6% | Follow-up mean 13 months Survival mean 19.0 months | 56.6 | Men: 40 (63.5%) Women: 23 (36.5%) | 77.8% | T3: 60.3% T4: 39.7% Poor-undifferentiated: 76.2% Size: 7.2 cm (mean) | Multiorgan resection 50.8% | NR | NR |
| 12 Li et al. [32] | 132 | Tumor location: proximal 36.2%, middle 20.2%, distal 30.8%, diffuse 12.8% Gastrectomy type: subtotal 28.7%, total 71.3% | 5 years | Mean: 58.6 Range: 31–75 | Men: 67 (71.3%) Women: 27 (28.7%) | 71.2% | T4a: 41.4% T4b: 68.6% Poor-undifferentiated: 78.7% Size: 7.3 cm (mean) | Pancreatectomy 26.6% Colectomy 18.0% Splenectomy 9.6% Liver resection 5.3% | Post-operative complications: 18.1% Post-operative death: 2.1% | Adjuvant: 100% (CapeOX, FOLFOX, SOX) |
| 13 Mita et al. [33] | 103 | Tumor location: NR Gastrectomy type: NR | Follow-up mean 23 months Survival mean 27 months | Mean 69.7 | Men: 81 (78.6%) Women: 22 (21.4%) | 82.5% | pT4a: 43.7% pT4b: 56.3% | Pancreatectomy 46.6% Splenectomy 29.1% Colectomy 13.6% Liver resection 11.7% | Post-operative complications: 37.9% Post-operative death: 1.0% | Adjuvant 100% S-1 alone or S-1 and cisplatin |
| 14 Xiao et al. [34] | 75 | Tumor location: upper 38.1%, middle 27.0%, lower 23.8%, diffuse 11.1% Gastrectomy type: subtotal 25.4%, total 74.6% | Median 32 months | Mean: 56.6 Range: 18–93 | Men: 58 (77.3%) Women: 17 (22.7%) | 86.7% | T3: 5.3% T4: 94.7% Size: 5.4 cm (mean) | Colectomy 22.7% Liver resection 20.0% Pancreatectomy + Splenectomy 17.3% | Post-operative complications: 9.6% Post-operative death: 0.7% | Adjuvant 69.3% |
| 15 Yang et al. [35] | 148 | Tumor location: cardia 24.3%, fundus 8.1%, body 27.0%, antrum 29.1%, total 11.5% Gastrectomy type: NR | Median 25.7 months | NR | NR | 85.6% | pT3: 9.8% pT4a: 47.1% pT4b: 43.1% | Pancreatectomy 52.9% Splenectomy 56.2% Colectomy 28.1% Liver resection 9.8% | Post-operative complications: 13.1% Post-operative death: 1.3% | Adjuvant 100% (CapeOX, FOLFOX 6–8 cycles) |
| 16 Dias et al. [36] | 58 | Tumor location: NR Gastrectomy type: subtotal 29.3%, total 70.7% | Median 19.3 months Range 1–106.7 months | Mean: 61.8 Range: 36–81 | Men: 41 (70.7%) Women: 17 (29.3%) | 87.9% | pT4a: 24.1% pT4b: 58.6% | Pancreatectomy 76% Splenectomy 56% Colectomy 50% Liver resection 24% | Post-operative complications: 53.5% Post-operative death: 8.6% | Neoadjuvant 27.6% |
| 17 Aversa et al. [37] | 347 | Tumor location: fundus 6.1%, body 13.3%, antrum/pylorus 35.7% Gastrectomy type: total 100% | Median 36 months Range 12–60 months | Median: 65 Range: 45–75 | Men: 195/347 Men: 195 (56.2%) Women: 152 (43.8%) | 60.8% | pT4b: 100% Size: 7.0 cm (median) Poor-undifferentiated: 82.7% | Multiorgan resection 44.2% | Post-operative complications: 28.6% | Adjuvant radiation 24.5% Adjuvant chemotherapy 43.8% Neoadjuvant chemoradiotherapy 28.5% |
| 18 Zhang et al. [38] | 210 | Tumor location: proximal 52.4%, distal 41.4%, diffuse 6.2% Gastrectomy type: total 14.3%, subtotal 85.7% | 3 to 5 years | Mean: 61 Range: 24–82 | Men: 153 (72.9%) Women: 57 (27.1%) | 94.3% | pT4b: 100% Poor-undifferentiated: 76.7% | Pancreatectomy 20.5% Colectomy 16.7% Liver resection 9.0% | Post-operative complications: 8.1% | Adjuvant chemotherapy 38.6% |
| 19 Bobrzyński et al. [39] | 218 | Tumor location: NR Gastrectomy type: total 85% | Follow-up median 101 months Survival median 10.6 months | NR | Men: 153 (72.9%) Women: 57 (27.1%) | 46% | cT4b: 100% | Colectomy 18.8% Splenectomy 57.8% Pancreatectomy 23.4% | Post-operative complications: 75% | Neodjuvant 11% (ECF, DCF, and FLOT regimens) |
| 20 Vladov et al. [40] | 101 | Tumor location: cardia 17.8%, fundus 5.0%, corpus 37.6%, antrum 22.8%, linitis plastica 16.8% Gastrectomy type: NR | Follow-up median 28.1 months | Median: 61 Range: 28–88 | Men: 73 (72.3%) Women: 28 (27.7%) | 84.2% | T3/T4a: 27.7% T4b 72.3% Poor-undifferentiated: 60.4% | Splenectomy 67.3% Pancreatectomy 32.7% Liver resection 20.8% En bloc resection 73.3% | Post-operative complications: 14.8% | No neoadjuvant |
| Study Number | R0 Survival | Non-Curative Survival | Significant Risk Factors (Mortality) | Conclusions |
|---|---|---|---|---|
| 1 Dhar et al. [21] | 1 year: 46.7% 3 years: 25.1% 5 years: 16.8% | 0% at 2 years | No splenectomy (RR = 2.18): 23.6% vs. 35.0% (splenectomy) at 1 year Esophageal invasion (RR = 2.11): 14.7% vs. 35.5% (no invasion) at 1 year | Pancreatic, double organ, and multiple organ involvement had no effect on patient survival. Splenectomy should be performed along with invaded organ resection. |
| 2 Kobayashi et al. [22] | 5 years: 36.9% | 0% at 1460 days after surgery | Peritoneal dissemination (RR = 2.22): 21.1% vs. 46.8% (no invasion) at 3 years | Aggressive surgery with curative intent (R0) resulted in a significantly higher survival. |
| 3 Kunisaki et al. [23] | 5 years: 32.2% | 9.5% at 5 years | Tumor size > 10 cm (HR = 4.79): 0% vs. 55.7% (smaller size) at 3 years Lymph node involvement > 6 (HR = 4.04): 7.0% vs. 55.4% (fewer nodes) at 3 years | Aggressive surgery with curative intent (R0) and lymph node dissection resulted in a significantly higher survival. |
| 4 Carboni et al. [24] | 5 years: 30.6% | 0% at 5 years | Non-resectability (HR = 3.17) | Aggressive surgical treatment of locally advanced gastric carcinoma with acceptable morbidity and mortality rates improves prognosis, especially when curative resection is achieved. |
| 5 Kim et al. [25] | 3 years: 19.9% | 5.4% at 3 years | Lymph node involvement (RR = 1.62): 10.1% vs. 21.9% (no involvement) at 3 years | Significant survival benefit of resection (both curative and non-curative) with lymph node invasion and curability as critical prognostic factors. |
| 6 Wang et al. [26] | 1 year: 77.0% 3 years: 34.0% | 1 year: 41.7% 3 years: 5.6% | NR | En bloc pancreaticoduodenectomy with gastrectomy can improve long-term survival for advanced gastric cancer patients with pancreaticoduodenal region involvement. |
| 7 Jeong et al. [27] | 1 year: 74.0% 3 years: 47.5% | 1 year: 66.7% 3 years: 15.5% | N3 Lymph node involvement (HR = 5.18) | Aggressive surgery with curative intent (R0) resulted in a significantly higher survival. |
| 8 Cheng et al. [28] | 1 year: 60.0% 3 years: 33.3% | 1 year: 28.0% 3 years: 0.0% | Liver invasion (RR = 4.49): 17.2% vs. 52.4% (no invasion) at 3 years N3 Lymph node involvement (RR = 9.05) | pT3 patients with MOR had significantly better long-term survival compared to pT4 with MOR and cT4 without MOR. A significantly improved survival for pT4 with R0 MOR resection. Lymph node status, liver invasion, and positive margins were independent prognostic factors. |
| 9 Mita et al. [29] | 1 year: 78.1% 3 years: 62.1% | 1 year: 28.6% 3 years: 0.0% | Tumor size > 10 cm (HR = 2.87) Non-resectability (HR = 4.46) | Aggressive surgery with curative intent (R0) resulted in a significantly higher survival. |
| 10 Pacelli et al. [30] | 5 years: 43.7% | 5 years (R1): 31.4% 5 years (R2): 0.0% | Positive peritoneal cytology (HR = 1.35): 16.7% vs. 31.4% (negative) at 3 years N3 Lymph node involvement (HR = 1.83): 21.5% vs. 53.3% (no involvement) | |
| 11 Xiao et al. [31] | 1 year: 61.6% | NR | Resectability (HR = 0.33): 68.2% vs. 41.9% (non-resectability) at 1 year Tumor size > 7 cm (HR = 3.58): 35.1% vs. 75.0% (smaller size) at 1 year | Aggressive surgery with curative intent (R0) resulted in a significantly higher survival. |
| 12 Li et al. [32] | 1 year: 54.6% 3 years: 22.9% 5 years: 13.8% | 1 year: 39.5% 3 years: 7.9% 5 years: 3.5% | N3 Lymph node involvement (HR = 2.49) | Curative resection (R0) with MOR significantly improves survival; lymph node metastasis associated with poorer survival. |
| 13 Mita et al. [33] | 1 year: 78.3% 3 years: 47.7% | 1 year: 46.6% 3 years: 14.3% | N3 Lymph node involvement (HR = 2.26): 12.1% vs. 51.9% (no involvement) at 3 years Splenectomy (HR = 0.58): 46.7% vs. 40.9% (no splenectomy) at 3 years | Curative resection (R0) with MOR significantly improves survival. |
| 14 Xiao et al. [34] | 1 year: 84.8% 3 years: 58.5% 5 years: 34.0% | 1 year: 69.8% 3 years: 40.6% 5 years: 29.8% | Linitis plastica (RR = 16.0): 11 months vs. 33 months survival Non-resectability (HR = 15.2): 11 months vs. 34 months survival | Curative resection (R0) with MOR significantly improves survival. |
| 15 Yang et al. [35] | 1 year: 59.5% 3 years: 26.0% 5 years: 14.5% | 1 year: 27.3% 3 years: 4.5% 5 years: 0.0% | Lymph node involvement > 15 (HR = 2.55): 2.2% vs. 17.1% (fewer nodes) at 5 years Resectability (HR = 2.36): 14.5% vs. 0.0% (non-resectability) at 5 years | Curative resection (R0) with MOR significantly improves survival. |
| 16 Dias et al. [36] | 5 years: 56.9% | 5 years: 28.6% | Tumor size > 5 cm (HR = 5.76) Lymph node involvement (HR = 13.8) | Curative resection (R0) with MOR significantly improves survival. |
| 17 Aversa et al. [37] | NR | NR | N3 Lymph node involvement (HR = 1.97) Resectability (HR = 1.63): 6.6 months longer survival | Curative resection (R0) with MOR significantly improves survival. |
| 18 Zhang et al. [38] | 3 years: 48.2% 5 years: 39.1% | NR | Nerve invasion (HR = 2.21) | Patients received combined resection of pancreas and multiple organs tend to have worse survival. |
| 19 Bobrzyński et al. [39] | Median 15.8 months | Median 7.9 months | Non-resectability (HR = 1.47) | Curative resection (R0) with MOR significantly improves survival but increased the risk of postoperative complications and prolonged hospital stay. |
| 20 Vladov et al. [40] | 1 year: 58.3% 3 years: 27.7% 5 years: 18.8% | NR | N3 Lymph node involvement (HR = 5.28) Non-resectability (HR = 4.63) | MOR shows poorer survival and higher complication rates compared to SGRs, but better long-term outcomes than palliative interventions. R0 resection is crucial for better survival, while R1 and N3 status are factors for unfavorable outcomes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dejeu, V.; Dejeu, P.; Muresan, A.; Bradea, P.; Dejeu, D. Analysis of Patient Outcomes following Curative R0 Multiorgan Resections for Locally Advanced Gastric Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 3010. https://doi.org/10.3390/jcm13103010
Dejeu V, Dejeu P, Muresan A, Bradea P, Dejeu D. Analysis of Patient Outcomes following Curative R0 Multiorgan Resections for Locally Advanced Gastric Cancer: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(10):3010. https://doi.org/10.3390/jcm13103010
Chicago/Turabian StyleDejeu, Viorel, Paula Dejeu, Anita Muresan, Paula Bradea, and Danut Dejeu. 2024. "Analysis of Patient Outcomes following Curative R0 Multiorgan Resections for Locally Advanced Gastric Cancer: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 10: 3010. https://doi.org/10.3390/jcm13103010
APA StyleDejeu, V., Dejeu, P., Muresan, A., Bradea, P., & Dejeu, D. (2024). Analysis of Patient Outcomes following Curative R0 Multiorgan Resections for Locally Advanced Gastric Cancer: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(10), 3010. https://doi.org/10.3390/jcm13103010





