Abstract
Background: Enhanced recovery after surgery (ERAS) protocols aim to reduce postoperative complications and promote earlier recovery. Although it is well established in noncardiac surgery fields, the ERAS approach has only recently been adopted in cardiac surgery. The aim of this review is to evaluate the status and implementation of ERAS protocols in patients undergoing heart valve surgery and to summarise associated clinical results. Methods: A literature search for the period January 2015 and January 2024 was performed through online databases. Clinical studies (randomised controlled trials and cohort studies) on patients undergoing heart valve surgical procedures and comparing ERAS and conventional approaches were included. The data extracted covered studies and populations characteristics, early outcomes and the features of each ERAS protocol. Results: There were 14 studies that fulfilled the final search criteria and were ultimately included in the review. Overall, 5142 patients were identified in the 14 studies, with 2501 in ERAS groups and 2641 patients who were representative of control groups. Seven experiences exclusively included patients who underwent heart valve surgery. Twelve out of fourteen protocols involved multiple interventions from the preoperative to postoperative phase, while two studies reported actions limited to intraoperative and postoperative care. We found high heterogeneity among the included protocols regarding key actions targeted for improvement and measured outcomes. All the studies showed that ERAS pathways can be safely adopted in cardiac surgery and in most of the experiences were associated with shorter mechanical ventilation time, reduced postoperative opioid use and reduced ICU and hospital stays. Conclusions: As demonstrated in noncardiac surgery, the adoption of structured ERAS protocols has the potential to improve results in patients undergoing heart valve surgery. Further evidence based on larger populations is needed, including more homogenous pathways and reporting further outcomes in terms of patient satisfaction, recovery and quality of life after surgery.
1. Introduction
Heart valve surgery is nowadays performed with a high safety profile, with real-world/national databases reporting a low rate of mortality after mitral valve surgery (~1%) [1] and for patients undergoing aortic valve replacement (<2%) [2]. Valve repair is largely performed in degenerative mitral valve disease, with evidence of excellent durability and freedom from symptoms, recurrence of mitral regurgitation and reoperation [3,4]. Aortic valve replacement also represents a safe treatment in elderly patients [5] and, in synergy with transcatheter procedures, can successfully address high-risk or technically demanding scenarios [6].
Having achieved excellent results in terms of safety and efficacy, new efforts have been put in place to reduce hospitalisation times and to promote prompt postoperative recovery after cardiac operations. Since the late 1990s, several experiences showed that fast-track programs, including the optimisation of intraoperative anaesthesia and targeting early extubation, were feasible and safe in cardiac surgery patients and allowed a reduction in ICU and hospital stays [7,8,9,10,11]. The establishment of a multidisciplinary approach that incorporates several actions and improvements throughout the entire surgical pathway—from the preoperative to postoperative phase—represents the core of enhanced recovery after surgery (ERAS). ERAS protocols aim to reduce postoperative complications and promote earlier recovery, as has already been demonstrated over the last decade in noncardiac surgery populations [12]. Although well established in general and thoracic surgery, this approach has only recently been adopted in cardiac surgery, with the first consensus guidelines only becoming available in 2019 [13].
The purpose of this narrative review is to evaluate the status and implementation of ERAS protocols in patients undergoing heart valve surgery; to summarise the aspects that have been targeted for improvement and optimisation in the preoperative, intraoperative and postoperative stages; and to report the surgical results in patients treated following an ERAS pathway compared to patients undergoing surgery following conventional perioperative care.
2. Methods
2.1. Definition
Enhanced recovery after surgery (ERAS) is a “multimodal, transdisciplinary care improvement initiative to promote recovery of patients undergoing surgery throughout their entire perioperative journey” aiming “to reduce complications and promote an earlier return to normal activities” [13]. ERAS programs incorporate multiple actions ranging from the preoperative to the postoperative period, including improving patients’ health status and physical and psychological conditions before surgery, providing a reduction in surgical tissue and biological trauma, enhancing pain control and ensuring a reduced mechanical ventilation time and early mobilisation aimed at rapid recovery and patients’ improved overall experience.
2.2. Literature Search
A literature search for the period January 2015 and January 2024 was performed through three online databases (PubMed, Medline, Google) according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria [14]. The following keywords were used: “ERAS” or “enhanced recovery” and “cardiac surgery”, “valve surgery”, “aortic”, “mitral”. The inclusion criteria were clinical studies (randomised controlled trials [RCTs] and cohort studies) on patients undergoing heart valve surgical procedures comparing conventional and ERAS approaches. Studies that included congenital or GUCH cardiac surgery, non-heart valve operation, non-cardiac surgery or transcatheter procedures were excluded. Similarly, comments, letters to the Editor, review articles, meta-analyses and conference abstracts were excluded. Studies with overlapping patient sets from the same institutions were reviewed to include only the largest or most recent data series.
Two reviewers (O.B. and M.G.) independently reviewed all records for inclusion and extracted data separately from relevant studies; divergences were resolved by consensus after discussion with a third reviewer (P.G.M.).
The data extracted from relevant studies included the following:
- Study period, number of patients, type of procedures;
- Patients’ characteristics: populations’ mean age, gender;
- Outcomes: early mortality (in-hospital or 30-day mortality), stroke, acute kidney injury, postoperative atrial fibrillation, postoperative pain management and use of opioids, time of mechanical ventilation, length of intensive care unit stay, overall postoperative hospital stay;
- Key actions included in the ERAS protocols.
In the case of studies that also included non-valvular procedures, we retrieved and presented data from patients who underwent heart valve surgery if provided separately. ERAS key actions were grouped according to the preoperative, intraoperative and postoperative phases. Data were presented as mean and standard deviation, as median and lower (Q1) and upper (Q3) quartiles or as proportions (percentage).
3. Results
3.1. Study Selection
We identified 2312 papers in the literature search, and their abstracts were reviewed. There were 14 studies that fulfilled the final search criteria and were ultimately included in the review [15,16,17,18,19,20,21,22,23,24,25,26,27,28] (Table 1). The PRISMA search flow diagram and checklist are available in Supplemental Figure S1 and Supplemental Table S1.
Overall, 5142 patients were identified in the 14 studies, with 2501 patients who underwent cardiac surgery following an ERAS protocol and 2641 patients representing control groups. One study was an RCT and included 209 patients [15], four studies provided propensity-match analyses [22,26,27,28] (2416 patients), and the remaining nine papers were observational cohort studies [16,17,18,19,20,21,23,24,25] (2495 patients). Seven experiences exclusively included patients who underwent heart valve surgery [20,21,23,24,26,27,28], while mixed case series were reported in the remaining papers with a prevalence of heart valve procedures ranging from 10% up to 50%.
3.2. ERAS Protocols
All the full texts provided details about the ERAS protocol that was adopted in each of the experiences. Table 2 and Figure 1, Figure 2 and Figure 3 summarise the key actions included in the proposed enhanced recovery pathways, which were furthermore grouped according to the preoperative, operative and postoperative phases.
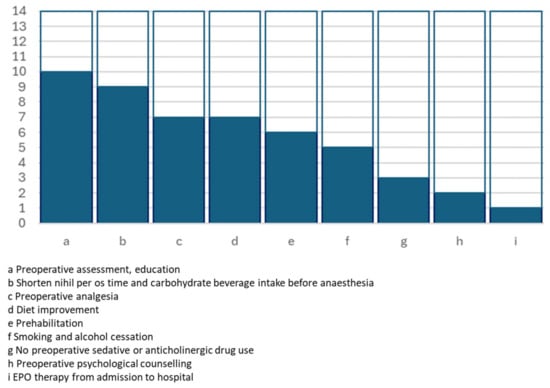
Figure 1.
Graphical representation of the number of studies including preoperative key actions.
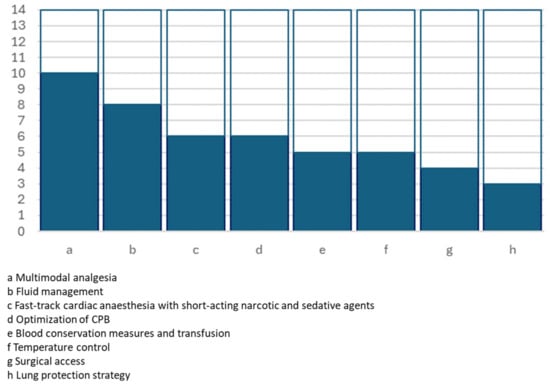
Figure 2.
Graphical representation of the number of studies including intraoperative key actions.
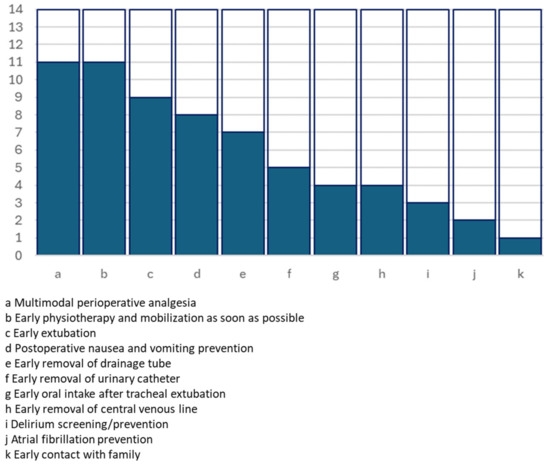
Figure 3.
Graphical representation of the number of studies including postoperative key actions.

Table 1.
List of the full-text papers included in the review summarising patients’ characteristics, postoperative outcomes and key findings associated with the ERAS approach.
Table 1.
List of the full-text papers included in the review summarising patients’ characteristics, postoperative outcomes and key findings associated with the ERAS approach.
| Author(s), Year | Study Period | Patients | Age (Years) | Sex (F/M) | Type of Valve Surgery | Mortality | Stroke | AKI | Postoperative AF | Time of Mechanical Ventilation (Hours) | ICU LOS (Hours) | Hospital LOS (Days) | Other(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li et al., 2018 RCT [15] | 2015–2016 | 104 ERAS | 51.0 ± 10.1 (25.0–69.0) | 53/51 | Aortic n = 11 (10.58%), mitral n = 11 (10.58%) | 0 | 0 | 2 (1.9%) | 4 (3.8%) | 7.2 (0.0–22.3) | 20.9 (13.5–69.3) | 6.0 (2.0–14.0) | ICU readmission 1 vs. 1 (ERAS vs. control) Reintubation 0 vs. 1 (ERAS vs. control) |
| 105 control | 52.2 ± 10.4 (23.0–69.0) | 56/49 | Aortic n = 17 (16.19%), mitral n = 8 (7.62%) | 0 | 1 (0.9%) | 3 (2.9%) | 12 (11.4%) | 8.8 (3.7–44.9) | 22.0 (13.4–212.3) | 7.0 (4.0–16.0) | |||
| p = 0.36 | p = 0.79 | - | - | - | p = 0.04 | p < 0.0001 | p = 0.001 | p = 0.07 | |||||
| Blitzer et al., 2022 OS [16] | 2019–2020 | 34 ERAS | 51.7 ± 14.83 | 9/34 | Valve surgery n = 15 (18%) | 1 (3%) | 0 | - | 5 (15%) | 70.6 ± 287.0 | 165 ± 312 | 15.4 ± 14.7 | |
| 49 control | 54.3 ± 13.6 | 19/30 | 1 (2%) | 0 | - | 6 (12%) | 59.3 ± 136.6 | 165 ± 192 | 16.8 ± 14.4 | ||||
| p = 0.41 | p = 0.24 | p = 0.79 | - | - | p = 0.75 | p = 0.81 | p = 0.99 | p = 0.66 | |||||
| Hendy et al., 2022 OS [17] | 2019–2020 vs. 2017 | 100 ERAS | 63.0 ± 10.7 | 18/82 | Aortic n = 26 (26%), mitral n = 5 (5%) | 0 | 0 | 0 | 13 (13%) | 6.7 ± 1.6 | - | 5.1 ± 1.3 vs. | Time to ambulation (hours) 9.78 ± 2.03 vs. 40.43 ± 46.70 (ERAS vs. control) p < 0.001 |
| 103 control | 64.1 ± 9.7 | 34/69 | Aortic n = 26 (25%), mitral n = 3 (2.9%) | 0 | 1 (0.97%) | 0 | 15 (14.6%) | 14.7 ± 33.1 | - | 8.9 ± 3.5 | |||
| p = 0.04 | p = 0.02 | - | - | p = 1 | - | p = 1 | p < 0.001 | - | p < 0.001 | ||||
| Fleming et al., 2016 OS [18] | 2010–2011 | 52 ERAS | 68.6 ± 11.1 | 14/38 | Aortic n = 5 (9.6%), mitral n = 7 (13.5%) | 1 (1.9%) | 0 | 2 (3.8%) | 7 (13.5%) | - | - | 6 (4–7) | First postoperative intake of enteral solids day 1: 42 (80.1%) vs. 29 (54.7%) (ERAS vs. control) p = 0.007 |
| 53 control | 66.5 ± 11.8 | 15/38 | Aortic n = 8 (15.1%), mitral n = 3 (5.7%) | 2 (3.8%) | 2 (3.8%) | 6 (7.5%) | 15 (28.3%) | - | - | 6 (5–9) | |||
| p = 0.34 | p = 0.87 | p = 0.71 | p = 0.57 | p = 0.16 | p = 0.27 | p = 0.06 | - | - | p = 0.31 | ||||
| Williams et al., 2019 OS [19] | 2017 | 443 ERAS | 65 vs. 65 (ERAS vs. control) | 31/34 | Mitral/tricuspid = 15%, aortic = 17% | - | - | - | - | 5.3 (3.9–6.9) | 28 (23–52) | 6 (5–8) | GI complications 3.6% vs. 6.8% (ERAS vs. control) (p = 0.04) |
| 489 control | 65 | 31/34 | - | - | - | - | 5.2 (3.9- 7.3) | 43 (25–74) | 7 (5–9) | ||||
| - | - | - | - | - | - | p = 0.53 | p < 0.01 | p < 0.01 | |||||
| Zaouter et al., 2019 OS [20] | 2014–2015 | 23 ERAS | 80 (74–82) | 14/9 | Aortic valve (mini-sternotomy) | - | - | 0 | 9 (39%) | - | 24 (24–28) | 7 (6.5–8) | Pulmonary infection 2 (9%) vs. 7 (30%) (ERAS vs. control) p = 0.06 |
| 23 control | 73 (68–82) | 7/16 | - | - | 2 (9%) | 6 (26%) | - | 28 (25–47) | 10 (9–13.5) | ||||
| p = 0.16 | p = 0.04 | - | - | p = 0.15 | p = 0.35 | - | p = 0.003 | p < 0.001 | |||||
| Petersen et al., 2021 OS [21] | 2018–2019 | 61 ERAS | 50.7 ± 12.9 | 14/47 | Aortic n = 37 (61%), mitral n = 24 (39%) | 0 | - | - | - | - | 26.5 ± 25.2 | 6.1 ± 2.6 | |
| 69 control | 54.1 ± 9.5 | 17/52 | Aortic n = 35 (51%), mitral n = 34 (49%) | 0 | - | - | - | - | 46.6 ± 44.9 | 7.7 ± 3.8 | |||
| p = 0.09 | p = 0.81 | p = 0.26 | - | - | - | - | - | p = 0.01 | 0.008 | ||||
| Yazdchi et al., 2021 OS [22] | 2017–2019 | 76 ERAS | 62.7 ± 9.7 | 25/51 | Aortic n = 22 (29%), mitral n = 14 (19%) | 0 | 0 | 0 | 11 (14.5%) | 3.5 (3.1–4.7) | 28 (23–47) | 5 (4–7) | Reoperation for bleeding 0 vs. 0 (ERAS vs. control) |
| 76 control | 63.2 ± 12.8 | 25/51 | Aortic n = 21 (28%), mitral n = 12 (16%) | 0 | 1 (1.3%) | 1 (1.3%) | 20 (26.3%) | 5.3 (3.7–7.5) | 48 (26–69) | 6 (5–8) | |||
| 0.76 | p = 1 | p = 0.4 | - | p = 1 | p = 1 | p = 0.1 | p = 0.01 | p = 0.005 | p = 0.03 | ||||
| Bills et al., 2022 OS [23] | 2019–2020 | 133 ERAS | 65 (60–72) | 41/92 | All valve procedures | - | - | - | - | 10.8 (9.3–12.9) | - | 5.8 (4.9–7.1) | Opioid-related complications ERAS 57% vs. control 63% |
| 185 control | 65 (58–71) | 54/131 | - | - | - | - | 8.85 (7.3–10.3) | - | 6.1 (5–8) | ||||
| p = 0.59 | p = 0.66 | - | - | - | - | p = 0.47 | - | p = 0.89 | |||||
| Loria, 2022 OS [24] | 2018 vs. 2020 | 216 ERAS | 62 (51–70) | 83/167 | Valve n = 54 (22%) | 8 (3.2%) | 2 (0.8%) | 8 (3.2%) | 69 (27.6%) | 4.9 | 74 | 6.5 | Chest tube removal postoperative day 3 vs. 4 (ERAS vs. control) p < 0.0001) |
| 250 control | 64 (57–70) | 66/150 | Valve n = 65 (30.1%) | 5 (2.3%) | 1 (0.5%) | 6 (2.8%) | 62 (28.7%) | 4.7 | 3.3 | 6 | |||
| p = 0.13 | p = 0.54 | p < 0.001 | p = 0.77 | p > 0.99 | p = 0.79 | p = 0.79 | p = 0.54 | p = 0.3 | p = 0.5 | ||||
| Gebauer et al., 2023 OS [25] | 2018–2020 | 101 ERAS | 56 ± 17 | 27/74 | Aortic n = 51 (52%), Mitral n = 49 (49%) | - | 2 (2%) | - | 25 (24.8%) | - | 18.5 ± 6 | 7 ± 3 | - |
| 111 control | 57.5 ± 13 | 32/79 | Aortic n = 49 (44%), mitral n = 62 (56%) | - | 2 (1.8%) | - | 17 (15.3%) | - | 26.5 ± 29 | 8 ± 4 | |||
| p = 0.015 | p = 0.73 | p = 0.28 | - | - | p = 0.08 | - | p < 0.01 | p < 0.01 | |||||
| Giger et al., 2023 OS [26] | 2015–2018 vs. 2018–2020 | 259 ERAS | 69.7 ± 8.3 | 111/148 | Aortic valve | 1 (0.4%) | 1 (0.2%) | 5 (1.9%) | 84 (32%) | 6.9 ± 25 | 1.7 ± 1.8 | 7.7 ± 6.7 | Mechanical ventilation time < 6 h 87% vs. 65% (ERAS vs. control) p < 0.001 Delirium 4 (1.5%) vs. 14 (5.4%) (ERAS vs. control) p = 0.028 |
| 229 control | 70.4 ± 9.7 | 114/115 | 1 (0.4%) | 2 (0.4%) | 1 (0.4%) | 88 (34%) | 6.5 ± 3.9 | 2.2 ± 3.1 | 7.4 ± 4.5 | ||||
| p = 0.39 | 0.79 | p = 1 | p = 0.56 | p = 0.1 | p = 0.79 | p = 0.86 | p = 0.039 | p = 0.5 | |||||
| Obafemi et al., 2023 OS [27] | 2017–2018 | 747 ERAS | 68.7 (61–75) | 157/590 | Aortic valve | 25 (3.3%) | - | - | - | 23.5 (9.6–122.6) | 54.0 (40.4–97.0) | 6.0 (4.9–8.8) | Days to first ambulation 1.6 (1.5–2.6) vs. 2.3 (1.6–3.5) (ERAS vs. Control) p < 0.001 |
| 747 control | 67.3 (59–75) | 165/582 | 30 (4%) | - | - | - | 272.4 (22.2–839.9) | 69.9 (40.8–116.7) | 7.0 (5.2–11.0) | ||||
| p = 0.16 | p = 0.61 | p = 0.47 | - | - | - | p < 0.001 | p = 0.01 | p < 0.001 | |||||
| Berretta et al., 2023 OS [28] | 2016–2020 | 152 ERAS | 69.6 ± 11.1 | 84/78 | Aortic n = 108 (71%) Mitral n = 44 (29%) | 0 | 0 | 2 (1.3%) | 41 (27%) | 0 | 30 (24–52) | 6 (5–7.7) | Respiratory insufficiency 1 (0.7%) vs. 5 (3.3%) (ERAS vs. Control) p = 0.04 |
| 152 control | 70 ± 11.9 | 78/84 | Aortic n = 107 (70%) Mitral n = 45 (30%) | 1 (0.7%) | 2 (1.3%) | 5 (3.3%) | 49 (32.3%) | 6 (4–9) | 40 (24–59) | 7 (6–8) | |||
| - | - | p = 0.9 | p = 0.5 | p = 0.4 | p = 0.4 | p < 0.001 | p = 0.003 | p = 0.04 |
AF: atrial fibrillation, AKI: acute kidney injury, ICU: intensive care unit, OS: observational study, RCT: randomised controlled trial.

Table 2.
Key actions included in the ERAS protocols grouped according to each perioperative stage and their level of evidence (LOE) as recognised by the latest Consensus Guidelines and Statement on ERAS in cardiac surgery.
Table 2.
Key actions included in the ERAS protocols grouped according to each perioperative stage and their level of evidence (LOE) as recognised by the latest Consensus Guidelines and Statement on ERAS in cardiac surgery.
| Action | Details | N of Studies | References | LOE in Engelman et al. [13] | LOE in Grant et al. [29] |
|---|---|---|---|---|---|
| Preoperative | |||||
| Preoperative assessment, education | Personal meeting, video tutorial, pamphlet | 10 | [15,17,18,19,20,21,22,24,25,26] | IIa C | Moderate Low |
| Preoperative psychological counselling | 2 | [15,21] | |||
| Prehabilitation | Pamphlet with exercises; in-person meeting with physiotherapist(s) | 6 | [17,19,20,21,25,26] | IIa B | Low |
| Diet improvement | High-energy, high-carbohydrate diet | 7 | [19,20,21,24,25,26,28] | IIa C | |
| Smoking and alcohol cessation | 5 | [17,19,22,26,28] | I C | ||
| EPO therapy from admission to hospital | 1 | [15] | |||
| Shorten nihil per os time and carbohydrate beverage intake before anaesthesia | Up to 2–6 h before anaesthesia | 9 | [15,16,17,18,19,22,25,26,28] | IIb C | Low |
| No preoperative sedative or anticholinergic drug use | 3 | [15,25,26] | |||
| Preoperative analgesia | Paracetamol, gabapentin, pregabalin | 7 | [16,17,18,19,20,22,24] | I B | |
| Intraoperative | |||||
| Multimodal analgesia | Including locoregional analgesia: paravertebral block, infiltration at the incision site | 10 | [15,19,20,21,22,23,24,25,26,28] | I B | Moderate |
| Fast-track cardiac anaesthesia with short-acting narcotic and sedative agents | 6 | [15,17,19,22,25,28] | IIa B | Moderate | |
| Optimisation of CPB | MIECC, fluid reduction, flow | 6 | [15,20,21,25,26,28] | Low | |
| Lung protection strategy | Low tidal volume (6–8 mL/kg) ventilation, positive end-expiratory pressure, lung recruitment manoeuvre | 3 | [15,20,26] | High | |
| Fluid management | Goal-directed (TOE-guided) | 8 | [15,19,20,21,22,24,25,26] | I B | Moderate |
| Blood conservation measures and transfusion | Cell saver, antiplasmin agent, antifibrinolytic and TEG monitor | 5 | [15,20,24,26,28] | I A | Moderate |
| Temperature control | 5 | [17,19,22,26,28] | I B | ||
| Surgical access | Sternotomy and minimally invasive access | 4 | [20,21,25,28] | ||
| Postoperative | |||||
| Early extubation | On table ICU early extubation | 9 | [17,19,20,21,22,25,26,27,28] | IIa B | Low Moderate |
| Multimodal postoperative analgesia | Patient-controlled analgesia, regional analgesia, infiltration at incision site | 11 | [15,17,18,19,21,22,23,25,26,27,28] | I B | Moderate |
| Postoperative nausea and vomiting prevention | Usually i.v. ondansetron | 8 | [15,16,17,18,19,22,25,26] | Moderate | |
| Atrial fibrillation prevention | 2 | [16,21] | Moderate | ||
| Delirium screening/prevention | 3 | [20,24,28] | I B | High | |
| Early oral intake after tracheal extubation | Usually from 6 h since extubation | 4 | [15,17,26,28] | ||
| Early removal of drainage tube | No clear criteria | 7 | [15,20,21,22,24,25,26,28] | ||
| Early removal of urinary catheter | No clear criteria | 5 | [20,21,22,25,26] | ||
| Early removal of central venous line | Venous line removed at discharge from ICU/removed approximately after 12 h | 4 | [20,21,22,26] | ||
| Early physiotherapy and mobilisation as soon as possible | From 3 h since extubation. Different protocols including chest physiotherapy, bed activities and full mobilisation within 12–24 h | 11 | [14,15,17,18,19,20,21,22,25,26,27,28] | Moderate | |
| Early contact with family | 1 | [28] |
3.3. Preoperative Phase
3.3.1. Assessment, Education and Psychological Counselling
Ten studies contemplated actions regarding patient’s assessment and counselling in their preoperative ERAS protocol [15,17,18,19,20,21,22,24,25,26]. The education of patients and families was usually carried out a few days or weeks before the hospital admission with an in-person meeting involving nurses or within a multidisciplinary team including all the healthcare figures who were expected to take care of the patient throughout the treatment journey. This included a detailed explanation of their pathologies, the perioperative care, the expectations from treatment and recovery. Booklet and video tutorials were used to facilitate communication and to reduce stress and anxiety by presenting the hospital environments and each step of the perioperative course [20]. Two studies provided evidence of the implementation of preoperative psychological counselling conducted by specialised and trained staff [15,21].
3.3.2. Prehabilitation and Diet Improvement
Six of the fourteen studies focused on prehabilitation before heart valve surgery [17,19,20,21,25,26]. Usually, this period started 2–3 weeks before surgery and included explanation and education on how respiratory exercises are performed properly, and the recommendation for daily training until admission [17,20,21,25,26]. None of the papers reported details regarding the type of exercises and the availability of different protocols based on the patients’ functional status and underlying pathologies. Most of these experiences provided in-person meetings with specialised personnel (nurses, physiotherapists, surgeons, anaesthetists) [20,21,25,26].
Screening of nutritional status and counselling was reported in three protocols [19,20,25]. Four experiences recommended a high-protein [19,26] or high-energy, high-carbohydrate diet [21,25] as supplemental nutrition from 1 week to 2 weeks before hospital admission.
The promotion of smoking cessation and improvement of diet before surgery was contemplated by all the studies supporting a prehabilitation period [17,19,20,21,22,25,26,28].
3.3.3. Preoperative Fasting
Several experiences recommended shortening the nihil per os period and allowed the consumption of clear fluid until 2–4 h before surgery [15,17,18,19,22,25,26,28] and solid food until 6–8 h preoperatively [19,26,28]. A carbohydrate drink of 200 mL or 400 mL was prescribed 2–6 h before surgery [15,16,17,18,19,22,25,26] as an adjunct to maintain gut motility and to be used as an energy source (protein-sparing effect) [17].
3.3.4. Preoperative Analgesia
Multimodal analgesia has been embedded in most of the studies included in this review [15,19,20,21,22,23,24,25,26,28], with wide heterogeneity according to the choice of drugs and the timing and dose of administration. Seven protocols contemplated preoperative analgesia starting before the transfer to the operative theatre, aiming for a reduction in opioid use in the postoperative period [16,17,18,19,20,22,24]. This pre-emptive analgesia usually included paracetamol (acetaminophen), gabapentin or pregabalin.
3.4. Intraoperative Phase
3.4.1. Chest Wall Analgesia
Locoregional analgesia was provided with different protocols and included paravertebral blocks [15,26,28], performed at T2–3 and T5–6 levels with the injection of 8–10 mL of 0.25% ropivacaine at each site before the induction of anaesthesia [15]; transverse thoracic plane and anterior serratus plane block [26,28]; local infiltration at the incision and drains insertion sites [15,20,24,28]; the continuous delivery of local anaesthetic through a fascial catheter during the first 24 h [25,28].
3.4.2. Fast Track Anaesthesia
Six studies provided a clear statement regarding the adoption of fast-track cardiac anaesthesia with short-acting narcotic and sedative agents [15,17,19,22,25,28].
Hendy et al. [17] provided details about their protocol including the induction of anaesthesia after 3 min of preoxygenation followed by IV injection of sufentanil (0.5–1 mcg/kg), ketamine (0.5 mg/kg), propofol (1–2 mg/kg) and rocuronium (1.5 mg/kg) sequentially; the maintenance of anaesthesia in the pre-bypass period using a continuous infusion of sufentanil 0.2–0.6 mcg/kg/h and sevoflurane anaesthetic gas (1.5–2.5%) to achieve a minimum alveolar concentration of 0.8; during the bypass time, sevoflurane was turned off and propofol was infused at 80–150 mcg/kg/min instead. Sufentanil infusion (0.2–0.6 mcg/kg/h) was continued until the end of surgery.
Williams et al. [19] administered i.v. fentanyl, typically <1 mg, for the entire case and hydromorphone (0.5–1 mg) near the completion of surgery.
Yazdchi et al. [22] used a 0.2 mcg/kg/hour sufentanil infusion started after induction and discontinued on transfer from the operating room to the ICU. A 0.5 mcg/kg bolus of sufentanil was given prior to sternotomy. Gebauer et al. [25] induced the anaesthesia with sufentanil (50 μg) and propofol (1.5 mg kg−1) and neuromuscular blocking with rocuronium (0.6 mg kg−1). The maintenance of anaesthesia was achieved with remifentanil (0.4–0.5 μg kg−1 min−1), propofol (2 mg kg−1 h−1) and a variable sevoflurane concentration (end-tidal Vol.% 0.6–1.8). On CPB, sevoflurane was administered via the CPB circuit.
3.4.3. Cardiopulmonary Bypass Management
Six studies included actions regarding cardiopulmonary bypass [15,20,21,25,26,28]. In their protocol, Li et al. [15] provided total priming fluid reduction to <1500 mL, retrograde oxygenated blood cardioplegia perfusion, modified ultrafiltration and albumin infusion to maintain a stable plasma colloid osmotic pressure. Gebauer et al. [25] primed the circuit with crystalloid solution, mannitol 20% (100 mL) and 100 mL of albumin 20%. Bypass flow was targeted to >3.2 l m2.min-1 and core temperature was lowered to 32–33 °C. For minimally invasive valve surgery, they routinely used crystalloid custodiol cardioplegia (>20–30 mL/kg) and, while on CPB, they routinely applied haemofiltration for the removal of the priming volume and cardioplegia targeting a zero to negative fluid balance. Similarly, other experiences reported a restrictive fluid substitution [21] and a reduction in total fluid priming to 900–1100 mL [26]. Zaouter et al. [20] and Berretta et al. [28] favoured the use of minimally invasive extracorporeal circulation (MiECC).
3.5. Postoperative Phase
3.5.1. Pain Control
Three studies reported methods of postoperative pain evaluation including a Likert scale [22], the use of a numeric rating scale [23,27] and through the Behaviour Pain Scale in intubated patients [23].
Table 3 provides details about the pain management protocols as reported in the five studies that also evaluated postoperative opioid use [17,18,19,20,24]. After surgery, paracetamol was generally used as the first-choice therapy, while some experiences reported the use of ketamine [26], oxycodone [20], NSAIDs [22,25,26] or tramadol [28], with the systematic [22] or as-needed (VAS > 3) [26] addition of low doses of opioids.

Table 3.
Study endpoints and principal findings.
Local [21] and locoregional anaesthesia [17,25,26,28] was continued until the first postoperative day in patients who underwent minithoracotomy surgery. Different protocols were proposed as concomitant therapies or in the case of breakthrough pain: the administration of fentanyl and acetaminophen [17] and the use of ketamine (0.05–0.15 mg/kg/h during the first 24 h) + paracetamol (1 g/6 h) + nefopam (20 mg IV/4 h during 2 days) + MgSO4 (3 g IV/24 h during 2 days) + ketoprofen (50 mg/6 h during 4 days if GFR > 60 mL/min/1.73 m2) + opioid [26], tramadol 4–8 μg/kg/min or morphine 10–20 mg/24 h [28].
3.5.2. Early Physiotherapy and Mobilisation
Early physiotherapy and mobilisation after surgery were covered in twelve experiences [15,17,18,19,20,21,22,24,25,26,27,28]. Some protocols reported that physiotherapy and mobilisation should begin as soon as possible without further details regarding timing and clinical criteria [15,22,24].
Fleming et al. [18] described the first mobilisation as “sitting regularly in a chair from the first postoperative morning onwards”.
An early mobilisation starting from 2 to 6 h since the extubation was advocated by Hendy et al., “early mobilization starting with sitting at the edge of the bed for 4 h after tracheal extubation” [17]; Williams et al. [19], “when hemodynamically stable and extubated, the patient is assisted out of bed to a chair and activity advanced as tolerated to ambulation 4 times daily”; Zaouter et al. [20], “mobilization on chair on the same day after surgery”.
In the experience of Petersen et al. [21], all patients received their first postoperative physiotherapy treatment in the recovery room 2–3 h after the operation, which included breathing exercises and active mobilisation in the sitting and upright position. The patients were usually transferred to the ICU for overnight monitoring and received their second physiotherapy session in the evening, guided by ICU nursing staff.
Gebauer et al. [25,30] reported that all their patients received the first postoperative physiotherapy treatment in the PACU, two to three hours after surgery with passive mobilisation in bed, respiratory exercises and active mobilisation to the upright position. The patient was then encouraged to sit down at the edge of the bed with the consensus of the responsible anaesthetist and after a careful evaluation of the motoric and sensory functions of the upper and lower extremities as well as the level of postoperative pain. After performing intensive respiratory therapy in the sitting position, the mobilisation was continued to the upright position at the bedside and a first step.
Giger et al. [26] proposed early postoperative active and passive cardio-muscular and respiratory rehabilitation, including respiratory exercises after extubation (passive with spirometer and active with the physiotherapist), assuming a sitting position after extubation and active ambulation on the first postoperative day. Finally, Berretta et al. [28] provided a detailed pathway of early mobilisation and physiotherapy including respiratory therapy (3–6 h after surgery), early mobilisation (6–12 h after surgery) with bed exercises, bed and chair sitting, standing position and ambulation with the possibility of immediate patient–family contact.
3.5.3. Lines and Chest Drains Removal
In order to facilitate mobilisation and to reduce the risk and the burden of perioperative infection, several experiences proposed an early removal of chest drains [15,20,21,22,24,25,26,28], urinary catheters [20,21,22,25,26] and venous and arterial lines [20,21,22,26]. Usually, drainage tubes were removed 12 h after surgery or on postoperative day 1 [15,21,25,28] or postoperative day 2 [22]. This was similar for arterial and venous lines and urinary catheters [21,22,25,26]. However, clear guidance on whether to remove or retain drains, lines and catheters has usually not been reported, with the exception of Zaouter et al. [20], who provided a detailed protocol with the removal of chest tubes when collecting <100 mL of blood in 8 h, the removal of urinary catheter if urinary output was >0.5 mL/h for 6 consecutive hours with no diuretic prescribed and the removal of the central venous line at discharge from the ICU.
3.5.4. Oral Intake and Nausea/Vomiting Prevention
Four papers included the early resumption of oral intake starting within/from 6 h since extubation in their ERAS protocol [15,17,26,28]. A complete diet was resumed from postoperative day 1 or day 2 [15,22,28].
The prevention of nausea and vomiting represented a key action in the postoperative period in several experiences [15,16,17,18,19,22,25,26]. Different protocols were provided with the use of stat doses or the continuous infusion of i.v. ondansetron [15,16,18,19,25] and the adjunct of promethazine when needed [16,19], or with the prescription of droperidol [25,26].
3.6. Outcomes
Ten papers reported data on early mortality (in-hospital or 30-day mortality) [15,16,17,18,21,22,24,26,27,28] and showed no difference between patients who underwent surgery following an ERAS protocol and patients operated on following a conventional approach. Similarly, there was no difference in perioperative cerebral stroke [15,16,17,18,26,28] that generally occurred in less than 1% of the cases. Postoperative atrial fibrillation was registered in 4% to 39% of patients in the ERAS cohorts and in 11% to 34% following conventional treatment [15,16,17,18,20,22,24,25,26,28]. The study of Li et al. [15] reported a significant difference in the occurrence of postoperative AF: 3.8% in the ERAS population vs. 11.4% in the control group (p = 0.04).
Five studies [15,17,22,27,28] found a significant reduction in mechanical ventilation time in patients undergoing an ERAS pathway, with a median time ranging from 0 (on table extubation) to 23.5 h versus 6 to 272 h in the conventional treatment cohorts. Giger et al. [26] reported no difference in terms of mean mechanical ventilation time (ERAS 6.9 h vs. conventional 6.5; p = 0.86) but they reported a higher rate of extubation within the first 6 postoperative hours in patients in the ERAS group (87% vs. 65%; p < 0.001).
Eleven full texts provided details about length of ICU stay. Among them, nine studies [15,19,20,21,22,25,26,27,28] reported a significantly shorter stay in the ICU for patients operated on following an ERAS protocol with a mean stay of 18 to 30 h vs. 22 to 48 h for conventional surgery. On the contrary, Loria et al. [24] (3.1 days vs. 3.3 days, respectively) and Blitzer et al. [16] (6.9 days vs. 6.9 days, respectively) found no difference.
Eight studies [17,19,20,21,22,25,27,28] found a shorter hospitalisation time in patients in ERAS cohorts when compared with patients in control groups. The remaining six papers showed no difference [15,16,18,23,24,26].
Two full texts included details of the medical costs associated with ERAS and conventional approaches. Li et al. [15] found a lower cost for patients in the ERAS group, CNY 69,202 (52,089–123,823) vs. CNY 77,058 (51,390–144,290) in the control group (11% of difference, p = 0.002), and Petersen et al. [21] reported medical costs of EUR 11,200.0 ± 3030 per patient in the ERAS group and EUR 13,110 ± 4528 per patient in the control group, with a financial advantage derived from the implementation of the ERAS program of EUR 1910 per patient (p = 0.006).
Table 1 summarises the data regarding postoperative outcomes. Table 3 provides details about endpoint(s) and key findings associated with the ERAS approach. Table 4 reports details of postoperative opioid use.

Table 4.
Postoperative opioid use.
4. Discussion
4.1. The Advent of ERAS in Cardiac Surgery
The ERAS approach aims to improve patient outcomes by optimising several strategies throughout the perioperative journey. This concept was introduced in 1997 by Henrik Kehlet, who suggested that incorporating multiple changes in the management of the surgical patients could significantly improve the surgical results. In 2001, the ERAS study group was formed with the aim of producing and interpreting the best available evidence to support the idea of fast-track surgery. The first consensus guidelines for colorectal surgery were published in 2005, and since 2010, the ERAS Society [31] has been dedicated to creating guidelines and promoting the broad implementation of ERAS pathways [32]. Although protocols to improve recovery after surgery are well established in general, breast and thoracic surgery, it was not until 2019 that the first consensus guidelines on ERAS in cardiac surgery became available [13]. In the subsequent years, a number of observational and RCT studies have reported satisfactory early results, reduced costs and increased satisfaction in cardiac surgery patients operated on following institutional ERAS protocols [15,19,22,30].
Previous reviews and meta-analyses included experiences mainly in CABG and OPCABG patients [33], reporting on both ERAS and fast-track pathways [34] or “ERAS-like” programmes that covered only some of the key actions of the perioperative management [35]. The purpose of our study was to provide an updated look at the current status of ERAS in heart valves surgery and to summarise the available evidence and results that could potentially support its wider implementation in daily clinical practice.
We have searched for all the experiences embedding an ERAS protocol and including surgical heart valve operations published within the last 10 years, from January 2015 to January 2024. Most of the retrieved abstracts related to non-cardiac surgery and we were able to select 14 articles that included patients operated on for heart valve disease. Ten of these papers were published during or after 2020; however, in six cases [15,18,19,20,27,28], an enhanced recovery pathway was already established before the publication of the 2019 ERAS guidelines in cardiac surgery as the first effort to systemise protocols and outcomes. Not surprisingly, our study showed high heterogeneity in the implementation of different key actions throughout the surgical journey. Twelve out of fourteen protocols involved multiple interventions from the preoperative to postoperative phase, while two studies reported actions that were limited to the management of intraoperative and postoperative analgesia [23] and postoperative care [27].
4.2. Communication and Prehabilitation
Ten studies [15,17,18,19,20,21,22,24,25,26] included a structured program of patients’ assessment and education through an in-person meeting or printed material. The goal of this intervention was to provide in-depth information regarding the pathology, the surgical care and the expectations of recovery. Making patients and their relatives familiar with the procedures and the hospital environments with a booklet describing every step of the protocol or a video describing the patient’s arrival in the operating room can decrease stress and reduce anxiety [20]. However, the inclusion of psychological assessment and support still appears limited, as it was only reported in two experiences from Li et al. [15] and Petersen et al. [22]. Near-future directions may see the progressive use of telemedicine in improving the preventative and pre-hospitalisation care of surgical candidates with electronic platforms that provide tailored information and guidance and encourage engagement in appropriate physical exercise.
Despite previous studies in non-cardiac surgery, although in the presence of a heterogeneous offering of treatment/exercises and delivery methods, having demonstrated some benefits of a pre-admission period of rehabilitation—prehabilitation—in terms of reducing the length of stay in the ICU [36] and postoperative complications [37,38], there is no univocal evidence regarding its efficacy. Prior experiences with cardiac surgery patients showed that a prehabilitation program was also feasible for patients scheduled for CABG surgery [39] and was associated with reduced ICU and hospital lengths of stays, faster postoperative recovery and improved postoperative quality of life [40,41]. Recent ERAS guidelines stated that a prehabilitation period “enables patients to withstand the stress of surgery by augmenting functional capacity. Preoperative exercise decreases sympathetic overreactivity, improves insulin sensitivity, and increases the ratio of lean body mass to body fat. It also improves physical and psychological readiness for surgery, reduces postoperative complications and the length of stay, and improves the transition from the hospital to the community. A cardiac prehabilitation program should include education, nutritional optimization, exercise training, social support, and anxiety reduction, although current existing evidence is limited” [13]. Despite these recommendations, only six [17,19,20,21,25,26] of the fourteen studies offered a prehabilitation program aimed at improving the patient’s physical fitness and providing training to facilitate postoperative physiotherapy.
4.3. Cardiopulmonary Bypass Management
Extracorporeal circulation remains a major determinant of patient outcomes. No recommendations on the management of CPB have been incorporated into the ERAS guidelines for cardiac surgery [13], while a more recent multidisciplinary consensus [29] suggested that goal-directed perfusion may play a role in preventing organ injury associated with cardiopulmonary bypass and that haemodilution and blood transfusion can be prevented by retrograde autologous priming and priming volume reduction. Our study demonstrated that only a minority of experiences with ERAS in valve surgery involved key actions addressing the optimisation of cardiopulmonary bypass conduit. Noteworthily, only two studies [20,28] reported the use of minimally invasive extracorporeal circulation (MiECC). MiECC combines several strategies including the prevention of haemodilution, limitation of cardiotomy aspiration, active normothermia and technologies to increase hemocompatibility and anticoagulation monitoring through a combined surgical approach, anaesthesiology and perfusion management. A recent position paper by the MiECTiS Society [42] stated that MiECC can reduce haemodilution, postoperative bleeding and the need for red-blood-cell transfusion. Alongside improved myocardial and organ protection, the use of MiECC systems has been associated with a reduced rate of postoperative atrial fibrillation, preserved neurocognitive function and attenuated inflammatory response [42] and can promote the adoption of ultra-fast-track pathways [43]. Despite progressive refinements to increase its safety and adoption, minimally invasive extracorporeal circulation remains a demanding procedure requiring multidisciplinary management by surgeons, anaesthetists and perfusionists.
4.4. Analgesia, Anaesthesia and Early Extubation
Pain control after surgery is essential for patient comfort, early extubation and mobilisation and contributes to reducing complications and facilitating postoperative recovery [44,45]. The administration of parenteral opioids has been the cornerstone in pain management after cardiac surgery; however, their use is associated with multiple side effects such as oversedation, respiratory depression and postoperative ileus. Multimodal opioid-reducing/sparing analgesia with the use of more than one pharmacological class of analgesic drugs, targeting different receptors along the pain pathway, aims at improved pain control while reducing adverse effects associated with each class of medication, especially those of opioids [17,18,19,20,23,24]. In most ERAS protocols, paracetamol (acetaminophen) represents the first-line medication for pain control after surgery because it does not interfere with the bowel function and provides superior analgesia when associated with low-dose opioids [13]. According to the ERAS guidelines, in cardiac surgery “there is sufficient evidence to recommend that cardiac surgery programs use acetaminophen, tramadol, dexmedetomidine, and pregabalin (or gabapentin) based on formulary availability”.
Despite the fact that adjuncts of loco-regional analgesia can be a valid tool for perioperative pain control after cardiac surgery [46], the cardiac ERAS guidelines do not provide any specific recommendation for their implementation [13]. Data on regional analgesia are extremely limited in the context of cardiac surgery, as they come from small sample sizes with high heterogeneity in techniques and the measurement of outcomes. Nevertheless, the available evidence indicates that loco-regional analgesia could facilitate enhanced recovery pathways [15,20,26,28,29,45,47]. The recent joint consensus from the ERAS Cardiac Society, the ERAS International Society and the Society of Thoracic Surgeons (STS) recommended chest wall regional analgesia as “an effective component of a multimodal approach to perioperative pain management” [29] and suggested that further research is required to establish delivery methods and the efficacy of locoregional pain control in cardiac surgery.
The adoption of fast-track cardiac anaesthesia with short-acting narcotic and sedative agents is advocated to reduce the time of mechanical ventilation [15,17,19,22,25,28,48]. Although early extubation represents one of the main goals in most experiences that include an ERAS program [17,19,20,21,22,25,26,27,28], the results of our review showed that there was wide heterogeneity in the duration of postoperative mechanical ventilation time, with some studies reporting a mean postoperative intubation time between 0 and 10 h [15,17,18,22,24,26,28], whereas others included patients with an average intubation time between 1 day and 3 days [16,27].
Several studies have already underscored the benefits associated with early/fast-track extubation, especially in OPCABG and CABG operations, mini heart valve surgery and transapical TAVI [10,11,49,50,51]. Two recent observational studies on minimally invasive mitral valve surgery showed that early extubation (within 6 to 10 h after the end of the surgical procedure) was associated with reduced stays in intensive care units and reduced overall hospitalisation [45,51]. A further step is represented by on-table extubation in the operating room at the end of the surgical procedure. Despite earlier concerns about safety issues [52], more recent evidence showed that on-table extubation can be safely achieved in cardiac surgery patients with no increased risk of reintubation for respiratory failure. Moreover, on-table extubation was shown to be associated with a further shortening of ICU and hospital stays [25,45,53,54,55]. Based on this growing evidence, the ERAS guidelines recommend strategies to ensure extubation within 6 h of surgery [13,29].
4.5. Early Mobilisation
One of the advantages associated with early extubation is the possibility to start respiratory therapy and to mobilise the patient as soon as possible. In non-cardiac surgery specialities, early ambulation and physiotherapy have been associated with reduced intensive care unit stays and better mobility at the time of discharge [56,57,58]. In cardiac surgery, early mobilisation can improve pulmonary function and reduce ICU lengths of stays, while promoting a better exercise capacity and a quicker functional recovery. Improved functional capacity is associated with a higher rate of home discharge without the need for any further period of rehabilitation [40,59,60].
The interpretation of the results coming from the literature remains difficult. As already highlighted in this review, there are no consistent details on the timing of early mobilisation; furthermore, clear eligibility criteria for this action are missing. According to the proposed protocols and keeping in mind the limitation of having selected mostly young/middle-aged low-risk patients, a safe approach under the care of physiotherapists and after a clinical evaluation by anaesthetists or intensivists would suggest starting “early physiotherapy” after 2–6 h from tracheal extubation [17,21,25,26,28]. Controlled breathing exercises (active or passive), bed activities with upper and lower limb exercises, followed by bed-sitting at three to six hours after surgery and mobilisation with upright position after 12 h or on postoperative day 1 seem to be perfectly safe.
Many factors can impact achieving early mobilisation, such as pain control, the need for intravenous fluid or medication support and the presence and maintenance of chest tubes or lines. Only one study [20] provided clear guidance regarding the decision of whether to remove or maintain chest tubes. The remaining experiences stated that usually, the drainage tubes and other lines were removed between 12 h after surgery and postoperative day 2 [15,21,22,25,26,28]. Another noteworthy finding was the limited availability of details regarding protocols for postoperative pain control and methods of pain assessment, with very little data on the means and timing of postoperative pain assessment.
All studies that included a structured protocol of early physiotherapy and mobilisation were characterised by shorter times of mechanical ventilation [17,28] and by reduced lengths of ICU [20,21,25,26,28] and hospital stays [17,20,21,25,28]. These actions are interdependent objectives and further highlight the importance of the ERAS concept of introducing multiple key actions which can act synergically to improve surgical outcomes. However, the positive impact of these interventions on mortality and major postoperative morbidity has not yet been clearly demonstrated by different authors. As already highlighted before, the lack of clear evidence may be due to the large inclusion of young, low-risk patients with an overall low incidence of adverse events.
4.6. The Role of Minimally Invasive Cardiac Surgery
Noncardiac surgery ERAS guidelines support, with a strong grade of recommendation, minimally invasive access in gynaecologic surgery as “an important tenet of ERAS” and an option to be “preferred for appropriate patients when feasible” [57]. Similarly, for general surgery, based on high-level evidence and a strong grade of recommendation, ERAS guidelines state that “a minimally invasive approach to colon and rectal cancer has clear advantages for improved and more rapid recovery, reduced general complications, reduced wound-related complications including incisional hernia and fewer adhesions. It is also an enabler for successful administration of many of the major components of ERAS such as opiate sparing analgesia and optimised fluid therapy” [58]. On the contrary, cardiac ERAS guidelines [13] do not provide any specific recommendation or suggestion regarding the adoption of minimally invasive surgery to improve patients’ outcomes and recovery, although a smaller incision, the avoidance of sternotomy and reduced surgical trauma could intuitively facilitate pain control, early mobilisation and lower wound complications. Heart valve surgery through reduced surgical access (mini-thoracotomy, mini-sternotomy) is nowadays well established in highly specialised heart valve centres and has proven to be as safe and effective as conventional treatment [61,62]. However, recent national and multi-institutional data showed that minimally invasive cardiac surgery techniques are not widely adopted and that mini-thoracotomy mitral surgery is performed only in 23% to 54% of the patients scheduled for mitral valve procedures [1,63,64,65]. Not surprisingly, only four studies in this review included patients operated on with minimal access approaches [20,21,25,28]. Reduced surgical access may be perceived as being difficult for adequate exposure and myocardial protection and can translate into longer cardiopulmonary bypass and cross-clamp times. Tissue trauma is important and undoubtedly affects the postoperative recovery; however, lengthy and complex procedures based on the only perspective of providing cardiac operations through small cuts can hardly be seen as minimally invasive surgery since biological trauma and operative times remain the main determinant of perioperative complications.
Recent experiences have highlighted that minimally invasive heart valve surgery, when integrated in an enhanced recovery after surgery pathway, while providing safe operations and durable results, can accomplish patients’ desire for easier mobilisation, faster recovery and return to normal activity, as it has been associated with reduced mechanical ventilation time and shorter ICU stays and hospitalisation times [45,51,66,67]. Nowadays, having achieved excellent results in terms of low mortality and low postoperative complications, minimally invasive cardiac surgery is called upon to address these new therapeutic targets that are of the utmost importance both for young and active patients who may experience the perioperative journey as the worst health issue that affects their lives, and for elderly and comorbid patients who could still benefit more from attenuated physical, physiological and psychological trauma.
5. Conclusions
In the context of heart valve surgery, there is limited evidence regarding the implementation and benefits of enhanced recovery after surgery pathways. Our study summarised relevant findings from initial ERAS experiences in cardiac surgery, which demonstrated its feasibility and potential for improving surgical outcomes in the treatment of patients undergoing heart valve operations. Multidisciplinary teamwork represents the core of the ERAS approach and allows the promotion of key actions for preoperative (education, prehabilitation, diet improvement), intraoperative (multimodal analgesia, fast-track anaesthesia, CPB conduit) and postoperative (pain control, early extubation, early mobilisation and feeding) management.
Despite the lack of impact of the ERAS programmes on early mortality and postoperative morbidity, these protocols have proven to be safe and were associated with shorter mechanical ventilation time, reduced ICU and hospital stays and reduced postoperative opioid use.
6. Future Directions
The present day is still to be considered the dawn of the ERAS approach in cardiac surgery. Next steps should include a broader implementation of ERAS protocols leading to the increased recruitment of patients and hence the availability of larger data sets to demonstrate feasibility and associated benefits. Further studies should focus on the impact of different surgical accesses and on the management of cardiopulmonary bypass. Additional patient-reported outcomes, beyond the usual hard endpoints, should be targeted in future studies to evaluate the impact of ERAS in cardiac surgery. It would be of utmost importance to address the evaluation of postoperative pain and patient satisfaction, assessing quality of life, recovery and psychological status. The inclusion of frail and higher-risk patients could further reveal the advantages to the prognosis and morbidity of a shared multidisciplinary ERAS approach in this less-healthy group of patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13102903/s1, Figure S1: Flow chart of literature search according to PRISMA guidelines; Table S1: PRISMA 2020 checklist [68].
Author Contributions
Conceptualisation, P.G.M., O.B. and M.D.E.; methodology, P.G.M., O.B. and M.G.; software, P.G.M.; validation, P.G.M., O.B., P.B., J.A., M.C., C.Z., H.M.Z., C.M. and M.D.E.; formal analysis, P.G.M.; investigation, O.B., P.G.M. and M.G.; resources, M.D.E.; data curation, O.B., M.G., P.G.M., P.B. and J.A.; writing—original draft preparation, O.B., P.G.M., C.M. and M.D.E.; writing—review and editing, P.G.M., M.D.E. and C.M.; supervision, P.G.M., C.M., H.M.Z. and M.D.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to its nature as a review study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gammie, J.S.; Chikwe, J.; Badhwar, V.; Thibault, D.P.; Vemulapalli, S.; Thourani, V.H.; Gillinov, M.; Adams, D.H.; Rankin, J.S.; Ghoreishi, M.; et al. Isolated Mitral Valve Surgery: The Society of Thoracic Surgeons Adult Cardiac Surgery Database Analysis. Ann. Thorac. Surg. 2018, 106, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, M.; Bilkhu, R.; Embleton-Thirsk, A.; Dehbi, H.-M.; Mani, K.; Anderson, J.; Avlonitis, V.; Baghai, M.; Birdi, I.; Booth, K.; et al. Surgical aortic valve replacement in the era of transcatheter aortic valve implantation: A review of the UK national database. BMJ Open 2021, 11, e046491. [Google Scholar] [CrossRef] [PubMed]
- David, T.E.; David, C.M.; Tsang, W.; Lafreniere-Roula, M.; Manlhiot, C. Long-Term Results of Mitral Valve Repair for Regurgitation Due to Leaflet Prolapse. J. Am. Coll. Cardiol. 2019, 74, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Javadikasgari, H.; Mihaljevic, T.; Suri, R.M.; Svensson, L.G.; Navia, J.L.; Wang, R.Z.; Tappuni, B.; Lowry, A.M.; McCurry, K.R.; Blackstone, E.H.; et al. Simple versus complex degenerative mitral valve disease. J. Thorac. Cardiovasc. Surg. 2018, 156, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Malvindi, P.G.; Luthra, S.; Giritharan, S.; Kowalewski, M.; Ohri, S. Long-term survival after surgical aortic valve replacement in patients aged 80 years and over. Eur. J. Cardio Thorac. Surg. 2021, 60, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Malvindi, P.G.; Bifulco, O.; Berretta, P.; Alfonsi, J.; Cefarelli, M.; Zingaro, C.; Capestro, F.; D’alfonso, A.; Di Eusanio, M. Improved Early Outcomes in Women Undergoing Aortic Valve Interventions. J. Clin. Med. 2023, 12, 5749. [Google Scholar] [CrossRef] [PubMed]
- Royse, C.F.; Royse, A.G.; Soeding, P.F. Routine immediate extubation after cardiac operation: A review of our first 100 patients. Ann. Thorac. Surg. 1999, 68, 1326. [Google Scholar] [CrossRef] [PubMed]
- Turfrey, D.J.; Ray, D.A.A.; Sutcliffe, N.P.; Ramayya, P.; Kenny, G.N.C.; Scott, N.B. Thoracic epidural anaesthesia for coronary artery bypass graft surgeryEffects on postoperative complications. Anaesthesia 1997, 52, 1090. [Google Scholar] [CrossRef]
- Straka, Z.; Brucek, P.; Vanek, T.; Votava, J.; Widimsky, P. Routine immediate extubation for off-pump coronary artery bypass grafting without thoracic epidural analgesia. Ann. Thorac. Surg. 2002, 74, 1544. [Google Scholar] [CrossRef]
- Reis, J.; Mota, J.; Ponce, P.; Costa-Pereira, A.; Guerreiro, M. Early extubation does not increase complication rates after coronary artery bypass graft surgery with cardiopulmonary bypass. Eur. J. Cardio Thorac. Surg. 2002, 21, 1026–1030. [Google Scholar] [CrossRef]
- Svircevic, V.; Nierich, A.P.; Moons, K.G.M.; Bruinsma, G.J.B.B.; Kalkman, C.J.; van Dijk, D. Fast-track anesthesia and cardiac surgery: A retrospective cohort study of 7989 patients. Obstet. Anesth. Dig. 2009, 108, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Thiele, R.H.; Rea, K.M.; Turrentine, F.E.; Friel, C.M.; Hassinger, T.E.; Goudreau, B.J.; Umapathi, B.A.; Kron, I.L.; Sawyer, R.G.; Hedrick, T.L.; et al. Standardization of care: Impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J. Am. Coll. Surg. 2015, 220, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Engelman, D.T.; Ben Ali, W.; Williams, J.B.; Perrault, L.P.; Reddy, V.S.; Arora, R.C.; Roselli, E.E.; Khoynezhad, A.; Gerdisch, M.; Levy, J.H.; et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery after Surgery Society Recommendations. JAMA Surg. 2019, 154, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, J.; Gan, T.J.; Qin, G.; Wang, L.; Zhu, M.; Zhang, Z.; Pan, Y.; Ye, Z.; Zhang, F.; et al. Enhanced recovery after surgery pathway for patients undergoing cardiac surgery: A randomized clinical trial. Eur. J. Cardio Thorac. Surg. 2018, 54, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, D.; Blackshear, C.T.; Stuckey, J.; Kruse, L.; Creswell, L.L.; Lirette, S.T.; Copeland, H. Enhanced recovery after surgery multi-modality pain regimen performs similar to PRN narcotics on outcomes and pain control after cardiac surgery: A quality improvement project. J. Card. Surg. 2022, 37, 1520–1522. [Google Scholar] [CrossRef] [PubMed]
- Hendy, A.; DiQuinzo, C.; O‘Reilly, M.; Hendy, A.; Vician, M.; Theriault, C.; Chedrawy, E.; Hirsch, G.; Aliter, H. Implementation of enhanced recovery in cardiac surgery: An experimental study with the control group. Asian Cardiovasc. Thorac. Ann. 2023, 31, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I.O.; Garratt, C.; Guha, R.; Desai, J.; Chaubey, S.; Wang, Y.; Leonard, S.; Kunst, G. Aggregation of Marginal Gains in Cardiac Surgery: Feasibility of a Perioperative Care Bundle for Enhanced Recovery in Cardiac Surgical Patients. J. Cardiothorac. Vasc. Anesth. 2016, 30, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.B.; McConnell, G.; Allender, J.E.; Woltz, P.; Kane, K.; Smith, P.K.; Engelman, D.T.; Bradford, W.T. One-year results from the first US-based enhanced recovery after cardiac surgery (ERAS Cardiac) program. J. Thorac. Cardiovasc. Surg. 2019, 157, 1881–1888. [Google Scholar] [CrossRef]
- Zaouter, C.; Oses, P.; Assatourian, S.; Labrousse, L.; Rémy, A.; Ouattara, A. Reduced Length of Hospital Stay for Cardiac Sur-gery-Implementing an Optimized Perioperative Pathway: Prospective Evaluation of an Enhanced Recovery After Surgery Program Designed for Mini-Invasive Aortic Valve Replacement. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3010–3019. [Google Scholar] [CrossRef]
- Petersen, J.; Kloth, B.; Konertz, J.; Kubitz, J.; Schulte-Uentrop, L.; Ketels, G.; Reichenspurner, H.; Girdauskas, E. Economic impact of enhanced recovery after surgery protocol in minimally invasive cardiac surgery. BMC Health Serv. Res. 2021, 21, 254. [Google Scholar] [CrossRef]
- Yazdchi, F.; Hirji, S.; Harloff, M.; McGurk, S.; Morth, K.; Zammert, M.; Shook, D.; Varelmann, D.; Shekar, P.; Kaneko, T.; et al. Enhanced Recovery After Cardiac Surgery: A Propensity-Matched Analysis. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Bills, S.; Wills, B.; Boyd, S.; Elbeery, J. Impact of an Enhanced Recovery after Surgery Protocol on Postoperative Outcomes in Cardiac Surgery. J. Pharm. Pract. 2023, 36, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Loria, C.M.; Zborek, K.; Millward, J.B.; Anderson, M.P.; Richardson, C.M.; Namburi, N.; Faiza, Z.; Timsina, L.R.; Lee, L.S. Enhanced recovery after cardiac surgery protocol reduces perioperative opioid use. JTCVS Open 2022, 12, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, A.; Konertz, J.; Petersen, J.; Brickwedel, J.; Köster, D.; Schulte-Uentrop, L.; Reichenspurner, H.; Girdauskas, E. The impact of a standardized Enhanced Recovery After Surgery (ERAS) protocol in patients undergoing minimally invasive heart valve surgery. PLoS ONE 2023, 18, e0283652. [Google Scholar] [CrossRef] [PubMed]
- Giger, A.; Schneider, C.; Marguerite, S.; Ramlugun, D.; Maechel, A.-L.; Collange, O.; Mertes, P.-M.; Mazzucotelli, J.-P.; Kindo, M. An enhanced recovery programme significantly improves postoperative outcomes after surgical aortic valve replacement. Eur. J. Cardio Thorac. Surg. 2023, 63, ezad125. [Google Scholar] [CrossRef]
- Obafemi, T.; Mullis, D.; Bajaj, S.; Krishna, P.; Boyd, J. Results following implementation of a cardiac surgery ERAS protocol. PLoS ONE 2023, 18, e0277868. [Google Scholar] [CrossRef]
- Berretta, P.; De Angelis, V.; Alfonsi, J.; Pierri, M.D.; Malvindi, P.G.; Zahedi, H.M.; Munch, C.; Di Eusanio, M. Enhanced recovery after minimally invasive heart valve surgery: Early and midterm outcomes. Int. J. Cardiol. 2023, 370, 98–104. [Google Scholar] [CrossRef]
- Grant, M.C.; Crisafi, C.; Alvarez, A.; Arora, R.C.; Brindle, M.E.; Chatterjee, S.; Ender, J.; Fletcher, N.; Gregory, A.J.; Gunaydin, S.; et al. Perioperative Care in Cardiac Surgery: A Joint Consensus Statement by the Enhanced Recovery After Surgery (ERAS) Cardiac Society, ERAS International Society, and The Society of Thoracic Surgeons (STS). Ann. Thorac. Surg. 2024, 117, 669–689. [Google Scholar] [CrossRef]
- Kubitz, J.C.; Schulte-Uentrop, L.; Zoellner, C.; Lemke, M.; Messner-Schmitt, A.; Kalbacher, D.; Sill, B.; Reichenspurner, H.; Koell, B.; Girdauskas, E. Establishment of an enhanced recovery after surgery protocol in minimally invasive heart valve surgery. PLoS ONE 2020, 15, e0231378. [Google Scholar] [CrossRef]
- Available online: https://erassociety.org/ (accessed on 14 February 2024).
- Golder, H.J.; Papalois, V. Enhanced Recovery after Surgery: History, Key Advancements and Developments in Transplant Sur-gery. J. Clin. Med. 2021, 10, 1634. [Google Scholar] [CrossRef]
- Baxter, R.; Squiers, J.; Conner, W.; Kent, M.; Fann, J.; Lobdell, K.; DiMaio, J.M. Enhanced Recovery After Surgery: A Narrative Review of its Application in Cardiac Surgery. Ann. Thorac. Surg. 2020, 109, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Maj, G.; Regesta, T.; Campanella, A.; Cavozza, C.; Parodi, G.; Audo, A. Optimal Management of Patients Treated with Minimally Invasive Cardiac Surgery in the Era of Enhanced Recovery After Surgery and Fast-Track Protocols: A Narrative Review. J. Cardiothorac. Vasc. Anesth. 2022, 36, 766–775. [Google Scholar] [CrossRef]
- Spadaccio, C.; Salsano, A.; Pisani, A.; Nenna, A.; Nappi, F.; Osho, A.; D’alessandro, D.; Sundt, T.M.; Crestanello, J.; Engelman, D.; et al. Enhanced recovery protocols after surgery: A systematic review and meta-analysis of randomized trials in cardiac surgery. World J. Surg. 2024, 48, 779–790. [Google Scholar] [CrossRef]
- Cambriel, A.; Choisy, B.; Hedou, J.; Bonnet, M.-P.; Fellous, S.; Lefevre, J.H.; Voron, T.; Gaudillière, D.; Kin, C.; Gaudillière, B.; et al. Impact of preoperative uni- or multimodal prehabilitation on postoperative morbidity: Meta-analysis. BJS Open 2023, 7, zrad129. [Google Scholar] [CrossRef]
- Moran, J.; Guinan, E.; McCormick, P.; Larkin, J.; Mockler, D.; Hussey, J.; Moriarty, J.; Wilson, F. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: A systematic review and meta-analysis. Surgery 2016, 160, 1189–1201. [Google Scholar] [CrossRef]
- Barberan-Garcia, A.; Ubré, M.; Roca, J.; Lacy, A.M.; Burgos, F.; Risco, R.; Momblán, D.; Balust, J.; Blanco, I.; Martínez-Pallí, G. Personalised Prehabilitation in High-risk Patients Undergoing Elective Major Abdominal Surgery: A Randomized Blinded Controlled Trial. Ann. Surg. 2018, 267, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Arthur, H.M.; Daniels, C.; McKelvie, R.; Hirsh, J.; Rush, B. Effect of a Preoperative intervention on preoperative and postoperative outcomes in low-risk patients awaiting elective coronary artery bypass graft surgery. A randomized, controlled trial. Ann. Intern. Med. 2000, 133, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Sawatzky, J.-A.V.; Kehler, D.S.; Ready, A.E.; Lerner, N.; Boreskie, S.; Lamont, D.; Luchik, D.; Arora, R.C.; A Duhamel, T. Prehabilitation program for elective coronary artery bypass graft surgery patients: A pilot randomized controlled study. Clin. Rehabil. 2014, 28, 648–657. [Google Scholar] [CrossRef]
- Waite, I.; Deshpande, R.; Baghai, M.; Massey, T.; Wendler, O.; Greenwood, S. Home-based preoperative rehabilitation (prehab) to improve physical function and reduce hospital length of stay for frail patients undergoing coronary artery bypass graft and valve surgery. J. Cardiothorac. Surg. 2017, 12, 91. [Google Scholar] [CrossRef]
- Anastasiadis, K.; Murkin, J.; Antonitsis, P.; Bauer, A.; Ranucci, M.; Gygax, E.; Schaarschmidt, J.; Fromes, Y.; Philipp, A.; Eberle, B.; et al. Use of minimal invasive extracorporeal circulation in cardiac surgery: Principles, definitions and potential benefits. A position paper from the Minimal invasive Extra-Corporeal Technologies international Society (MiECTiS). Interact. Cardiovasc. Thorac. Surg. 2016, 22, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Berretta, P.; Cefarelli, M.; Montecchiani, L.; Alfonsi, J.; Vessella, W.; Zahedi, M.H.; Carozza, R.; Munch, C.; Di Eusanio, M. Minimally invasive versus standard extracorporeal circulation system in minimally invasive aortic valve surgery: A propensity score-matched study. Eur. J. Cardio Thorac. Surg. 2019, 57, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Zubrzycki, M.; Liebold, A.; Skrabal, C.; Reinelt, H.; Ziegler, M.; Perdas, E.; Zubrzycka, M. Assessment and pathophysiology of pain in cardiac surgery. J. Pain Res. 2018, 11, 1599–1611. [Google Scholar] [CrossRef] [PubMed]
- Malvindi, P.G.; Bifulco, O.; Berretta, P.; Galeazzi, M.; Zingaro, C.; D’alfonso, A.; Zahedi, H.M.; Munch, C.; Di Eusanio, M. On-table extubation is associated with reduced intensive care unit stay and hospitalization after trans-axillary minimally invasive mitral valve surgery. Eur. J. Cardio Thorac. Surg. 2024, 65, ezae010. [Google Scholar] [CrossRef] [PubMed]
- Balan, C.; Bubenek-Turconi, S.I.; Tomescu, D.R.; Valeanu, L. Ultrasound-Guided Regional Anesthesia-Current Strategies for En-hanced Recovery after Cardiac Surgery. Medicina 2021, 57, 312. [Google Scholar] [CrossRef]
- Jiang, T.; Ting, A.; Leclerc, M.; Calkins, K.; Huang, J. Regional Anesthesia in Cardiac Surgery: A Review of the Literature. Cureus 2021, 13, 18808. [Google Scholar] [CrossRef]
- Ender, J.; Borger, M.A.; Scholz, M.; Funkat, A.K.; Anwar, N.; Sommer, M.; Mohr, F.W.; Fassl, J. Cardiac surgery fast-track treatment in a postanesthetic care unit: Six-month results of the Leipzig fast-track concept. Anesthesiology 2008, 109, 61–66. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, Y.; Hu, Z.; Mao, H.; Xu, Q.; Wu, Q. Clinical Evaluation of on-Table Extubation in Patients Aged over 60 Years Un-dergoing Minimally Invasive Mitral or Aortic Valve Replacement Surgery. Front. Surg. 2022, 9, 934044. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, L.; Teng, H.; Lou, X.; Wei, H.; Yan, M. The Clinical Application of Ultra-Fast-Track Cardiac Anesthesia in Right-Thoracoscopic Minimally Invasive Cardiac Surgery: A Retrospective Observational Study. J. Cardiothorac. Vasc. Anesth. 2023, 37, 700–706. [Google Scholar] [CrossRef]
- Van Praet, K.M.; Kofler, M.; Hirsch, S.; Akansel, S.; Hommel, M.; Sündermann, S.H.; Meyer, A.; Jacobs, S.; Falk, V.; Kempfert, J. Factors associated with an unsuccessful fast-track course following minimally invasive surgical mitral valve repair. Eur. J. Cardio Thorac. Surg. 2022, 62, ezac451. [Google Scholar] [CrossRef]
- Montes, F.R.; Sanchez, S.I.; Giraldo, J.C.; Rincón, J.D.; Rincón, I.E.; Vanegas, M.V.; Charris, H. The lack of benefit of tracheal extubation in the operating room after coronary artery bypass surgery. Obstet. Anesth. Dig. 2000, 91, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Badhwar, V.; Esper, S.; Brooks, M.; Mulukutla, S.; Hardison, R.; Mallios, D.; Chu, D.; Wei, L.; Subramaniam, K. Extubating in the operating room after adult cardiac surgery safely improves outcomes and lowers costs. J. Thorac. Cardiovasc. Surg. 2014, 148, 3101–3109.e1. [Google Scholar] [CrossRef] [PubMed]
- Totonchi, Z.; Azarfarin, R.; Jafari, L.; Ghavidel, A.A.; Baharestani, B.; Alizadehasl, A.; Alasti, F.M.; Ghaffarinejad, M.H. Feasibility of On-table Extubation After Cardiac Surgery with Cardiopulmonary Bypass: A Randomized Clinical Trial. Anesthesiol. Pain Med. 2018, 8, e80158. [Google Scholar] [CrossRef] [PubMed]
- James, L.; Smith, D.E.; Galloway, A.C.; Paone, D.; Allison, M.; Shrivastava, S.; Vaynblat, M.; Swistel, D.G.; Loulmet, D.F.; Grossi, E.A.; et al. Routine Extubation in the Operating Room after Isolated Coronary Artery Bypass. Ann. Thorac. Surg. 2024, 117, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, E.; dos Reis Falcao, L.F.; O’Kane, M.; Liem, R.; Pournaras, D.J.; Salminen, P.; Urman, R.D.; Wadhwa, A.; Gustafsson, U.O.; Thorell, A. Guidelines for perioperative care in bariatric surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations: A 2021 update. World J. Surg. 2022, 46, 729–751. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Bakkum-Gamez, J.; Kalogera, E.; Glaser, G.; Altman, A.; A Meyer, L.; Taylor, J.S.; Iniesta, M.; Lasala, J.; Mena, G.; et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations—2019 update. Int. J. Gynecol. Cancer 2019, 29, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced recovery after surgery (ERAS®) society recommendations: 2018. World J. Surg. 2018, 43, 659–695. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P.; Indraratna, P.; Tardo, D.T.; Peeceeyen, S.C.; E Peoples, G. Safety and efficacy of aerobic exercise commenced early after cardiac surgery: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2019, 26, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Ramos Dos Santos, P.M.; Aquaroni Ricci, N.; Aparecida Bordignon Suster, É.; de Moraes Paisani, D.; Dias Chiavegato, L. Effects of early mobilisation in patients after cardiac surgery: A systematic review. Physiotherapy 2017, 103, 1–12. [Google Scholar] [CrossRef]
- Sá, M.P.B.O.; Eynde, J.V.D.; Cavalcanti, L.R.P.; Kadyraliev, B.; Enginoev, S.; Zhigalov, K.; Ruhparwar, A.; Weymann, A.; Dreyfus, G. Mitral valve repair with minimally invasive approaches vs sternotomy: A meta-analysis of early and late results in randomized and matched observational studies. J. Card. Surg. 2020, 35, 2307–2323. [Google Scholar] [CrossRef]
- Eqbal, A.J.; Gupta, S.; Basha, A.; Qiu, Y.; Wu, N.; Rega, F.; Chu, F.V.; Belley-Cote, E.P.; Whitlock, R.P. Minimally invasive mitral valve surgery versus conventional sternotomy mitral valve surgery: A systematic review and meta-analysis of 119 studies. J. Card. Surg. 2022, 37, 1319–1327. [Google Scholar] [CrossRef]
- Beckmann, A.; Meyer, R.; Lewandowski, J.; Markewitz, A.; Blaßfeld, D.; Böning, A. German Heart Surgery Report 2021: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac. Cardiovasc. Surg. 2022, 70, 362–376. [Google Scholar] [CrossRef]
- Olsthoorn, J.R.; Heuts, S.; Houterman, S.; Maessen, J.G.; Sardari Nia, P.; Cardiothoracic Surgery Registration Committee of The Netherlands Heart Registration. Effect of minimally invasive mitral valve surgery compared to sternotomy on short- and long-term outcomes: A retrospective multicentre interventional cohort study based on Netherlands Heart Registration. Eur. J. Cardiothorac. Surg. 2022, 61, 1099–1106. [Google Scholar] [CrossRef]
- Badhwar, V.; Vemulapalli, S.; Mack, M.A.; Gillinov, A.M.; Chikwe, J.; Dearani, J.A.; Grau-Sepulveda, M.V.; Habib, R.; Rankin, J.S.; Jacobs, J.P.; et al. Volume-Outcome Association of Mitral Valve Surgery in the United States. JAMA Cardiol. 2020, 5, 1092–1101. [Google Scholar] [CrossRef]
- Malvindi, P.G.; Wilbring, M.; De Angelis, V.; Bifulco, O.; Berretta, P.; Kappert, U.; Di Eusanio, M. Transaxillary approach enhances postoperative recovery after mitral valve surgery. Eur. J. Cardio Thorac. Surg. 2023, 64, ezad207. [Google Scholar] [CrossRef]
- Bifulco, O.; Malvindi, P.G.; Berretta, P.; Brugiatelli, L.; Cefarelli, M.; Alfonsi, J.; D’alfonso, A.; Zingaro, C.; Di Eusanio, M. Minimally Invasive Trans-Axillary versus Full Sternotomy Mitral Valve Repair: A Propensity Score-Matched Analysis on Mid-Term Outcomes. Medicina 2023, 60, 29. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).