How Will Artificial Intelligence Shape the Future of Decision-Making in Congenital Heart Disease?
Abstract
1. Introduction
2. Role of Statistical Tools in the Management of Congenital Heart Disease
- Overfitting occurs when a model captures random fluctuations or noise in the data rather than underlying patterns. In the context of CHD analysis, overfitting can lead to overly complex models that perform well on the training data but fail when generalised to real life. This can result in misleading conclusions about the relationships between variables and the prediction of outcomes. Overfitting could be mitigated by strategies like cross-validation, regularisation and feature selection to ensure that the model is not overly sensitive to noise in the data and could be generalised well to new patients.
- Collinearity turns up when two or more predictors are highly correlated with each other in a regression model. In CHD analysis, collinearity can distort the estimated coefficients, making it difficult to assess their effect on the outcome. Collinearity can arise due to the complex interplay of genetic, environmental and clinical factors influencing the development and progression of the disease. To address collinearity, researchers can perform diagnostic tests such as variance inflation factor analysis to identify highly correlated predictors. Moreover, methods such as variable transformation or variable selection could be used to mitigate the effects of collinearity.
3. Application of Artificial Intelligence in Diagnosis, Management and Follow-Up of CHD
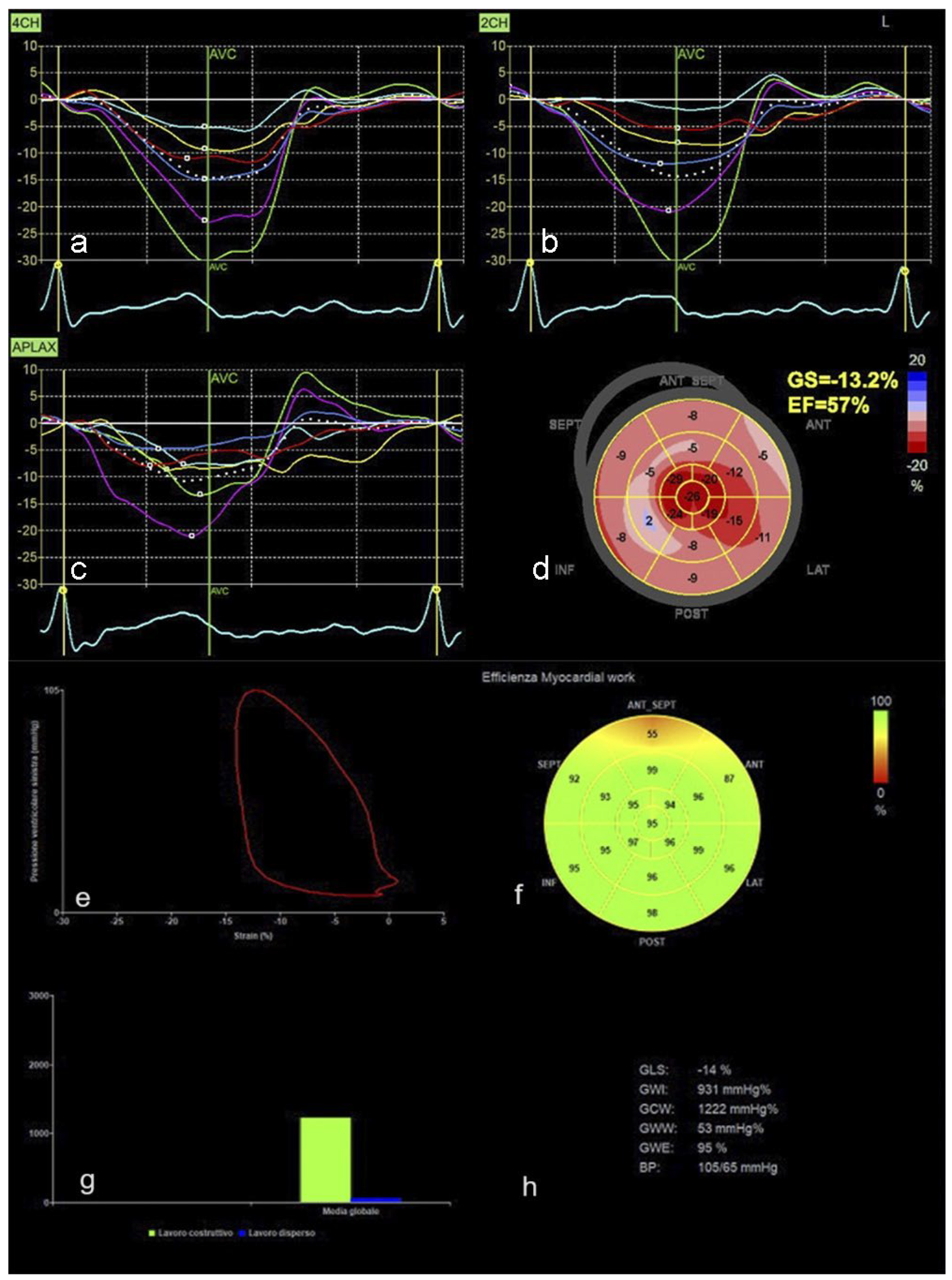
Cardiac Imaging Evolution: Artificial Intelligence-Guided Advancement
4. Artificial Intelligence in Clinical Decision-Making: From Datasets to Solutions
5. Artificial Intelligence and Outcome Prediction
6. Artificial Intelligence to Unveil Omics Medicine
7. Insights of Artificial Intelligence in Continuous Monitoring
8. Artificial Intelligence and Machine Learning Applied to Congenital Cardiac Surgery and Interventional Cardiology
9. Exploring Boundaries: Challenges in Applying Artificial Intelligence
10. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Frogoudaki, A.A. Congenital heart disease prevalence: What does the future hold? Eur. J. Prev. Cardiol. 2023, 30, 167–168. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.; Di Salvo, G. The new pandemic: ACHD HF. Int. J. Cardiol. 2022, 356, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Zühlke, L.; Black, G.C.; Choy, M.-K.; Li, N.; Keavney, B.D. Global birth prevalence of congenital heart defects 1970–2017: Updated systematic review and meta-analysis of 260 studies. Leuk. Res. 2019, 48, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Gaffar, S.; Gearhart, A.S.; Chang, A.C. The Next Frontier in Pediatric Cardiology. Pediatr. Clin. N. Am. 2020, 67, 995–1009. [Google Scholar] [CrossRef] [PubMed]
- Jone, P.-N.; Gearhart, A.; Lei, H.; Xing, F.; Nahar, J.; Lopez-Jimenez, F.; Diller, G.-P.; Marelli, A.; Wilson, L.; Saidi, A.; et al. Artificial Intelligence in Congenital Heart Disease. JACC Adv. 2022, 1, 100153. [Google Scholar] [CrossRef]
- Ledziński, Ł.; Grześk, G. Artificial Intelligence Technologies in Cardiology. J. Cardiovasc. Dev. Dis. 2023, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, S.N.; Gapizov, A.; Ekhator, C.; Ain, N.U.; Ahmad, S.; Khan, M.; Barker, C.; Hussain, M.; Malineni, J.; Ramadhan, A.; et al. The Role of Artificial Intelligence in Prediction, Risk Stratification, and Personalized Treatment Planning for Congenital Heart Diseases. Cureus 2023, 15, e44374. [Google Scholar] [CrossRef]
- Yang, H. Application of Artificial Intelligence-Based Auxiliary Diagnosis in Congenital Heart Disease Screening. Anatol. J. Cardiol. 2023, 27, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jimenez, F.; Attia, Z.; Arruda-Olson, A.M.; Carter, R.; Chareonthaitawee, P.; Jouni, H.; Kapa, S.; Lerman, A.; Luong, C.; Medina-Inojosa, J.R.; et al. Arti ficial Intelligence in Cardiology: Present and Future. Mayo Clin. Proc. 2020, 95, 1015–1039. [Google Scholar] [CrossRef]
- Reddy, C.D.; Eynde, J.V.D.; Kutty, S. Artificial intelligence in perinatal diagnosis and management of congenital heart disease. Semin. Perinatol. 2022, 46, 151588. [Google Scholar] [CrossRef]
- Ejaz, H.; Thyyib, T.; Ibrahim, A.; Nishat, A.; Malay, J. Role of artificial intelligence in early detection of congenital heart diseases in neonates. Front. Digit. Health 2023, 5, 1345814. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yin, Y.; Yang, Q.; Huo, T. Artificial intelligence in cardiovascular diseases: Diagnostic and therapeutic perspectives. Eur. J. Med. Res. 2023, 28, 242. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.M.S.; Krittanawong, C.; Narula, S.; Narula, J.; Argulian, E. Echocardiographic Data in Artificial Intelligence Research. JACC Cardiovasc. Imaging 2020, 13, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Nabi, W.; Bansal, A.; Xu, B. Applications of artificial intelligence and machine learning approaches in echocardiography. Echocardiography 2021, 38, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Canadilla, P.; Isabel-Roquero, A.; Aurensanz-Clemente, E.; Valls-Esteve, A.; Miguel, F.A.; Ormazabal, D.; Llanos, F.; Sanchez-De-Toledo, J. Machine Learning-Based Systems for the Anticipation of Adverse Events After Pediatric Cardiac Surgery. Front. Pediatr. 2022, 10, 930913. [Google Scholar] [CrossRef]
- Dreiseitl, S.; Ohno-Machado, L. Logistic regression and artificial neural network classification models: A methodology review. J. Biomed. Informatics 2002, 35, 352–359. [Google Scholar] [CrossRef]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 1st ed.; Wiley Series in Probability and Statistics; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Ruiz-Fernández, D.; Torra, A.M.; Soriano-Payá, A.; Marín-Alonso, O.; Palencia, E.T. Aid decision algorithms to estimate the risk in congenital heart surgery. Comput. Methods Programs Biomed. 2016, 126, 118–127. [Google Scholar] [CrossRef]
- Sarris, G.E.; Zhuo, D.; Mingardi, L.; Dunn, J.; Levine, J.; Tobota, Z.; Maruszewski, B.; Fragata, J.; Bertsimas, D. Congenital Heart Surgery Machine Learning-Derived In-Depth Benchmarking Tool. Ann. Thorac. Surg. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Rigatti, S.J.; Rigatti, S.J.; Dbim, D. Random Forest. J. Insur. Med. 2017, 47, 31–39. [Google Scholar] [CrossRef]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Geenen, L.W.; Opotowsky, A.R.; Lachtrupp, C.; Baggen, V.J.M.; Brainard, S.; Landzberg, M.J.; van Klaveren, D.; Lingsma, H.F.; Boersma, E.; Roos-Hesselink, J.W. Tuning and external validation of an adult congenital heart disease risk prediction model. Eur. Hear. J. Qual. Care Clin. Outcomes 2022, 8, 70–78. [Google Scholar] [CrossRef] [PubMed]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef] [PubMed]
- De Pretis, F.; Landes, J.; Peden, W. Artificial intelligence methods for a Bayesian epistemology-powered evidence evaluation. J. Evaluation Clin. Pr. 2021, 27, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Elkin, P.L.; Schlegel, A.R.; Anderson, M.; Komm, J.; Ficheu, G.; Bisson, L. Artificial Intelligence: Bayesian versus Heuristic Method for Diagnostic Decision Support. Appl. Clin. Informatics 2018, 9, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Al’aref, S.J.; Anchouche, K.; Singh, G.; Slomka, P.J.; Kolli, K.K.; Kumar, A.; Pandey, M.; Maliakal, G.; van Rosendael, A.R.; Beecy, A.N.; et al. Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. Eur. Heart J. 2018, 40, 1975–1986. [Google Scholar] [CrossRef]
- Arafati, A.; Hu, P.; Finn, J.P.; Rickers, C.; Cheng, A.L.; Jafarkhani, H.; Kheradvar, A. Artificial intelligence in pediatric and adult congenital cardiac MRI: An unmet clinical need. Cardiovasc. Diagn. Ther. 2019, 9, S310–S325. [Google Scholar] [CrossRef]
- Pozza, A.; Reffo, E.; Castaldi, B.; Cattapan, I.; Avesani, M.; Biffanti, R.; Cavaliere, A.; Cerutti, A.; Di Salvo, G. Utility of Fetal Cardiac Resonance Imaging in Prenatal Clinical Practice: Current State of the Art. Diagnostics 2023, 13, 3523. [Google Scholar] [CrossRef]
- Chessa, M.; Van De Bruaene, A.; Farooqi, K.; Valverde, I.; Jung, C.; Votta, E.; Sturla, F.; Diller, G.P.; Brida, M.; Sun, Z.; et al. OUP accepted manuscript. Eur. Heart J. 2022, 43, 2672–2683. [Google Scholar] [CrossRef] [PubMed]
- Brida, M.; Chessa, M.; Celermajer, D.; Li, W.; Geva, T.; Khairy, P.; Griselli, M.; Baumgartner, H.; A Gatzoulis, M. Atrial septal defect in adulthood: A new paradigm for congenital heart disease. Eur. Heart J. 2022, 43, 2660–2671. [Google Scholar] [CrossRef]
- Oktay, O.; Rueckert, D.; Bai, W.; Guerrero, R.; Rajchl, M.; de Marvao, A.; O’Regan, D.P.; Cook, S.A.; Heinrich, M.P.; Glocker, B. Stratified Decision Forests for Accurate Anatomical Landmark Localization in Cardiac Images. IEEE Trans. Med. Imaging 2016, 36, 332–342. [Google Scholar] [CrossRef]
- Asch, F.M.; Poilvert, N.; Abraham, T.; Jankowski, M.; Cleve, J.; Adams, M.; Romano, N.; Hong, H.; Mor-Avi, V.; Martin, R.P.; et al. Automated Echocardiographic Quantification of Left Ventricular Ejection Fraction Without Volume Measurements Using a Machine Learning Algorithm Mimicking a Human Expert. Circ. Cardiovasc. Imaging 2019, 12, e009303. [Google Scholar] [CrossRef]
- Nedadur, R.; Wang, B.; Tsang, W. Artificial intelligence for the echocardiographic assessment of valvular heart disease. Heart 2022, 108, 1592–1599. [Google Scholar] [CrossRef]
- Narula, S.; Shameer, K.; Omar, A.M.S.; Dudley, J.T.; Sengupta, P.P. Machine-Learning Algorithms to Automate Morphological and Functional Assessments in 2D Echocardiography. J. Am. Coll. Cardiol. 2016, 68, 2287–2295. [Google Scholar] [CrossRef]
- Veronese, P.; Guariento, A.; Cattapan, C.; Fedrigo, M.; Gervasi, M.T.; Angelini, A.; Riva, A.; Vida, V. Prenatal Diagnosis and Fetopsy Validation of Complete Atrioventricular Septal Defects Using the Fetal Intelligent Navigation Echocardiography Method. Diagnostics 2023, 13, 456. [Google Scholar] [CrossRef] [PubMed]
- Medvedofsky, D.; Mor-Avi, V.; Amzulescu, M.; Fernández-Golfin, C.; Hinojar, R.; Monaghan, M.J.; Otani, K.; Reiken, J.; Takeuchi, M.; Tsang, W.; et al. Three-dimensional echocardiographic quantification of the left-heart chambers using an automated adaptive analytics algorithm: Multicentre validation study. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gajjala, S.; Agrawal, P.; Tison, G.H.; Hallock, L.A.; Beussink-Nelson, L.; Lassen, M.H.; Fan, E.; Aras, M.A.; Jordan, C.; et al. Fully Automated Echocardiogram Interpretation in Clinical Practice. Circulation 2018, 138, 1623–1635. [Google Scholar] [CrossRef]
- Davies, R.; Babu-Narayan, S.V. Deep learning in congenital heart disease imaging: Hope but not haste. Heart 2020, 106, 960–961. [Google Scholar] [CrossRef]
- Shortliffe, E.H.; Sepúlveda, M.J. Clinical Decision Support in the Era of Artificial Intelligence. JAMA 2018, 320, 2199–2200. [Google Scholar] [CrossRef]
- Shah, N.H.; Milstein, A.; Bagley, S.C. Making Machine Learning Models Clinically Useful. JAMA 2019, 322, 1351–1352. [Google Scholar] [CrossRef] [PubMed]
- Vayena, E.; Blasimme, A.; Cohen, I.G. Machine learning in medicine: Addressing ethical challenges. PLOS Med. 2018, 15, e1002689. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Srivastava, S.; Xu, X.; Mehta, J.L. Artificial Intelligence, Machine Learning, and Cardiovascular Disease. Clin. Med. Insights Cardiol. 2020, 14, 1179546820927404. [Google Scholar] [CrossRef] [PubMed]
- Moroz, H.; Li, Y.; Marelli, A. hART: Deep learning-informed lifespan heart failure risk trajectories. Int. J. Med. Informatics 2024, 185, 105384. [Google Scholar] [CrossRef] [PubMed]
- Jacquemyn, X.; Kutty, S.; Manlhiot, C. The Lifelong Impact of Artificial Intelligence and Clinical Prediction Models on Patients with Tetralogy of Fallot. CJC Pediatr. Congenit. Heart Dis. 2023, 2, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Diller, G.P.; Orwat, S.; Vahle, J.; Bauer, U.M.M.; Urban, A.; Sarikouch, S.; Berger, F.; Beerbaum, P.; Baumgartner, H. Prediction of prognosis in patients with tetralogy of Fallot based on deep learning imaging analysis. Heart 2020, 106, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Leo, I.; Sabatino, J.; Avesani, M.; Moscatelli, S.; Bianco, F.; Borrelli, N.; De Sarro, R.; Leonardi, B.; Calcaterra, G.; Surkova, E.; et al. Non-Invasive Imaging Assessment in Patients with Aortic Coarctation: A Contemporary Review. J. Clin. Med. 2023, 13, 28. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Yang, M.; Huang, M.; Li, J.; Jia, Q.; Zhuang, J.; Huang, L. Evaluating the severity of aortic coarctation in infants using anatomic features measured on CTA. Eur. Radiol. 2021, 31, 1216–1226. [Google Scholar] [CrossRef]
- Diller, G.P.; Babu-Narayan, S.; Li, W.; Radojevic, J.; Kempny, A.; Uebing, A.; Dimopoulos, K.; Baumgartner, H.; A Gatzoulis, M.; Orwat, S. Utility of machine learning algorithms in assessing patients with a systemic right ventricle. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 925–931. [Google Scholar] [CrossRef]
- Naruka, V.; Rad, A.A.; Ponniah, H.S.; Francis, J.; Vardanyan, R.; Tasoudis, P.; Magouliotis, D.E.; Lazopoulos, G.L.; Salmasi, M.Y.; Athanasiou, T. Machine learning and artificial intelligence in cardiac transplantation: A systematic review. Artif. Organs 2022, 46, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Squiers, J.J.; Saracino, G.; Chamogeorgakis, T.; MacHannaford, J.C.; Rafael, A.E.; Gonzalez-Stawinski, G.V.; Hall, S.A.; DiMaio, J.M.; Lima, B. Application of the International Society for Heart and Lung Transplantation (ISHLT) criteria for primary graft dysfunction after cardiac transplantation: Outcomes from a high-volume centre. Eur. J. Cardio Thoracic Surg. 2017, 51, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Ayers, B.; Sandholm, T.; Gosev, I.; Prasad, S.; Kilic, A. Using machine learning to improve survival prediction after heart transplantation. J. Card. Surg. 2021, 36, 4113–4120. [Google Scholar] [CrossRef] [PubMed]
- Kampaktsis, P.N.; Siouras, A.; Doulamis, I.P.; Moustakidis, S.; Emfietzoglou, M.; Eynde, J.V.D.; Avgerinos, D.V.; Giannakoulas, G.; Alvarez, P.; Briasoulis, A. Machine learning-based prediction of mortality after heart transplantation in adults with congenital heart disease: A UNOS database analysis. Clin. Transplant. 2023, 37, e14845. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Gray, G.M.; Ashfaq, A.; Asante-Korang, A.; Rehman, M.A.; Ahumada, L.M. Using machine learning to predict five-year transplant-free survival among infants with hypoplastic left heart syndrome. Sci. Rep. 2024, 14, 4512. [Google Scholar] [CrossRef] [PubMed]
- Boskovski, M.T.; Homsy, J.; Nathan, M.; Sleeper, L.A.; Morton, S.; Manheimer, K.B.; Tai, A.; Gorham, J.; Lewis, M.; Swartz, M.; et al. De Novo Damaging Variants, Clinical Phenotypes, and Post-Operative Outcomes in Congenital Heart Disease. Circ. Genom. Precis. Med. 2020, 13, e002836. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Laser, K.T.; Dubowy, K.-O.; Scholl-Bürgi, S.; Michel, E. Metabolomics and random forests in patients with complex congenital heart disease. Front. Cardiovasc. Med. 2022, 9, 994068. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.; Kumar, C.; Zeng, W.-F.; Strauss, M.T. Artificial intelligence for proteomics and biomarker discovery. Cell Syst. 2021, 12, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Y.; Yang, L.; Huang, M.; Zhang, X.; Wang, X.; Guan, X.; Yang, P.; Wang, Y.; Meng, L.; et al. Analysis of Biomarkers for Congenital Heart Disease Based on Maternal Amniotic Fluid Metabolomics. Front. Cardiovasc. Med. 2021, 8, 671191. [Google Scholar] [CrossRef]
- Bassareo, P.P.; McMahon, C.J. Metabolomics: A New Tool in Our Understanding of Congenital Heart Disease. Children 2022, 9, 1803. [Google Scholar] [CrossRef]
- Feng, B.; Li, X.; Zhang, Q.; Wang, Y.; Gu, S.; Yao, R.-E.; Li, Z.; Gao, S.; Chang, G.; Li, Q.; et al. Molecular and phenotypic spectrum of cardio-facio-cutaneous syndrome in Chinese patients. Orphanet J. Rare Dis. 2023, 18, 284. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, R.; Tan, X.; Li, N.; Ding, Y.; Li, J.; Chang, G.; Chen, Y.; Ma, L.; Wang, J.; et al. Molecular and phenotypic spectrum of Noonan syndrome in Chinese patients. Clin. Genet. 2019, 96, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Ameen, M.; Sundaram, L.; Shen, M.; Banerjee, A.; Kundu, S.; Nair, S.; Shcherbina, A.; Gu, M.; Wilson, K.D.; Varadarajan, A.; et al. Integrative single-cell analysis of cardiogenesis identifies developmental trajectories and non-coding mutations in congenital heart disease. Cell 2022, 185, 4937–4953.e23. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Liu, S.; Chen, Z.; Xia, Y.; Xie, J.; Fu, M.; Lu, D.; Wu, Y.; Shen, H.; Yang, P.; et al. Anatomically resolved transcriptome and proteome landscapes reveal disease-relevant molecular signatures and systematic changes in heart function of end-stage dilated cardiomyopathy. View 2022, 4, 40. [Google Scholar] [CrossRef]
- Rose, S.M.S.-F.; Contrepois, K.; Moneghetti, K.J.; Zhou, W.; Mishra, T.; Mataraso, S.; Dagan-Rosenfeld, O.; Ganz, A.B.; Dunn, J.; Hornburg, D.; et al. A longitudinal big data approach for precision health. Nat. Med. 2019, 25, 792–804. [Google Scholar] [CrossRef]
- Shcherbina, A.; Mattsson, C.M.; Waggott, D.; Salisbury, H.; Christle, J.W.; Hastie, T.; Wheeler, M.T.; Ashley, E.A. Accuracy in Wrist-Worn, Sensor-Based Measurements of Heart Rate and Energy Expenditure in a Diverse Cohort. J. Pers. Med. 2017, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimkhani, M.; Johnson, E.M.I.; Sodhi, A.; Robinson, J.D.; Rigsby, C.K.; Allen, B.D.; Markl, M. A Deep Learning Approach to Using Wearable Seismocardiography (SCG) for Diagnosing Aortic Valve Stenosis and Predicting Aortic Hemodynamics Obtained by 4D Flow MRI. Ann. Biomed. Eng. 2023, 51, 2802–2811. [Google Scholar] [CrossRef]
- Sachdeva, R.; Armstrong, A.K.; Arnaout, R.; Grosse-Wortmann, L.; Han, B.K.; Mertens, L.; Moore, R.A.; Olivieri, L.J.; Parthiban, A.; Powell, A.J. Novel Techniques in Imaging Congenital Heart Disease. J. Am. Coll. Cardiol. 2024, 83, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.H.; el Mathari, S.; Abjigitova, D.; Bogers, A.J.J.C.; Mahtab, E.A.F. Current and Future Applications of Virtual, Augmented, and Mixed Reality in Cardiothoracic Surgery. Ann. Thorac. Surg. 2022, 113, 681–691. [Google Scholar] [CrossRef]
- Meier, L.M.; Meineri, M.; Hiansen, J.Q.; Horlick, E.M. Structural and congenital heart disease interventions: The role of three-dimensional printing. Neth. Heart J. 2017, 25, 65–75. [Google Scholar] [CrossRef]
- Seymour, N.E.; Gallagher, A.G.; Roman, S.A.; O’brien, M.K.; Bansal, V.K.; Andersen, D.K.; Satava, R.M. Virtual Reality Training Improves Operating Room Performance. Ann. Surg. 2002, 236, 458–463, discussion 463–464. [Google Scholar] [CrossRef] [PubMed]
- Reznick, R.K.; MacRae, H. Teaching Surgical Skills—Changes in the Wind. N. Engl. J. Med. 2006, 355, 2664–2669. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, F.; Vida, V.; Godard, C.; Bertelli, F.; Reffo, E.; Boddaert, N.; El Beheiry, M.; Masson, J. Fast-track virtual reality for cardiac imaging in congenital heart disease. J. Card. Surg. 2021, 36, 2598–2602. [Google Scholar] [CrossRef]
- Stephenson, N.; Pushparajah, K.; Wheeler, G.; Deng, S.; Schnabel, J.A.; Simpson, J.M. Extended reality for procedural planning and guidance in structural heart disease—A review of the state-of-the-art. Int. J. Cardiovasc. Imaging 2023, 39, 1405–1419. [Google Scholar] [CrossRef] [PubMed]
- Cattapan, C.; Guariento, A.; Bertelli, F.; Galliotto, F.; Vazzoler, C.; Magagna, P.; Gerosa, G.; Vida, V. The introduction of surgical simulation on three-dimensional-printed models in the cardiac surgery curriculum: An experimental project. J. Cardiovasc. Med. 2024, 25, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Muzio, F.P.L.; Rozzi, G.; Rossi, S.; Luciani, G.B.; Foresti, R.; Cabassi, A.; Fassina, L.; Miragoli, M. Artificial Intelligence Supports Decision Making during Open-Chest Surgery of Rare Congenital Heart Defects. J. Clin. Med. 2021, 10, 5330. [Google Scholar] [CrossRef]
- Lu, Y.; Li, B.; Liu, N.; Chen, J.-W.; Xiao, L.; Gou, S.; Chen, L.; Huang, M.; Zhuang, J. CT-TEE Image Registration for Surgical Navigation of Congenital Heart Disease Based on a Cycle Adversarial Network. Comput. Math. Methods Med. 2020, 2020, 4942121. [Google Scholar] [CrossRef] [PubMed]
- Laux, J.; Wachter, S.; Mittelstadt, B. Trustworthy artificial intelligence and the European Union AI act: On the conflation of trustworthiness and acceptability of risk. Regul. Gov. 2024, 18, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Májovský, M.; Černý, M.; Kasal, M.; Komarc, M.; Netuka, D. Artificial Intelligence Can Generate Fraudulent but Authentic-Looking Scientific Medical Articles: Pandora’s Box Has Been Opened. J. Med. Internet Res. 2024, 25, e46924. [Google Scholar] [CrossRef] [PubMed]
- Boscardin, C.K.; Gin, B.; Golde, P.B.; Hauer, K.E. ChatGPT and Generative Artificial Intelligence for Medical Education: Potential Impact and Opportunity. Acad. Med. 2023, 99, 22–27. [Google Scholar] [CrossRef]

| Tools | Applications in Congenital Heart Disease | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Logistic Regression Analysis of variables related to binary outcomes | Prediction of mortality or hospital readmission based on clinical data, or reintervention in patients treated by surgery |
|
| [16,17] |
| Decision trees Series of hierarchical decisions (nodes of the tree) based on features |
|
|
| [18,19] |
| Random Forests Multiple decision trees for classification or prediction | Outcome prediction based on clinical data |
|
| [16,20,21] |
| Gradient Boosting Machines Sequentially decision trees | Comprehension of complex feature interactions |
|
| [22] |
| Survival Analysis Models Cox proportional Hazard models or Kaplan-Meier estimators |
|
|
| [21,23] |
| Neural Networks Interconnected nodes inspired by the structure of the human brain |
|
|
| [24] |
| Support Vector Machines Algorithms for dichotomous classification | Outcome prediction |
|
| [25] |
| Long Short-Term Memory (LSTM) Algorithms with recurrent nodes, memory cells and gating mechanisms | Analysis of time-series measurements for future outcome prediction |
|
| [26] |
| Bayesian Networks Models of probabilistic dependencies between variables | Personalised risk assessment and decision support |
|
| [27,28] |
| Study | Year of Publication | Key Findings | AI Algorithm/Model Used |
|---|---|---|---|
| Narula et al. [37] | 2016 |
| Support vector machine Random forests Artificial neural networks |
| Oktay et al. [34] | 2017 | Anatomical landmark localisation in cardiac imaging | Stratified decision forests |
| D. Medvedofsky et al. [39] | 2018 | Three-dimensional echocardiographic quantification of left-heart chambers | Automated adaptive analytics algorithm |
| X. Li et al. [63] | 2019 | Analysis of the molecular and phenotypic spectrum of Noonan syndrome in Chinese patients | Neural networks |
| F. M. Asch et al. [35] | 2019 | Automated quantification of left ventricular ejection fraction without volume measurements | Neural network |
| G.P. Diller et al. [51] | 2019 |
| Convolutional neural networks |
| Y. Lu et al. [78] | 2020 | Perform CT-TEE image registration for surgical navigation of congenital heart disease | Cycle adversarial network (deep learning) |
| F. P. Lo Muzio et al. [77] | 2021 | Support decision-making in cardiac surgical practice | Supervised machine learning |
| M. Mann et al. [59] | 2021 | Facilitate proteomics and biomarker discovery to improve disease diagnosis and therapeutic targeting | Artificial neural networks |
| B. Ayers et al. [54] | 2021 | Development of predictive models to improve survival prediction after heart transplant | Artificial neural network, Support vector machine Random forest |
| Y. Li et al. [60] | 2021 | Identification of biomarkers for CHD using maternal amniotic fluid metabolomics | Logistic regression |
| Nedadur et al. [36] | 2022 |
| Convolutional neural network (CNN) |
| V. Naruka et al. [52] | 2022 | Identification of AI-driven approaches for risk prediction, patient selection and post-transplant outcomes assessment | Deep neural networks |
| M. Michel et al. [58] | 2022 |
| Random forests |
| B. Feng et al. [62] | 2023 | Analysis of the molecular and phenotypic spectrum of cardio-facio-cutaneous syndrome in Chinese patients | Neural networks |
| Li Lin et al. [65] | 2023 | Identification of disease-relevant molecular signatures and changes in heart function in dilated cardiomyopathy | Neural networks |
| M. Ebrahimkhani et al. [68] | 2023 | Analysis of wearable seismocardiography (SCG) data for diagnosing aortic valve stenosis and predicting aortic hemodynamics obtained by 4D flow MRI. | Convolutional neural network |
| P. N. Kampaktsis et al. [55] | 2023 | Development of a risk-prediction model for assessment of 1-year mortality post-heart transplantation | Logistic regression Adaptative boosting Random forests |
| H. Morotz et al. [46] | 2024 | Prediction of lifespan heart failure risk trajectories | Logistic regression Support vector machine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozza, A.; Zanella, L.; Castaldi, B.; Di Salvo, G. How Will Artificial Intelligence Shape the Future of Decision-Making in Congenital Heart Disease? J. Clin. Med. 2024, 13, 2996. https://doi.org/10.3390/jcm13102996
Pozza A, Zanella L, Castaldi B, Di Salvo G. How Will Artificial Intelligence Shape the Future of Decision-Making in Congenital Heart Disease? Journal of Clinical Medicine. 2024; 13(10):2996. https://doi.org/10.3390/jcm13102996
Chicago/Turabian StylePozza, Alice, Luca Zanella, Biagio Castaldi, and Giovanni Di Salvo. 2024. "How Will Artificial Intelligence Shape the Future of Decision-Making in Congenital Heart Disease?" Journal of Clinical Medicine 13, no. 10: 2996. https://doi.org/10.3390/jcm13102996
APA StylePozza, A., Zanella, L., Castaldi, B., & Di Salvo, G. (2024). How Will Artificial Intelligence Shape the Future of Decision-Making in Congenital Heart Disease? Journal of Clinical Medicine, 13(10), 2996. https://doi.org/10.3390/jcm13102996







