Comprehensive Review of the Imaging Recommendations for Diagnosis, Staging, and Management of Thyroid Carcinoma
Abstract
1. Introduction
2. Risk Factors and Clinical Presentations
3. Epidemiology and Etiopathogenesis
4. Imaging Referral Guidelines
5. Clinical/Non-Imaging Diagnostic Workup
6. Imaging Guidelines
6.1. Diagnosis
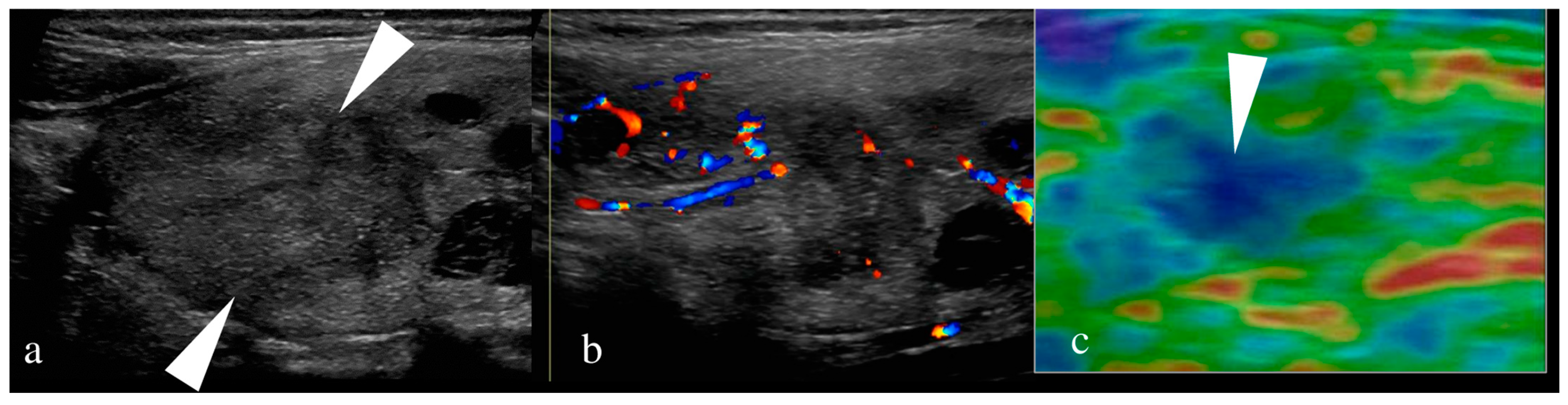
6.1.1. US of Thyroid
6.1.2. US of Neck Nodes
6.1.3. US-Guided FNA of Thyroid Nodule/Neck Nodes
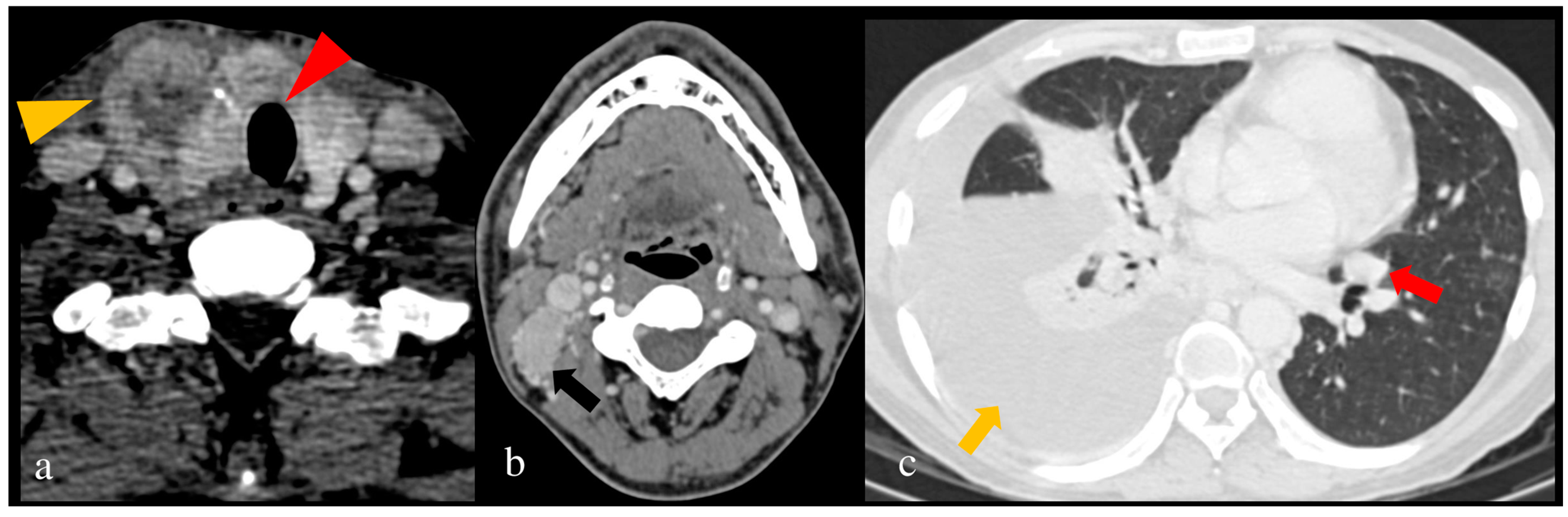
6.2. Staging
7. Principles of Management
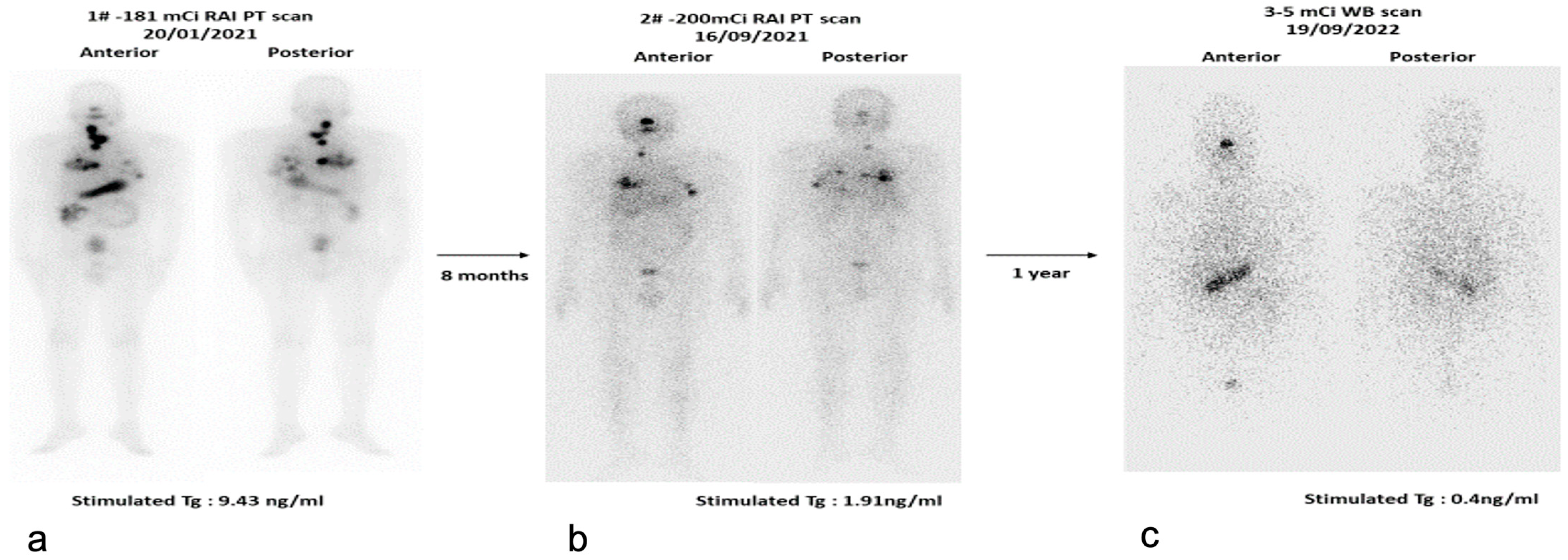
7.1. Radioactive Iodine (RAI) Scan
7.2. Role of FDG-PET/CT
8. Follow-Up
9. Management of Recurrent Disease
Recurrence in MTC
10. Management of Papillary Microcarcinoma
11. Percutaneous Ablation
12. Imaging Recommendations for Pediatric Thyroid Carcinoma
13. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| HNC | Head and neck cancer |
| PTC | Papillary thyroid carcinoma |
| FTC | Follicular thyroid carcinoma |
| MTC | Medullary thyroid carcinoma |
| PDTC | Poorly differentiated thyroid carcinoma |
| ATC | Anaplastic thyroid carcinoma |
| WHO | World Health Organization |
| PMC | Papillary microcarcinoma |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| FDG PET | Fluorodeoxyglucose positron emission tomography |
| RT | Radiation therapy |
| MEN | Multiple endocrine neoplasia |
| MAPK | Mitogen-activated protein kinase |
| PI3K | Phosphatidylinositol-3 kinase |
| RAS | Rat sarcoma virus |
| PTEN | Phosphatase and tensin homolog |
| ETE | Extrathyroidal extension |
| ATA | American Thyroid Association |
| AACE | American Association of Clinical Endocrinologists |
| NCCN | National Comprehensive Cancer Network |
| ESMO | European Society of Medical Oncology |
| FNA | Fine needle aspiration |
| ACR TI-RADS | American College of Radiology Thyroid Imaging Reporting and Data Systems |
| K-TIRADS | Korean Society of Thyroid Radiology Thyroid Imaging, Reporting and Data Systems |
| EU-TIRADS | European Thyroid Association |
| BTA | British Thyroid Association |
| SRU | Society of Radiologists in Ultrasound |
| ACE | American College of Endocrinology |
| AME | Associazione Medici Endocrinologi |
| F-TIRADS | French-TIRADS |
| TMC-RSS | Thyroid Multimodal-imaging Comprehensive Risk Stratification Scoring |
| TSH | Thyrotropin |
| US | Ultrasound |
| CEA | Carcinoembryonic antigen |
| IHC | Immunohistochemistry |
| ENE | Extranodal extension |
| Tg | Thyroglobulin |
| TNM | Tumor, Node, Metastasis |
| AJCC | American Joint Committee on Cancer |
| UICC | Union for International Cancer Control |
| CECT | Contrast-enhanced computed tomography |
| WBS | Whole body scintigraphy |
| SNMMI | Society of Nuclear Medicine and Molecular Imaging |
| EANM | European Association of Nuclear Medicine |
| SUV | Standardized uptake value |
| MTV | Metabolic tumor volume |
| TLG | Total lesion glycolysis |
| RLN | Recurrent laryngeal nerve |
| EBSLN | External branch of the superior laryngeal nerve |
| EBRT | External beam radiotherapy |
| IMRT | Intensity modulated radiotherapy |
| SPECT | Single photon emission computed tomography |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| iRECIST | Immune RECIST |
| RANO BM | Response assessment in neuro-oncology brain metastases |
| SSTR | Somatostatin receptor |
| PRRT | Peptide receptor radionuclide therapy |
References
- Global Cancer Observatory. Available online: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf (accessed on 5 February 2024).
- National Cancer Institute. Surveillance Epidemiology and End Results Data 1983 to 2009. Available online: http://seer.cancer.gov/ (accessed on 5 February 2024).
- Hoang, J.K.; Branstetter, B.F.; Gafton, A.R.; Lee, W.K.; Glastonbury, C.M. Imaging of thyroid carcinoma with CT and MRI: Approaches to common scenarios. Cancer Imaging 2013, 13, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Fagin, J.A.; Wells, S.A. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef] [PubMed]
- Younis, E. Oncogenesis of Thyroid Cancer. Asian Pac. J. Cancer Prev. 2017, 18, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Juhlin, C.C.; Mete, O.; Baloch, Z.W. The 2022 WHO Classification of Thyroid Tumors: Novel Concepts in Nomenclature and Grading. Endocr.-Relat. Cancer 2023, 30, e220293. [Google Scholar] [CrossRef] [PubMed]
- John, A.M.; Jacob, P.M.; Oommen, R.; Nair, S.; Nair, A.; Rajaratnam, S. Our experience with papillary thyroid microcancer. Indian J. Endocrinol. Metab. 2014, 18, 410–413. [Google Scholar] [PubMed]
- Mahajan, A.; Suryavanshi, S.; Shukla, S.; Vaish, R.; Agarwal, U.; D’Cruz, A. Active Surveillance of Low-Risk Papillary Microcarcinoma of the Thyroid in Indian Scenario: Are we Ready for it? A Narrative Review. Indian J. Endocrinol. Metab. 2022, 26, 119. [Google Scholar] [CrossRef] [PubMed]
- Dralle, H.; Machens, A.; Basa, J.; Fatourechi, V.; Franceschi, S.; Hay, I.D.; Nikiforov, Y.E.; Pacini, F.; Pasieka, J.L.; Sherman, S.I. Follicular cell-derived thyroid cancer. Nat. Rev. Dis. Primers 2015, 1, 15077. [Google Scholar] [CrossRef] [PubMed]
- Kushchayev, S.V.; Kushchayeva, Y.S.; Tella, S.H.; Glushko, T.; Pacak, K.; Teytelboym, O.M. Medullary Thyroid Carcinoma: An Update on Imaging. J. Thyroid Res. 2019, 2019, 1893047. [Google Scholar] [CrossRef] [PubMed]
- Bonnefond, S.; Davies, T.F. Thyroid cancer-risks and causes. Oncol. Hematol. Rev. 2014, 10, 14451. [Google Scholar] [CrossRef]
- Curtis, R.E.; Rowlings, P.A.; Deeg, H.J.; Shriner, D.A.; Socié, G.; Travis, L.B.; Horowitz, M.M.; Witherspoon, R.P.; Hoover, R.N.; Sobocinski, K.A.; et al. Solid cancers after bone marrow transplantation. N. Engl. J. Med. 1997, 336, 897–904. [Google Scholar] [CrossRef]
- Pacini, F.; Vorontsova, T.; Demidchik, E.P.; Molinaro, E.; Agate, L.; Romei, C.; Shavrova, E.; Cherstvoy, E.D.; Ivashkevitch, Y.; Kuchinskaya, E.; et al. Post-Chernobyl thyroid carcinoma in Belarus children and adolescents: Comparison with naturally occurring thyroid carcinoma in Italy and France. J. Clin. Endocrinol. Metab. 1997, 82, 3563–3569. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Kebebew, E.; Brierley, J.; Brito, J.P.; Cabanillas, M.E.; Clark, T.J., Jr.; Di Cristofano, A.; Foote, R.; Giordano, T.; Kasperbauer, J.; et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 2021, 31, 337–386. [Google Scholar] [CrossRef]
- LeClair, K.; Bell, K.J.L.; Furuya-Kanamori, L.; Doi, S.A.; Francis, D.O.; Davies, L. Evaluation of Gender Inequity in Thyroid Cancer Diagnosis: Differences by Sex in US Thyroid Cancer Incidence Compared With a Meta-analysis of Subclinical Thyroid Cancer Rates at Autopsy. JAMA Intern. Med. 2021, 181, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.A.; Feuer, E.J.; Hankey, B.F. Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. CA Cancer J. Clin. 1993, 43, 27–41. [Google Scholar] [CrossRef]
- Longheu, A.; Medas, F.; Pisano, G.; Gordini, L.; Nicolosi, A.; Sorrenti, S.; Erdas, E.; Calò, P.G. Differentiated thyroid cancer in patients ≥75 years: Histopathological features and results of surgical treatment. Int. J. Surg. 2016, 33, S159–S163. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef] [PubMed]
- Nagaiah, G.; Hossain, A.; Mooney, C.J.; Parmentier, J.; Remick, S.C. Anaplastic thyroid cancer: A review of epidemiology, pathogenesis, and treatment. J. Oncol. 2011, 2011, 542358. [Google Scholar] [CrossRef] [PubMed]
- Prete, A.; de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol. 2020, 11, 517263. [Google Scholar] [CrossRef]
- Eng, C.; Clayton, D.; Schuffenecker, I.; Lenoir, G.; Cote, G.; Gagel, R.F.; van Amstel, H.K.P.; Lips, C.J.M.; Nishisho, I.; Takai, S.-I.; et al. The Relationship Between Specific RET Proto-Oncogene Mutations and Disease Phenotype in Multiple Endocrine Neoplasia Type 2: International RET Mutation Consortium Analysis. JAMA 1996, 276, 1575–1579. [Google Scholar] [CrossRef]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, R.; Romei, C.; Ramone, T.; Prete, A.; Tacito, A.; Cappagli, V.; Bottici, V.; Viola, D.; Torregrossa, L.; Ugolini, C.; et al. Genetic Landscape of Somatic Mutations in a Large Cohort of Sporadic Medullary Thyroid Carcinomas Studied by Next-Generation Targeted Sequencing. iScience 2019, 20, 324–336. [Google Scholar] [CrossRef]
- Elisei, R.; Tacito, A.; Ramone, T.; Ciampi, R.; Bottici, V.; Cappagli, V.; Viola, D.; Matrone, A.; Lorusso, L.; Valerio, L.; et al. Twenty-Five Years Experience on RET Genetic Screening on Hereditary MTC: An Update on The Prevalence of Germline RET Mutations. Genes 2019, 10, 698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ito, Y.; Miyauchi, A. Prognostic factors and therapeutic strategies for differentiated carcinomas of the thyroid. Endocr. J. 2009, 56, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Nath, M.C.; Erickson, L.A. Aggressive Variants of Papillary Thyroid Carcinoma: Hobnail, Tall Cell, Columnar, and Solid. Adv. Anat. Pathol. 2018, 25, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Roman, S.; Lin, R.; Sosa, J.A. Prognosis of medullary thyroid carcinoma: Demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 2006, 107, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Rufini, V.; Salvatori, M.; Giordano, A.; Giovanella, L. PET imaging in recurrent medullary thyroid carcinoma. Int. J. Mol. Imaging 2012, 2012, 324686. [Google Scholar] [CrossRef] [PubMed]
- Gharib, H.; Papini, E.; Garber, J.R.; Duick, D.S.; Harrell, R.M.; Hegedus, L.; Paschke, R.; Valcavi, R.; Vitti, P. American association of clinical endocrinologists, American college of endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules-2016 update appendix. Endocr. Pract. 2016, 22, 1–60. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology: Thyroid Carcinoma, Version 2. 2024—12 March 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf (accessed on 15 March 2024).
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up †. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef]
- Horvath, E.; Majlis, S.; Rossi, R.; Franco, C.; Niedmann, J.P.; Castro, A.; Dominguez, M. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J. Clin. Endocrinol. Metab. 2009, 94, 1748–1751. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Jung, I.; Baek, J.H.; Baek, S.M.; Choi, N.; Choi, Y.J.; Jung, S.L.; Kim, E.K.; Kim, J.A.; Kim, J.H.; et al. Image reporting and characterization system for ultrasound features of thyroid nodules: Multicentric Korean retrospective study. Korean J. Radiol. 2013, 14, 110–117, Erratum in: Korean J. Radiol. 2013, 14, 389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR thyroid imaging, reporting and data system (TI-RADS): White paper of the ACR TI-RADS committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G. Thyroid imaging reporting and data system (TI-RADS): A user’s guide. Radiology 2018, 287, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.-H.; Lee, Y.H.; Lim, H.K.; Moon, W.-J.; Na, D.G.; Park, J.S.; et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: Revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef] [PubMed]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: The EU-TIRADS. Eur. Thyroid. J. 2017, 6, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Grani, G.; Lamartina, L.; Ascoli, V.; Bosco, D.; Biffoni, M.; Giacomelli, L.; Maranghi, M.; Falcone, R.; Ramundo, V.; Cantisani, V.; et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: Toward the “right” TIRADS. J. Clin. Endocrinol. Metab. 2019, 104, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Vaidya, T.; Vaish, R.; Sable, N. The Journey of Ultrasound-based Thyroid Nodule Risk Stratification Scoring Systems: Do All Roads Lead to Thyroid Imaging, Reporting and Data System (TIRADS)? J. Head Neck Physicians Surg. 2017, 5, 57–65. [Google Scholar] [CrossRef]
- Mahajan, A.; Vaish, R.; Arya, S.; Sable, N.; Pande, S.; Paul, P.; Kane, S.; Chaukar, D.; Chaturvedi, P.; Pai, P.S.; et al. Diagnostic performance of thyroid multimodal-imaging comprehensive risk stratification scoring (TMC-RSS) system in characterising thyroid nodules. J. Head Neck Physicians Surg. 2017, 35, e17588. [Google Scholar] [CrossRef]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017, 27, 1341–1346. [Google Scholar] [CrossRef]
- Elisei, R.; Romei, C.; Cosci, B.; Agate, L.; Bottici, V.; Molinaro, E.; Sculli, M.; Miccoli, P.; Basolo, F.; Grasso, L.; et al. RET genetic screening in patients with medullary thyroid cancer and their relatives: Experience with 807 individuals at one center. J. Clin. Endocrinol. Metab. 2007, 92, 4725–4729. [Google Scholar] [CrossRef]
- Eng, C.; Mulligan, L.M.; Smith, D.P.; Healey, C.S.; Frilling, A.; Raue, F.; Neumann, H.P.; Ponder, M.A.; Ponder, B.A. Low frequency of germline mutations in the RET proto-oncogene in patients with apparently sporadic medullary thyroid carcinoma. Clin. Endocrinol. 1995, 43, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Romei, C.; Cosci, B.; Renzini, G.; Bottici, V.; Agate, L.; Passannanti, P.; Viola, D.; Biagini, A.; Materazzi, G.; Pinchera, A.; et al. RET genetic screening of sporadic medullary thyroid cancer (MTC) allows the preclinical diagnosis of unsuspected gene carriers and the identification of a relevant percentage of hidden familial MTC (FMTC). Clin. Endocrinol. 2011, 74, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Shukla, S.; Ankathi, S.K.; Shukla, A.; Vaish, R.; Suryavanshi, S.; Agarwal, U.; Patil, V.; Sahu, A.; Padashetty, S.; et al. Imaging Recommendations for Diagnosis, Staging, and Management of Cancer of the Thyroid, Parathyroid, and Salivary Glands. Indian J. Med. Paediatr. Oncol. 2023, 44, 159–174. [Google Scholar] [CrossRef]
- Vaish, R.; Mahajan, A.; Sable, N.; Dusane, R.; Deshmukh, A.; Bal, M.; D’cruz, A.K. Role of computed tomography in the evaluation of regional metastasis in well-differentiated thyroid cancer. Front. Radiol. 2023, 3, 1243000. [Google Scholar] [CrossRef] [PubMed]
- Alabousi, M.; Alabousi, A.; Adham, S.; Pozdnyakov, A.; Ramadan, S.; Chaudhari, H.; Young, J.E.M.; Gupta, M.; Harish, S. Diagnostic Test Accuracy of Ultrasonography vs Computed Tomography for Papillary Thyroid Cancer Cervical Lymph Node Metastasis: A Systematic Review and Meta-analysis. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Shin, J.H.; Kim, J.H.; Jung, S.L.; Son, E.J.; Oh, Y.L. Tall cell variant of papillary thyroid carcinoma: Sonographic and clinical findings. J. Ultrasound Med. 2011, 30, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, W.; Liu, C.; Li, J. Tall cell variant of papillary thyroid carcinoma: Current evidence on clinicopathologic features and molecular biology. Oncotarget 2016, 7, 40792–40799. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H. Ultrasonographic imaging of papillary thyroid carcinoma variants. Ultrasonography 2017, 36, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, E.K.; Kim, S.J.; Kwak, J.Y. Thyroid ultrasonography: Pitfalls and techniques. Korean J. Radiol. 2014, 15, 267–276. [Google Scholar] [CrossRef]
- Vaidya, T.P.; Mahajan, A.; Thakur, M.; Kembhavi, S.A. Revisiting thyroid imaging: Approach to risk stratification of thyroid nodules. In Proceedings of the European Congress of Radiology-ECR 2018, Vienna, Austria, 28 February–4 March 2018. [Google Scholar]
- Mahajan, A.; Vaidya, T.P.; Sable, N.; Chand, A.; Gupta, A.; Agarwal, U.; Kania, V. Journey from TIRADS to ACR-TIRADS-Review of Existing Ultrasonographic Imaging Scoring Systems and Current Practice Guidelines for Risk Stratification of Thyroid Nodules. In Proceedings of the European Congress of Radiology-ECR 2019, Vienna, Austria, 27 February–3 March 2019. [Google Scholar]
- Peng, J.-Y.; Pan, F.-S.; Wang, W.; Wang, Z.; Shan, Q.-Y.; Lin, J.-H.; Luo, J.; Zheng, Y.-L.; Hu, H.-T.; Ruan, S.-M.; et al. Malignancy risk stratification and FNA recommendations for thyroid nodules: A comparison of ACR TI-RADS, AACE/ACE/AME and ATA guidelines. Am. J. Otolaryngol. 2020, 41, 102625. [Google Scholar] [CrossRef]
- Chng, C.L.; Tan, H.C.; Too, C.W.; Lim, W.Y.; Chiam, P.P.S.; Zhu, L.; Nadkarni, N.V.; Lim, A.Y.Y. Diagnostic performance of ATA, BTA and TIRADS sonographic patterns in the prediction of malignancy in histologically proven thyroid nodules. Singap. Med. J. 2018, 59, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Liu, M.; He, J.; Wu, S.; Chen, M.; Wan, Y.; Gao, L.; Cai, X.; Ding, J.; Fu, X. Comparison of Different Risk-Stratification Systems for the Diagnosis of Benign and Malignant Thyroid Nodules. Front. Oncol. 2019, 9, 378. [Google Scholar] [CrossRef]
- Xu, T.; Wu, Y.; Wu, R.-X.; Zhang, Y.-Z.; Gu, J.-Y.; Ye, X.-H.; Tang, W.; Xu, S.-H.; Liu, C.; Wu, X.-H. Validation and comparison of three newly-released Thyroid Imaging Reporting and Data Systems for cancer risk determination. Endocrine 2019, 64, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Marukatat, N.; Parklug, P.; Chanasriyotin, C. Comparison of the diagnostic accuracy of K-TIRADS and EU-TIRADS guidelines for detection of thyroid malignancy on ultrasound. Radiography 2023, 29, 862–866. [Google Scholar] [CrossRef]
- King, A.D. Imaging for staging and management of thyroid cancer. Cancer Imaging 2008, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Kebebew, E.; Clark, O.H. Differentiated thyroid cancer: ‘complete’ rational approach. World J. Surg. 2000, 24, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.T.; Chow, L.; Chik, W.; King, W.; Metreweli, C. Metastatic cervical nodes in papillary carcinoma of the thyroid: Ultrasound and histological correlation. Clin. Radiol. 1995, 50, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Rosário, P.W.S.; de Faria, S.; Bicalho, L.; Alves, M.F.G.; Borges, M.A.R.; Purisch, S.; Padrão, E.L.; Rezende, L.L.; Barroso, L. Ultrasonographic differentiation between metastatic and benign lymph nodes in patients with papillary thyroid carcinoma. J. Ultrasound Med. 2005, 24, 1385–1389. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, E.-K.; Kim, B.M.; Kwak, J.Y.; Kim, S.J.; Youk, J.H.; Park, S.H. US-guided Fine-Needle Aspiration of Thyroid Nodules: Indications, Techniques, Results. RadioGraphics 2008, 28, 1869–1886. [Google Scholar] [CrossRef] [PubMed]
- Bhatki, A.M.; Brewer, B.; Robinson-Smith, T.; Nikiforov, Y.; Steward, D.L. Adequacy of surgeon-performed ultrasound-guided thyroid fine-needle aspiration biopsy. Otolaryngol. Head Neck Surg. 2008, 139, 27–31. [Google Scholar] [CrossRef]
- Deacu, L.; Niculescu, D.A.; Caragheorgheopol, A.; Terzea, D.; Poiană, C. Thyroglobulin in lymph node fine-needle aspiration biopsy washout fluid. A tertiary center experience. Arch. Clin. Cases 2021, 8, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Konca Degertekin, C.; Yalcin, M.M.; Cerit, T.; Ozkan, C.; Kalan, I.; Iyidir, O.T.; Altinova, A.E.; Akturk, M.; Toruner, F.; Akin, M.; et al. Lymph node fine-needle aspiration washout thyroglobulin in papillary thyroid cancer: Diagnostic value and the effect of thyroglobulin antibodies. Endocr. Res. 2016, 41, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, D.K.; Patel, S.G.; Shah, J.P. Changes in the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging of Head and Neck Cancer: Rationale and Implications. Curr. Oncol. Rep. 2019, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, D.; Zeng, W.; Chen, S.; Huang, Y.; Zhou, L.; Zhou, W.; Wei, W.; Zhang, C.; Liu, Z.; et al. New proposed tumor-node-metastasis staging system for medullary thyroid carcinoma based on the Surveillance, Epidemiology, and End Results database. Am. J. Transl. Res. 2020, 12, 2703–2710. [Google Scholar] [PubMed]
- Mahajan, A.; Vaish, R.; Sable, N.; Arya, S.; Kane, S.V.; D’Cruz, A. 391P Incremental value of preoperative CT in the surgical management of papillary thyroid cancer. Ann. Oncol. 2016, 27, ix121. [Google Scholar] [CrossRef]
- Mahajan, A.; Agarwal, U.; Padashetty, S.; Shukla, S.; Smriti, V.; Rastogi, S.; Vaish, R.; Kumar, S.; D’Cruz, A. A narrative review of the role of cross-sectional imaging in the management of thyroid carcinoma: Imaging guidelines and T-CIRADS. Cancer Res. Stat. Treat. 2022, 5, 490–498. [Google Scholar] [CrossRef]
- Mahajan, A.; Sable, N.; Vaish, R.; Chaukar, D.; Chaturvedi, P.; Pai, P. CT based modified SHIN classification for grading trachea Invasion: Addressing the resectability issues in Thyroid cancer. In Proceedings of the European Congress of Radiology-ECR 2019, Vienna, Austria, 27 February–3 March 2019. [Google Scholar]
- Kim, H.; Kim, J.-A.; Son, E.J.; Youk, J.H.; Chung, T.-S.; Park, C.S.; Chang, H.-S. Preoperative prediction of the extrathyroidal extension of papillary thyroid carcinoma with ultrasonography versus MRI: A retrospective cohort study. Int. J. Surg. 2014, 12, 544–548. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.; Takashima, S.; Matsushita, T.; Takayama, F.; Kobayashi, T.; Kadoya, M. Esophageal invasion by thyroid carcinomas: Prediction using magnetic resonance imaging. J. Comput. Assist. Tomogr. 2003, 27, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Shindo, M.L.; Caruana, S.M.; Kandil, E.; McCaffrey, J.C.; Orloff, L.A.; Porterfield, J.R.; Shaha, A.; Shin, J.; Terris, D.; Randolph, G. Management of invasive well-differentiated thyroid cancer: An American head and neck society consensus statement: AHNS consensus statement. Head Neck 2014, 36, 1379–1390. [Google Scholar] [CrossRef]
- Seo, Y.L.; Yoon, D.Y.; Lim, K.J.; Cha, J.H.; Yun, E.J.; Choi, C.S.; Bae, S.H. Locally Advanced Thyroid Cancer: Can CT Help in Prediction of Extrathyroidal Invasion to Adjacent Structures? Am. J. Roentgenol. 2010, 195, W240–W244. [Google Scholar] [CrossRef]
- Mancuso, A.A. The neck. In Computed Tomography and Magnetic Resonance Imaging of the Head and Neck, 2nd ed.; Stamathis, G., Eckhart, C., Nolley, C.S., Eds.; Williams & Wilkins: Baltimore, MD, USA, 1985; pp. 169–191. [Google Scholar]
- Takashima, S.; Morimoto, S.; Ikezoe, J.; Takai, S.; Kobayashi, T.; Koyama, H.; Nishiyama, K.; Kozuka, T. CT evaluation of anaplastic thyroid carcinoma. AJNR 1990, 11, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Takashima, S.; Takayama, F.; Wang, J.; Kobayashi, S.; Kadoya, M. Using MR imaging to predict invasion of the recurrent laryngeal nerve by thyroid carcinoma. AJR 2003, 180, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Tamaki, Y.; Takahashi, M.; Higuchi, K.; Sakaino, K.; Nonaka, T.; Shioya, M.; Mitsuhashi, N.; Niibe, H. Comparison of primary thyroid lymphoma with anaplastic thyroid carcinoma on computed tomographic imaging. Radiat. Med. 2002, 20, 9–15. [Google Scholar] [PubMed]
- Luster, M.; Clarke, S.E.; Dietlein, M.; Lassmann, M.; Lind, P.; Oyen, W.J.G.; Tennvall, J.; Bombardieri, E. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1941–1959. [Google Scholar] [CrossRef] [PubMed]
- Nimmons, G.L.; Funk, G.F.; Graham, M.M.; Pagedar, N.A. Urinary Iodine Excretion After Contrast Computed Tomography Scan: Implications for Radioactive Iodine Use. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Padovani, R.P.; Kasamatsu, T.S.; Nakabashi, C.C.D.; Camacho, C.P.; Andreoni, D.M.; Malouf, E.Z.; Marone, M.M.S.; Maciel, R.M.B.; Biscolla, R.P.M. One Month Is Sufficient for Urinary Iodine to Return to Its Baseline Value After the Use of Water-Soluble Iodinated Contrast Agents in Post-Thyroidectomy Patients Requiring Radioiodine Therapy. Thyroid 2012, 22, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Shalash, A.M.; Elahmadawy, M.A.; Heikal, S.Y.; Amin, A.A.; Youssef, A.A. Value of diffusion MRI versus [18F]FDG PET/CT in detection of cervical nodal metastases in differentiated thyroid cancer patients. Nucl. Med. Rev. Cent. East Eur. 2022, 25, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Qiao, Y.; Zhang, H. Value of CT Features in the Diagnosis of Papillary Thyroid Tumors in Incidental Thyroid Nodules. Int. J. Endocrinol. 2020, 2020, 9342317. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, H.; Sun, Z.; Ge, Y.; Li, J.; Yu, C.; Deng, Z.; Dou, W.; Wang, X. Preoperative assessment of extrathyroidal extension of papillary thyroid carcinomas by ultrasound and magnetic resonance imaging: A comparative study. Radiol. Med. 2020, 125, 870–876. [Google Scholar] [CrossRef]
- Cho, S.J.; Suh, C.H.; Baek, J.H.; Chung, S.R.; Choi, Y.J.; Lee, J.H. Diagnostic performance of MRI to detect metastatic cervical lymph nodes in patients with thyroid cancer: A systematic review and meta-analysis. Clin. Radiol. 2020, 75, e1–e562.10. [Google Scholar] [CrossRef]
- Giannoula, E.; Melidis, C.; Papadopoulos, N.; Bamidis, P.; Raftopoulos, V.; Iakovou, I. Dynamic Risk Stratification for Predicting Treatment Response in Differentiated Thyroid Cancer. J. Clin. Med. 2020, 9, 2708. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.A.; Pura, J.; Goffredo, P.; Dinan, M.A.; Reed, S.D.; Scheri, R.P.; Hyslop, T.; Roman, S.A.; Sosa, J.A. Presence and Number of Lymph Node Metastases Are Associated With Compromised Survival for Patients Younger Than Age 45 Years With Papillary Thyroid Cancer. J. Clin. Oncol. 2015, 33, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Ranade, R.; Pawar, S.; Mahajan, A.; Basu, S. Unusual false positive radioiodine uptake on 131I whole body scintigraphy in three unrelated organs with different pathologies in patients of differentiated thyroid carcinoma: A case series. World J. Nucl. Med. 2016, 15, 137–141. [Google Scholar]
- Avram, A.M.; Giovanella, L.; Greenspan, B.; A Lawson, S.; Luster, M.; Van Nostrand, D.; Peacock, J.G.; Ovčariček, P.P.; Silberstein, E.; Tulchinsky, M.; et al. SNMMI Procedure Standard/EANM Practice Guideline for Nuclear Medicine Evaluation and Therapy of Differentiated Thyroid Cancer: Abbreviated Version. J. Nucl. Med. 2022, 63, 15N–35N. [Google Scholar]
- Basu, S.; Kalshetty, A. Monitoring metastatic lesions in TENIS, initiating multi-targeted tyrosine kinase inhibitors and follow-up: Should the newer FDG PET-CT quantitative indices be the defining objective parameter in clinical trials? Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1092–1094. [Google Scholar] [CrossRef]
- Basu, S.; Dandekar, M.; Joshi, A.; D’Cruz, A. Defining a rational step-care algorithm for managing thyroid carcinoma patients with elevated thyroglobulin and negative on radioiodine scintigraphy (TENIS): Considerations and challenges towards developing an appropriate roadmap. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1167–1171. [Google Scholar] [CrossRef][Green Version]
- Zampella, E.; Klain, M.; Pace, L.; Cuocolo, A. PET/CT in the management of differentiated thyroid cancer. Diagn. Interv. Imaging 2021, 102, 515–523. [Google Scholar] [CrossRef]
- Basu, S.; Mahajan, A. Discordant and aggressive tumor biology of solitary scalp metastasis amidst widespread skeletal metastases in differentiated thyroid carcinoma: Functional radionuclide and MR imaging features and clinical correlates. Indian J. Cancer 2014, 51, 613–614. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Abraham, G.; Patil, V.M.; Menon, N.; Mandal, T.; Jacob, S.; Garg, K.; Sekar, A.; Sarma, R.J.; Reddy, L.; et al. Audit of Demographics, Treatment Patterns and Outcomes of Differentiated Thyroid Cancers Treated with Tyrosine Kinase Inhibitors. Indian J. Surg. Oncol. 2022, 13, 81–86. [Google Scholar] [CrossRef]
- Abhishek, M.; Renuka, A.; Ujjwal, A.; Amit, C.; Vijay, P.; Vanita, N.; Nandini, M.; Kumar, P. Atypical posterior reversible encephalopathy syndrome associated with Lenvatinib therapy in a patient with metastatic thyroid cancer—A case report. Cancer Rep. 2022, 5, e1605. [Google Scholar] [CrossRef]
- Kanteti, A.P.K.; Abraham, G.; Patil, V.M.; Menon, N.; Mandal, T.; Jacob, S.V.; Garg, K.; Sekar, A.; Sarma, R.J.; Mekala, L.R.; et al. An Audit of Systemic Therapy in Medullary Carcinoma Thyroid. Indian J. Surg. Oncol. 2022, 13, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Mahajan, A. Ovarian dermoid cyst serendipitously detected by pelvic radioiodine-(131) I uptake and by diffusion weighted MRI in a post-thyroidectomy case of papillary thyroid carcinoma. Hell. J. Nucl. Med. 2013, 16, 62–63. [Google Scholar] [PubMed]
- Chalian, H.; Töre, H.G.; Horowitz, J.M.; Salem, R.; Miller, F.H.; Yaghmai, V. Radiologic assessment of response to therapy: Comparison of RECIST Versions 1.1 and 1.0. Radiographics 2011, 31, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, N.; Mahajan, A.; Baheti, A.D.; Choudhari, A.; Patil, V.; Popat, P.; Unde, H. A Radiologist’s Perspective on Treatment-Related Pseudoprogression: Clues and Hues. Indian J. Med. Paediatr. Oncol. 2022, 43, 052–059. [Google Scholar] [CrossRef]
- Nishino, M.; Hatabu, H.; Hodi, F.S. Imaging of cancer immunotherapy: Current approaches and future directions. Radiology 2019, 290, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Borcoman, E.; Nandikolla, A.; Long, G.; Goel, S.; Le Tourneau, C. Patterns of response and progression to immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 169–178. [Google Scholar] [CrossRef]
- Persigehl, T.; Lennartz, S.; Schwartz, L.H. iRECIST: How to do it. Cancer Imaging 2020, 20, 2. [Google Scholar] [CrossRef]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Bendszus, M.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; et al. Response assessment in neurooncology, response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015, 16, e270e8. [Google Scholar] [CrossRef]
- Yang, J.H.; Camacho, C.P.; Lindsey, S.C.; Valente, F.O.; Andreoni, D.M.; Yamaga, L.Y.; Wagner, J.; Biscolla, R.P.M.; Maciel, R.M. The combined use of calcitonin doubling time and 18f-fdg pet/ct improves prognostic values in medullary thyroid carcinoma: The clinical utility of 18F-FDG PET/CT. Endocr. Pract. 2017, 23, 942–948. [Google Scholar] [CrossRef]
- Gubbi, S.; Koch, C.A.; Klubo-Gwiezdzinska, J. Peptide Receptor Radionuclide Therapy in Thyroid Cancer. Front. Endocrinol. 2022, 13, 896287. [Google Scholar] [CrossRef]
- Ito, Y.; Miyauchi, A.; Oda, H. Low-risk papillary microcarcinoma of the thyroid: A review of active surveillance trials. Eur. J. Surg. Oncol. 2018, 44, 307–315. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Li, D.; Ridouani, F. Percutaneous ablation of low-risk papillary thyroid cancer. Endocr.-Relat. Cancer 2023, 30, e220244. [Google Scholar] [CrossRef]
- Mauri, G.; Hegedüs, L.; Bandula, S.; Cazzato, R.L.; Czarniecka, A.; Dudeck, O.; Fugazzola, L.; Netea-Maier, R.; Russ, G.; Wallin, G.; et al. European Thyroid Association and Cardiovascular and Interventional Radiological Society of Europe 2021 Clinical Practice Guideline for the Use of Minimally Invasive Treatments in Malignant Thyroid Lesions. Eur. Thyroid. J. 2021, 10, 185–197. [Google Scholar] [CrossRef]
- Lee, M.K.; Baek, J.H.; Chung, S.R.; Choi, Y.J.; Lee, J.H.; Jung, S.L. Radiofrequency ablation of recurrent thyroid cancers: Anatomy-based management. Ultrasonography 2022, 41, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.L.; Waguespack, S.G.; Bauer, A.J.; Angelos, P.; Benvenga, S.; Cerutti, J.M.; Dinauer, C.A.; Hamilton, J.; Hay, I.D.; Luster, M.; et al. American Thyroid Association Guidelines Task Force. Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2015, 25, 716–759. [Google Scholar] [CrossRef] [PubMed]



| Categories | ||||||
|---|---|---|---|---|---|---|
| TIRADS (Horvath et al. [33]) | Normal (0%) | Benign (5%) | Probably benign (<5%) | Suspicious (5–80%) 4a (5–10%) 4b (10–80%) | Probably malignant (>80%) | Biopsy proven malignancy |
| TIRADS (Kwak et al. [34]) | Negative | Benign (0%) | Probably benign (1.7%) | Suspicious 4a (3–30%) 4b (9.2%) 4c (44.4–72.4%) | Highly suggestive of malignancy (87.5%) | |
| F-TIRADS | Normal | Benign (0%) | Very probably benign (0.25%) | 4a: Suspicious, low risk of malignancy (6%) 4b: Suspicious, high risk of malignancy (69%) | Effectively certainly malignant nodules (100%) | |
| BTA | U1: Normal | U2: Benign | U3: Indeterminate | U4: Suspicious | U5: Malignant | |
| ATA | Benign (<1%) | Very low suspicion (<3%) | Low suspicion (5–10%) | Indeterminate suspicion (10–20%) | High suspicion (>70–90%) | |
| K-TIRADS | Category 1: Benign (0%) | Category 2: Probably benign (<5%) | Category 3: Indeterminate (5–50%) | Category 4: Suspicious for malignancy (>50%) | ||
| ACR-TIRADS | TR 1: Benign | TR 2: Not suspicious | TR3: Mildly suspicious | TR4: Moderately suspicious | TR5: Highly suspicious | |
| TMC-RSS | Group 1: Low risk (2.4%) | Group 2: Intermediate risk (18%) | Group 3: High risk (80%) | |||
| AACE/ACE-AME | Class 1: Low-risk thyroid lesion Malignancy risk: 1% | Class 2: Intermediate-risk thyroid lesion Malignancy risk: 5–15% | Class 3: High-risk thyroid lesion Malignancy risk: 50–90% | |||
| EU-TIRADS | EU-TIRADS 1 (Normal) | EU-TIRADS 2 (Benign category) Malignancy risk: 0% EU-TIRADS 3 (Low-risk category) Malignancy risk: 2–4% | EU-TIRADS 4 (Intermediate-risk category) Malignancy risk: 6–17% | EU-TIRADS 5 (High-risk category) Malignancy risk: 26–87% | ||
| Studies | BTA | EU-TIRADS | K-TIRADS | ACR-TIRADS | Kwak-TIRADS | ATA | AACE/ACE/AME |
|---|---|---|---|---|---|---|---|
| Grani et al. [39] Best performance by ACR-TIRADS with FNR of 2.2% | Unnecessary FNA avoided in 30.7% S = 86.1% Sp = 32% PPV = 8.9% NPV = 96.7% | Unnecessary FNA avoided in 17.1% S = 91.7% Sp = 17.8% PPV = 7.9% NPV = 96.5% | Unnecessary FNA avoided in 53.4% S = 83.3% Sp = 56.2% PPV = 12.8% NPV = 97.8% | Unnecessary FNA avoided in 34.9% S = 86.1% Sp = 36.5% PPV = 9.5% NPV = 97.1% | Unnecessary FNA avoided in 43.8% S = 75% Sp = 45.3% PPV = 9.6% NPV = 95.9% | ||
| Peng et al. [55] Good performance by ACR-TIRADS, ATA, AACE/ACE/AME | S = 94.9% Sp = 58.1% PPV = 76.9% NPV = 88.5% A = 80.0% | S = 92.5% Sp = 68.4% PPV = 79.7% NPV = 87.% A = 82.2% | S = 88.3% Sp = 75.3% PPV = 84.0% NPV = 81.4% A = 83.0% | ||||
| Chng et al. [56] BTA, Kwak-TIRADS, and ATA have high S and NPV. | S = 90% Sp = 50.9% PPV = 45.5% NPV = 91.8% | S = 94% Sp = 28.2% PPV = 37.3% NPV = 91.2% | S = 98% Sp = 17.3% PPV = 35% NPV = 95% | ||||
| Shen et al. [57] Good diagnostic performances by EU-TITADS, ACR-TIRADS, Kwak-TIRADS, and ATA (AUCs > 86%). | S = 93.4% Sp = 81.1% PPV = 81.9% NPV = 92.9% | S = 88.2% Sp = 87.5% PPV = 86.7% NPV = 89% | S = 93.5% Sp = 85.8% PPV = 86% NPV = 93.4% | S = 91.7% Sp = 82% PPV = 82.4% NPV = 92.9% | |||
| Xu et al. [58] -Lowest rate of unnecessary FNA by ACR-TIRADS -Most effective diagnostic performance in specificity by K-TIRADS | S = 83.2% Sp = 79.4% PPV = 73.5% NPV = 87.3% | S = 71.4% Sp = 87.4% PPV = 79.6% NPV = 81.6% | S = 96.6% Sp = 52.9% PPV = 58.6% NPV = 95.8% | ||||
| Marukatat et al. [59] Similar results by K-TIRADS and EU-TIRADS for predicting malignancy | S = 86.2% Sp = 75.5% PPV = 56.6% NPV = 93.7% | S = 83.5% Sp = 76.8% PPV = 57.8% NPV = 93.4% |
| Type of Thyroid Carcinoma | Imaging Recommendations |
|---|---|
| Medullary thyroid carcinoma |
|
| Anaplastic thyroid carcinoma |
|
| Author | Trachea | Esophagus | CCA/IJV/Vessel | RLN |
|---|---|---|---|---|
| Wang et al. [74] | Outer layer encasement | |||
| Seo et al. [76] | One of the following on CT: ≥180° contact Deformity of the tracheal lumen at the level of the mass. Focal irregularity, thickening, or bulge in the mucosa adjacent to the mass. | One of the following on CT: ≥180° contact Loss of normal esophageal wall and lumen. | ≥180° contact | Two of the following: Completely effaced fat in the tracheoesophageal groove >25% abutment at the posterior portions of the thyroid (posterior extracapsular invasion) Ipsilateral vocal cord palsy on CT seen as paramedian cord, anteromedial deviation of the arytenoid cartilage, enlarged pyriform sinus, or enlarged laryngeal ventricle |
| Mancuso et al. [77] | ≥90° contact | |||
| Takashima et al. [78] | ≥180° contact | ≥180° contact | ≥180° contact | |
| Takashima et al. [79] | Effaced fat in the tracheoesophageal groove | |||
| Ishikawa et al. [80] | ≥180° contact | ≥180° contact | ≥180° contact |
| Imaging Findings | Implications in Thyroid Carcinoma Management |
|---|---|
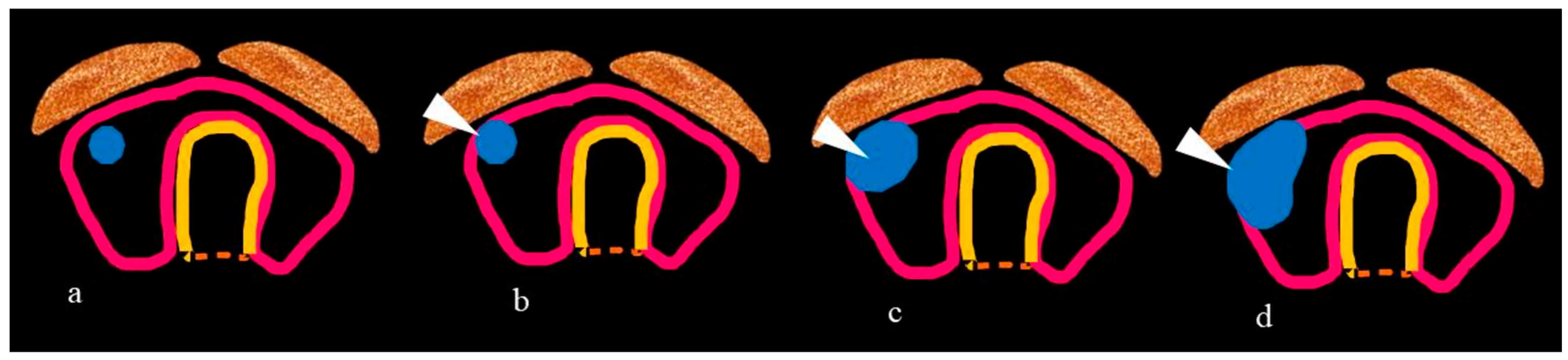
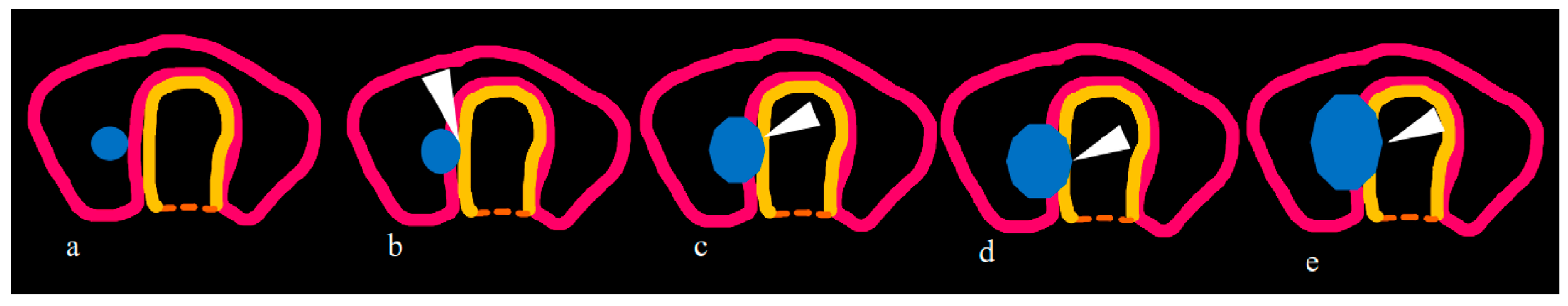
| ETE infiltrating strap muscles | -Total thyroidectomy with/without RAI -Complete resection without reconstruction. |
| Tracheal invasion as per SHIN grading (Figure 3) | -Total thyroidectomy with/without RAI -Grade 1: Shave procedure without any residual disease -Remaining grades: window resection/circumferential tracheal resection and re-anastomosis. |
| Soft tissue in tracheoesophageal groove suggesting RLN involvement with vocal cord paralysis | -Total thyroidectomy with/without RAI -RLN resected only if soft tissue adherent to RLN and its function is compromised preoperatively. |
| Esophageal encasement > 180 degree or frank infiltration | -Total thyroidectomy with/without RAI -Extensive involvement till mucosa and submucosa: Segmental resection with flap reconstruction -Involvement of only muscularis layer: Margins possible without segmental resection. |
| Involvement of larynx | -Total thyroidectomy with RAI -Extensive involvement: Partial/total laryngectomy -Superficial involvement: Shave excision. |
| Prevertebral fascia infiltration | Total thyroidectomy with RAI. |
| Encasement of carotid artery by >270 degree | Total thyroidectomy with RAI. |
| Aberrant right subclavian artery suggesting non-recurrent inferior laryngeal nerve | -Total thyroidectomy with/without RAI -Careful dissection to preserve the nerve. |
| Internal jugular vein tumor thrombosis/involvement | -Total thyroidectomy with RAI -If bilateral IJV involved, then resected with reconstruction, provided adequate proximal and distal stump present. -If unilateral IJV involved, then resected without reconstruction, provided adequate proximal and distal stump present. |
| Nodal burden | -No nodes: only total thyroidectomy -Nodal involvement: Total thyroidectomy with RAI + neck dissection, including central compartment clearance. |
| Distant metastasis | -Total thyroidectomy with RAI. |
| Authors | Nature of Study | Sample Size | Imaging Modality | Outcome | Comments |
|---|---|---|---|---|---|
| Alabousi et al. (2022) [48] | Systematic review | 31,942 | CT and US | For central compartment nodes: CT S = 39% Sp = 87% US S = 28% Sp = 95% For lateral compartment nodes: CT S = 77% Sp = 88% US S = 73% Sp = 89% For ETE: CT and US: S = 86–91% Sp = 30–47% |
CT was more sensitive for central compartment neck nodal metastasis, whereas US was more specific. No significant difference in the diagnostic accuracy for lateral compartment neck nodal metastasis between US and CT. |
| Seo et al. (2010) [76] | Diagnostic accuracy for ETE | 84 | CT | For tracheal invasion: S = 59.1%, Sp = 91.4%, A = 83.2% For esophageal invasion: S = 28.6%, Sp = 96.2%, A = 90.7% For invasion of CCA: S = 75.0%, Sp = 99.4%, A = 98.8% For invasion of IJV: S = 33.3%, Sp = 98.8%, A = 97.1% For invasion of RLN: S = 78.2%, Sp = 89.8%, A = 85.5% | Despite a low sensitivity, CT can be a valuable modality for ETE detection. |
| Shalash et al. (2022) [84] | Comparative study for detection of cervical nodal metastasis in DTC | 30 | DW-MRI and 18F-FDG PET/CT | PET-CT S = 84% Sp = 80% NPV = 50% PPV = 95% A = 83% DW-MRI S = 84% Sp = 60% NPV = 42.8% PPV = 91.3% A = 80% Combined PET-CT and DW-MRI S = 96% NPV = 80% | 18F-FDG PET/CT outperforms DW-MRI for the assessment of neck nodal deposits. |
| Zhang et al. (2020) [85] | Diagnostic study for PTC | 82 | CT | S = 87.8%, Sp = 94.2%, A = 91.1% | Irregular ring, marginal defects, and enhanced blurring on CT were strongly correlated with PTC. |
| Hu et al. (2020) [86] | Comparative study for ETE in PTC | 225 | MRI and US | For minimal ETE: MRI S = 71.3% Sp = 77.1% PPV = 83.8% NPV = 61.7% A = 73.4% US S = 87.5% Sp = 66.6% PPV = 81.4% NPV = 76.2% A = 79.7% For extensive ETE: MRI S = 85.4% Sp = 76.2% PPV = 68.3% NPV = 89.7% A = 79.7% US S = 87.5% Sp = 66.6% PPV = 81.4% NPV = 76.2% A = 79.7% Overall ETE: MRI S = 76.6% Sp = 93.8% PPV = 89.1% NPV = 85.7% A = 86.9% US S = 79.7% Sp = 83.3% PPV = 76.1% NPV = 86% A = 81.9% |
For minimal ETE prediction: preoperative US should be used as the first-line imaging. For extensive ETE evaluation: MRI should be added. For overall ETE: MRI had higher specificity and PPV than US. |
| Cho et al. (2020) [87] | Systematic review and meta-analysis | 504 | MRI | S = 80% Sp = 85% |
Moderate diagnostic performance of MRI for neck nodal metastasis in thyroid cancer. May be an optional or complementary imaging method to US or CT. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakrabarty, N.; Mahajan, A.; Basu, S.; D’Cruz, A.K. Comprehensive Review of the Imaging Recommendations for Diagnosis, Staging, and Management of Thyroid Carcinoma. J. Clin. Med. 2024, 13, 2904. https://doi.org/10.3390/jcm13102904
Chakrabarty N, Mahajan A, Basu S, D’Cruz AK. Comprehensive Review of the Imaging Recommendations for Diagnosis, Staging, and Management of Thyroid Carcinoma. Journal of Clinical Medicine. 2024; 13(10):2904. https://doi.org/10.3390/jcm13102904
Chicago/Turabian StyleChakrabarty, Nivedita, Abhishek Mahajan, Sandip Basu, and Anil K. D’Cruz. 2024. "Comprehensive Review of the Imaging Recommendations for Diagnosis, Staging, and Management of Thyroid Carcinoma" Journal of Clinical Medicine 13, no. 10: 2904. https://doi.org/10.3390/jcm13102904
APA StyleChakrabarty, N., Mahajan, A., Basu, S., & D’Cruz, A. K. (2024). Comprehensive Review of the Imaging Recommendations for Diagnosis, Staging, and Management of Thyroid Carcinoma. Journal of Clinical Medicine, 13(10), 2904. https://doi.org/10.3390/jcm13102904







