Three-Dimensional Cone Beam Computed Tomography (CBCT)-Derived Soft Tissue Changes in Patients with Unilateral Cleft Lip, Alveolus, and Palate with Midfacial Deficiency after 1.5 Years of Bone-Anchored Maxillary Protraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Registration and Ethical Approval
2.2. Study Subjects
2.3. Inclusion Criteria
2.4. Bone-Anchored Maxillary Protraction (BAMP)
2.5. CBCT Acquisition
2.6. Segmentation, Superimposition, and Measurement of the CBCT 3D Surface Models
2.7. Statistics
3. Results
3.1. Sample
3.2. Intra-Observer Reliability
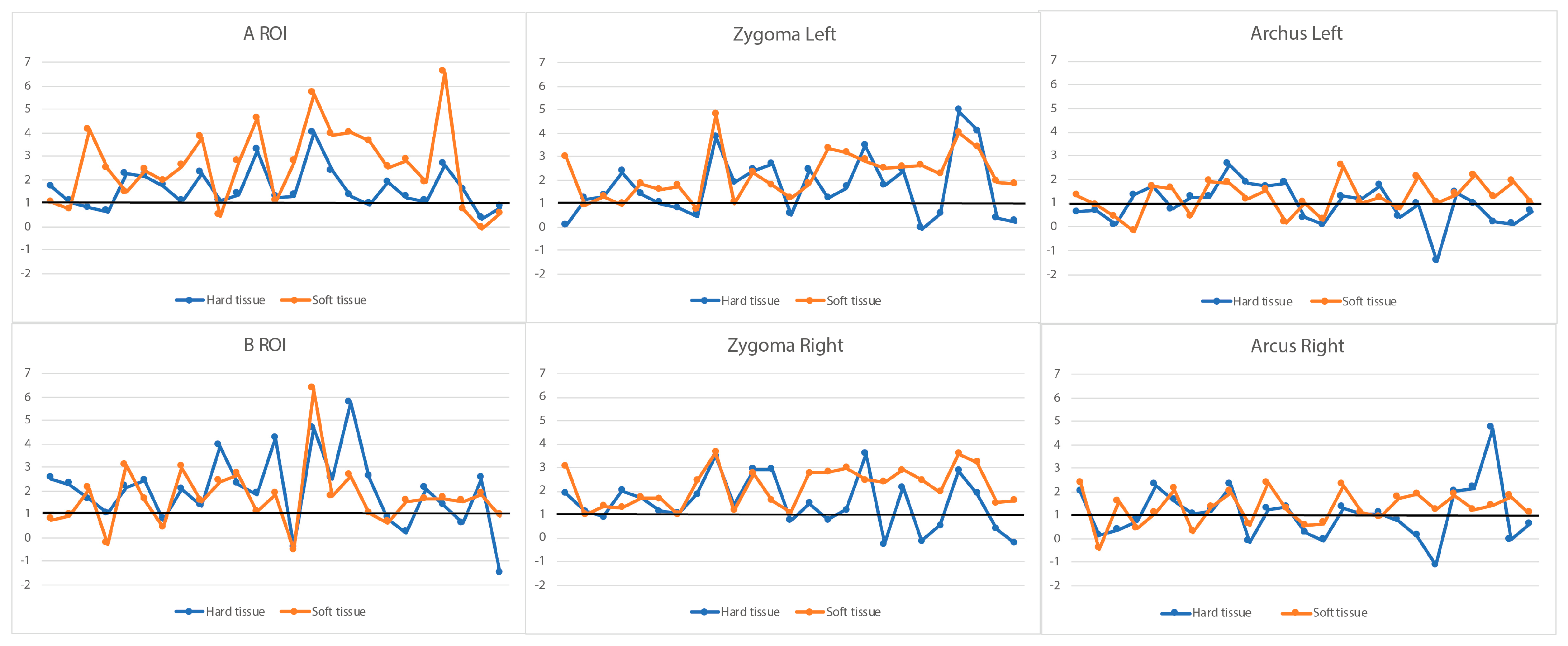
3.3. Soft Tissue and Corresponding Hard Tissue Changes
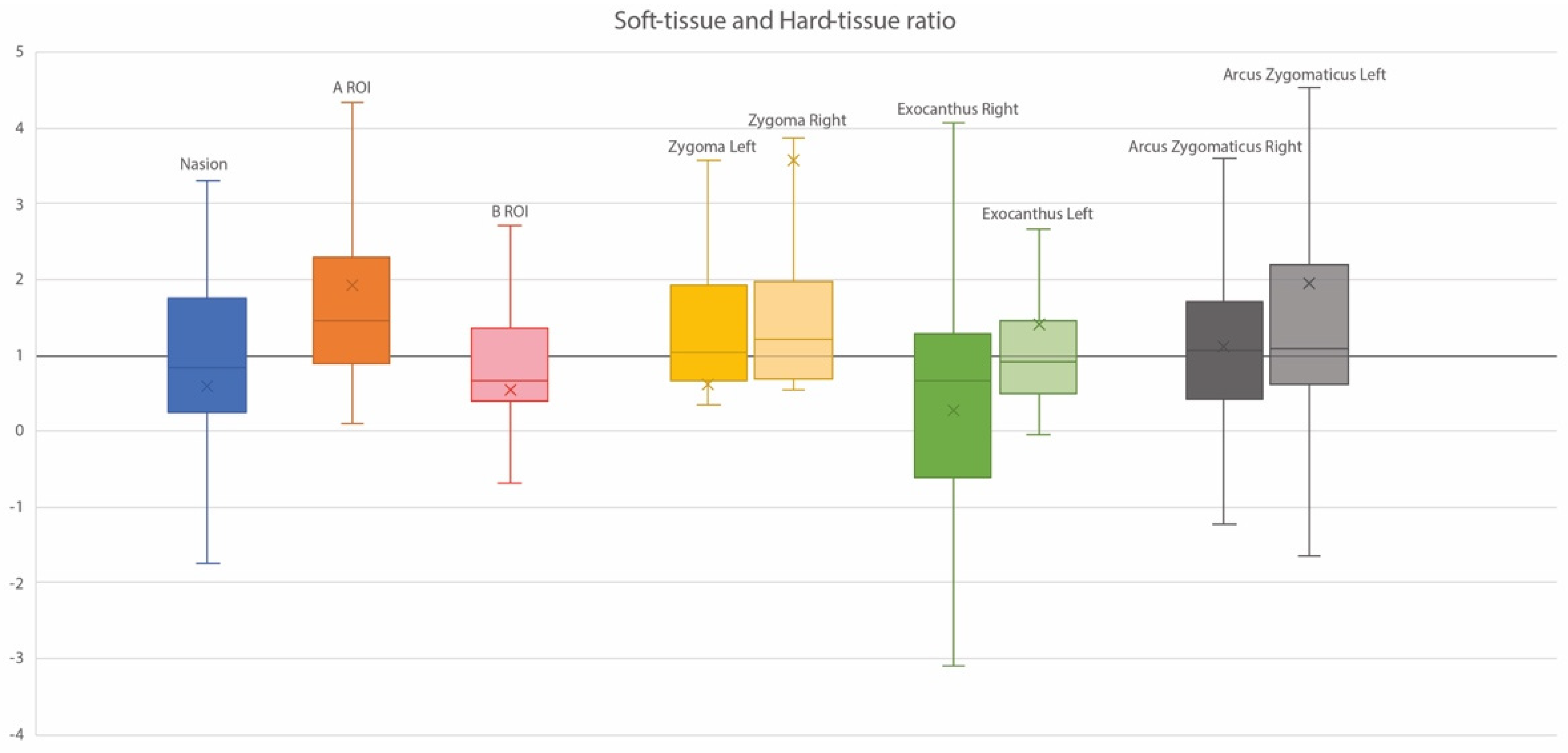
3.4. Ratios of Soft Tissue and Corresponding Hard Tissue Changes
3.5. Regression Analyses of Patient-Related Factors in Treatment Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Chen, Z.Q.; Qian, Y.F.; Wang, G.M.; Shen, G. Sagittal Maxillary Growth in Patients with Unoperated Isolated Cleft Palate. Cleft Palate Craniofac. J. 2009, 46, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.W.; Fang, Y.M.; Lin, C.X. Isolated Influences of Surgery Repair on Maxillofacial Growth in Complete Unilateral Cleft Lip and Palate. J. Oral. Maxillofac. Surg. 2016, 74, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Tindlund, R.S.; Holmefjord, A.; Eriksson, J.-C.H.; Johnson, G.E.; Vindenes, H. Interdisciplinary Evaluation of Consecutive Patients With Unilateral Cleft Lip and Palate at Age 6, 15, and 25 Years: A Concurrent Standardized Procedure and Documentation by Plastic Surgeon; Speech and Language Pathologist; Ear, Nose, and Throat Specialist; and Orthodontist. J. Craniofac. Surg. 2009, 20, 1687–1698. [Google Scholar] [CrossRef] [PubMed]
- Susami, T.; Okayasu, M.; Inokuchi, T.; Ohkubo, K.; Uchino, N.; Uwatoko, K.; Takahashi-Ichikawa, N.; Nagahama, K.; Takato, T. Maxillary Protraction in Patients with Cleft Lip and Palate in Mixed Dentition: Cephalometric Evaluation after Completion of Growth. Cleft Palate-Craniofac. J. 2014, 51, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Tome, W.; Yashiro, K.; Kogo, M.; Yamashiro, T. Cephalometric Outcomes of Maxillary Expansion and Protraction in Patients with Unilateral Cleft Lip and Palate after Two Types of Palatoplasty. Cleft Palate-Craniofac. J. 2016, 53, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, F.; Chang, Q.; Bai, Y. Three-Dimensional Comparative Evaluation of Customized Bone-Anchored vs Tooth-Borne Maxillary Protraction in Patients with Skeletal Class III Malocclusion. Am. J. Orthod. Dentofac. Orthop. 2021, 160, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Toffol, L.D.; Pavoni, C.; Baccetti, T.; Franchi, L.; Cozza, P. Orthopedic Treatment Outcomes in Class III Malocclusion. Angle Orthod. 2008, 78, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; An, K.; Peng, Y. Comparative Efficacy of the Bone-anchored Maxillary Protraction Protocols for Orthopaedic Treatment in Skeletal Class III Malocclusion: A Bayesian Network Meta-analysis. Orthod. Craniofac. Res. 2022, 25, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Steegman, R.; Dieters, A.; Jansma, J.; Stamatakis, H. Bone-Anchored Maxillary Protraction in Patients with Unilateral Complete Cleft Lip and Palate and Class III Malocclusion. Clin. Oral. Investig. 2019, 23, 2429–2441. [Google Scholar] [CrossRef]
- Steegman, R.M.; Meulekamp, A.F.K.; Dieters, A.; Jansma, J.; van der Meer, W.J.; Ren, Y. Skeletal Changes in Growing Cleft Patients with Class III Malocclusion Treated with Bone Anchored Maxillary Protraction—A 3.5-Year Follow-Up. JCM 2021, 10, 750. [Google Scholar] [CrossRef]
- Yatabe, M.; Garib, D.G.; Faco, R.A.S.; de Clerck, H.; Janson, G.; Nguyen, T.; Cevidanes, L.H.S.; Ruellas, A.C. Bone-Anchored Maxillary Protraction Therapy in Patients with Unilateral Complete Cleft Lip and Palate: 3-Dimensional Assessment of Maxillary Effects. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Antoun, J.S.; Fowler, P.V.; Jack, H.C.; Farella, M. Oral Health–Related Quality of Life Changes in Standard, Cleft, and Surgery Patients after Orthodontic Treatment. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Nolte, F.M.; Bos, A.; Prahl, C. Quality of Life Among Dutch Children With a Cleft Lip and/or Cleft Palate: A Follow-Up Study. Cleft Palate-Craniofac. J. 2019, 56, 1065–1071. [Google Scholar] [CrossRef]
- De Queiroz Herkrath, A.P.C.; Herkrath, F.J.; Rebelo, M.A.B.; Vettore, M.V. Measurement of Health-Related and Oral Health–Related Quality of Life among Individuals with Nonsyndromic Orofacial Clefts: A Systematic Review and Meta-Analysis. Cleft Palate-Craniofac. J. 2015, 52, 157–172. [Google Scholar] [CrossRef]
- Steegman, R.; Schoeman, A.; Dieters, A.; Jongsma, B.; Jansma, J.; van der Meer, J.; Ren, Y. Three-Dimensional Volumetric Changes in the Airway of Growing Unilateral Complete Cleft Lip and Palate Patients after Bone-Anchored Maxillary Protraction. Am. J. Orthod. Dentofac. Orthop. 2022, 162, 850–860. [Google Scholar] [CrossRef]
- Vermeulen, F.M.J. Interceptieve Behandeling van Een Maxillaire Hypoplasie Met Behulp van Botankers. Een Literatuuronderzoek. NTvT 2014, 121, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Gkantidis, N.; Schauseil, M.; Pazera, P.; Zorkun, B.; Katsaros, C.; Ludwig, B. Evaluation of 3-Dimensional Superimposition Techniques on Various Skeletal Structures of the Head Using Surface Models. PLoS ONE 2015, 10, e0118810. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- De Clerck, H.; Cevidanes, L.; Baccetti, T. Dentofacial Effects of Bone-Anchored Maxillary Protraction: A Controlled2 Study of Consecutively Treated Class III Patients. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 577–581. [Google Scholar] [CrossRef]
- Ngan, P.; Hägg, U.; Yiu, C.; Merwin, D.; Wei, S.H.Y. Soft Tissue and Dentoskeletal Profile Changes Associated with Maxillary Expansion and Protraction Headgear Treatment. Am. J. Orthod. Dentofac. Orthop. 1996, 109, 38–49. [Google Scholar] [CrossRef]
- Sade Hoefert, C.; Bacher, M.; Herberts, T.; Krimmel, M.; Reinert, S.; Göz, G. 3D Soft Tissue Changes in Facial Morphology in Patients with Cleft Lip and Palate and Class III Mal Occlusion under Therapy with Rapid Maxillary Expansion and Delaire Facemask. J. Orofac. Orthop. 2010, 71, 136–151. [Google Scholar] [CrossRef]
- Elnagar, M.H.; Elshourbagy, E.; Ghobashy, S.; Khedr, M.; Kusnoto, B.; Evans, C.A. Three-Dimensional Assessment of Soft Tissue Changes Associated with Bone-Anchored Maxillary Protraction Protocols. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Möhlhenrich, S.C.; Kötter, F.; Peters, F.; Kniha, K.; Chhatwani, S.; Danesh, G.; Hölzle, F.; Modabber, A. Effects of Different Surgical Techniques and Displacement Distances on the Soft Tissue Profile via Orthodontic-Orthognathic Treatment of Class II and Class III Malocclusions. Head. Face Med. 2021, 17, 13. [Google Scholar] [CrossRef]
- Soncul, M.; Bamber, M.A. Evaluation of Facial Soft Tissue Changes with Optical Surface Scan after Surgical Correction of Class III Deformities. J. Oral. Maxillofac. Surg. 2004, 62, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.P.S.; Machado-Fernández, A.; Guijarro-Martínez, R.; Hernández-Alfaro, F.; Haas Jr, O.L.; De Oliveira, R.B. Le Fort I Osteotomy and Soft Tissue Response: A Retrospective Cohort Study Comparing Three Different Techniques. J. Cranio-Maxillofac. Surg. 2022, 50, 107–113. [Google Scholar] [CrossRef]
- Rupperti, S.; Winterhalder, P.; Krennmair, S.; Holberg, S.; Holberg, C.; Mast, G.; Rudzki, I. Changes in the Facial Soft Tissue Profile after Maxillary Orthognathic Surgery. J. Orofac. Orthop. 2022, 83, 215–220. [Google Scholar] [CrossRef]
- Olivetti, E.C.; Nicotera, S.; Marcolin, F.; Vezzetti, E.; Sotong, J.P.A.; Zavattero, E.; Ramieri, G. 3D Soft-Tissue Prediction Methodologies for Orthognathic Surgery—A Literature Review. Appl. Sci. 2019, 9, 4550. [Google Scholar] [CrossRef]
- Vasudavan, S.; Jayaratne, Y.S.N.; Padwa, B.L. Nasolabial Soft Tissue Changes After Le Fort I Advancement. J. Oral. Maxillofac. Surg. 2012, 70, e270–e277. [Google Scholar] [CrossRef]
- Metzger, T.E.; Kula, K.S.; Eckert, G.J.; Ghoneima, A.A. Orthodontic soft-tissue parameters: A comparison of cone-beam computed tomography and the 3dMD imaging system. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 672–681. [Google Scholar] [CrossRef]

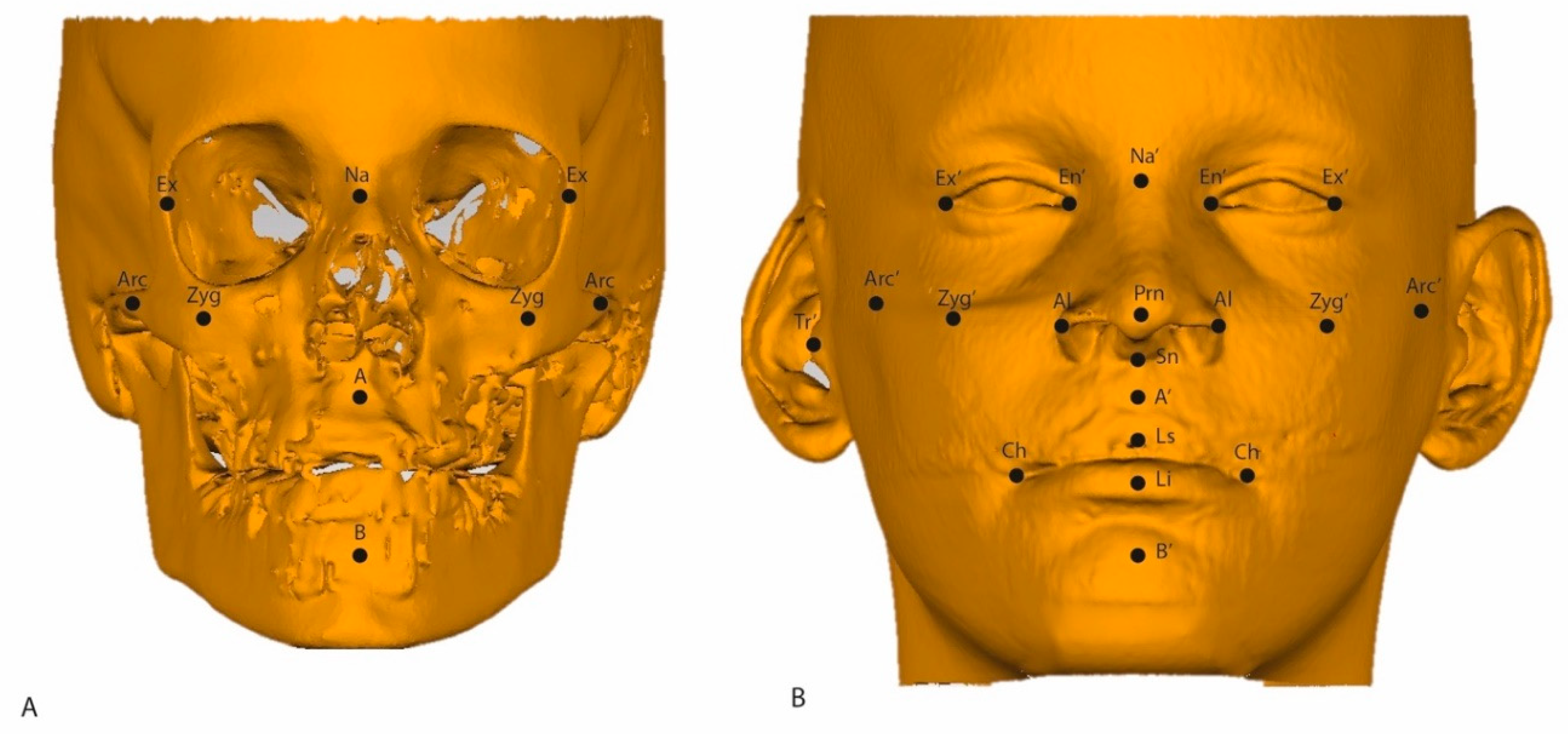
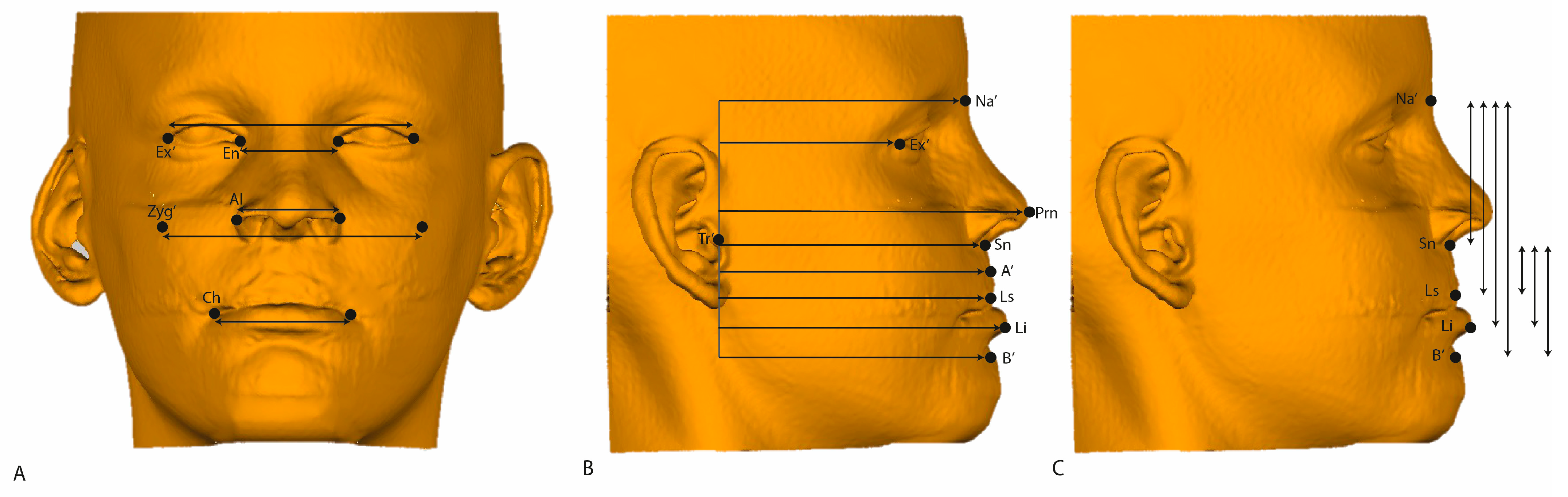
| ROI | Abbreviation | Description |
|---|---|---|
| Soft tissue Nasion | Na’ | Midpoint on the soft tissue contour of the base of the nasal root at the level of the frontonasal suture. |
| A point | A’ | Most posterior midpoint of the philtrum. |
| B point | B’ | Most posterior midpoint on the labiomental soft tissue contour that defines the border between the lower lip and the chin. |
| Pronasale | Prn | Most anterior midpoint of the nasal tip. |
| Subnasale | Sn’ | Midpoint on the nasolabial soft tissue contour between the columella crest and the upper lip. |
| Alare | Al | Most lateral point on each alar contour. |
| Exocanthion left and right | Ex’ | Soft tissue point located at the outer commissure of each eye fissure. |
| Endocanthion left and right | En’ | Soft tissue point located at the inner commissure of each eye fissure. |
| Labrale Superius | Ls | Midpoint of the vermilion line of the upper lip. |
| Labrale Inferius | Li | Midpoint of the vermilion line of the lower lip. |
| Cheilion left and right | Ch | Point located at each labial commissure. |
| Soft tissue Zygoma left and right | Zyg’ | Most anterior party of the left/right zygomatic process; the skeletal, vertical, and transversal coordinates count as a reference. |
| Soft tissue Arcus left and right | Arc’ | Most transversal point of the arcus zygomaticus; the skeletal, vertical, and transversal coordinates count as a reference. |
| Soft tissue Tragus | Tr’ | Most outward-pointed eminence of the external right ear. |
| ROI | Hard Tissue Increment | Soft Tissue Increment | Paired Difference | 95% CI | p Value | Correlation | |
|---|---|---|---|---|---|---|---|
| Nasion | Overall | 0.89 ± 0.62 | 0.98 ± 1.35 | 0.09 ± 1.28 | −0.27; 0.45 | 0.629 | 0.346 |
| Horizontal | 0.85 ± 0.60 | 0.94 ± 1.29 | −0.09 ± 1.21 | −0.43; 0.25 | 0.604 | 0.360 | |
| Vertical | 0.12 ± 0.18 | 0.07 ± 0.28 | 0.05 ± 0.31 | −0.04; 0.14 | 0.241 | 0.160 | |
| A | Overall | 1.60 ± 0.94 | 2.58 ± 1.74 | 0.97 ± 1.46 | 0.55; 1.39 | <0.001 | 0.560 # |
| Horizontal | 1.50 ± 0.88 | 2.27 ± 1.60 | −0.77 ±1.36 | −1.15; −0.38 | <0.001 | 0.552 # | |
| Vertical | −0.14 ±0.42 | −0.44 ±1.08 | 0.31 ± 1.13 | −0.01; 0.63 | 0.059 | 0.090 | |
| B | Overall | 1.69 ±1.42 | 1.99 ± 1.63 | 0.30 ± 1.47 | −0.11; 0.72 | 0.154 | 0.542 # |
| Horizontal | 1.56 ± 1.32 | 1.83 ± 1.59 | −0.26 ± 1.48 | −0.86; 0.16 | 0.216 | 0.497 | |
| Vertical | 0.45 ± 0.47 | 0.15 ± 0.85 | 0.30 ± 0.76 | 0.09; 0.52 | 0.007 | 0.454 | |
| Zygoma L | Overall | 2.21 ± 1.05 | 1.72 ± 1.40 | −0.49 ± 1.27 | −0.85; −0.13 | 0.008 | 0.495 |
| Horizontal | 1.78 ± 0.84 | 1.54 ± 0.13 | 0.24 ± 1.14 | −0.08; 0.57 | 0.139 | 0.483 | |
| Vertical | 0.80 ± 0.56 | −0.38 ± 0.41 | 1.19 ± 0.79 | 0.96; 1.41 | <0.001 | −0.294 | |
| Transversal | 0.93 ± 0.54 | 0.60 ± 0.49 | −0.33 ± 0.57 | −0.49; −0.17 | <0.001 | 0.397 | |
| Zygoma R | Overall | 2.19 ± 0.90 | 1.51 ± 1.16 | −0.68 ± 1.18 | −1.01; −0.35 | <0.001 | 0.356 |
| Horizontal | 1.84 ± 0.77 | 1.32 ± 1.03 | 0.52 ± 1.05 | 0.22; 0.82 | <0.001 | 0.347 | |
| Vertical | 0.68 ± 0.49 | −0.32 ± 0.36 | 1.00 ± 0.67 | 0.81; 1.19 | <0.001 | −0.187 | |
| Transversal | 0.81 ± 0.51 | 0.61 ± 0.47 | 0.20 ± 0.51 | 0.05; 0.35 | 0.008 | 0.447 | |
| Arcus L | Overall | 1.24 ± 0.75 | 0.96 ± 0.87 | −0.28 ± 1.03 | −0.57; 0.01 | 0.059 | 0.203 |
| Horizontal | 0.20 ± 0.17 | 0.16 ± 0.26 | 0.037 ± 0.27 | −0.4; 0.11 | 0.335 | 0.291 | |
| Vertical | 0.17 ± 0.25 | 0.10 ± 0.13 | 0.079 ± 0.27 | 0.00; 0.15 | 0.043 | 0.157 | |
| Transversal | 1.22 ± 0.67 | 0.93 ± 0.84 | −0.30 ± 0.92 | −0.56; −0.04 | 0.026 | 0.281 | |
| Arcus R | Overall | 1.30 ± 0.75 | 1.07 ± 1.20 | −0.23 ± 1.20 | −0.57; 0.11 | 0.182 | 0.310 |
| Horizontal | 0.25 ± 0.19 | 0.19 ± 0.27 | 0.06 ± 0.30 | −0.03; 0.14 | 0.207 | 0.00 | |
| Vertical | 0.13 ± 0.22 | 0.10 ± 020 | 0.03 ± 0.30 | −0.06; 0.11 | 0.493 | 0.155 | |
| Transversal | 1.20 ± 0.82 | 1.04 ± 1.16 | 0.16 ± 1.16 | −0.17; 0.49 | 0.345 | 0.345 | |
| Exo L | Overall | 1.14 ± 0.53 | 1.02 ± 0.93 | −0.12 ± 0.86 | −0.36; 0.13 | 0.342 | 0.428 |
| Horizontal | 0.88 ± 0.46 | 0.65 ± 0.66 | 0.23 ± 0.66 | 0.04; 0.42 | 0.017 | 0.362 | |
| Vertical | 0.15 ± 0.15 | 0.27 ± 0.55 | −0.12 ± 0.51 | −0.27; 0.02 | 0.101 | 0.371 | |
| Transversal | −0.46± 0.57 | 0.56 ±0.61 | 1.03 ±0.95 | 0.75; 1.30 | <0.001 | 0.326 | |
| Exo R | Overall | 1.07 ± 0.65 | 0.81 ± 1.36 | −0.26 ± 1.29 | −0.63; 0.10 | 0.157 | 0.360 |
| Horizontal | 0.85 ± 0.53 | 0.58 ± 0.95 | 0.27 ± 0.89 | 0.01; 0.52 | 0.039 | 0.368 | |
| Vertical | 0.12 ± 0.15 | 0.07 ± 0.42 | 0.05 ± 0.41 | −0.06; 0.17 | 0.353 | 0.222 | |
| Transversal | 0.46 ± 0.57 | 0.56 ± 0.90 | −0.10 ± 0.85 | −0.34; 0.14 | 0.407 | 0.399 |
| ROI | Dimension | Mean ± SD | Range | ROI | Mean ± SD | Range |
|---|---|---|---|---|---|---|
| Soft tissue ROIs midsagittal plane | ||||||
| Prn | Overall | 2.78 ± 1.63 | 0.04; 6.93 | |||
| Horizontal | 2.68 ± 1.59 | 0.04; 6.87 | ||||
| Vertical | −0.14 ± 0.39 | −1.27; 0.81 | ||||
| Ls | Overall | 2.32 ± 1.92 | −0.72; 6.96 | |||
| Horizontal | 2.21 ± 1.87 | −0.71; 6.86 | ||||
| Vertical | 0.04 ± 0.55 | −2.05; 1.73 | ||||
| Li | Overall | 1.67 ±2.20 | −4.23; 5.94 | |||
| Horizontal | 1.58 ± 2.10 | −4.18; 5.81 | ||||
| Vertical | −0.22 ± 0.66 | −2.83; 1.20 | ||||
| Soft tissue ROIs left | Soft tissue ROIs right | |||||
| Al L | Overall | 0.83 ± 1.98 | −5.27; 4.24 | Al R | 1.83 ± 2.21 | −4.09; 6.86 |
| Horizontal | 0.43 ± 0.88 | −1.85; −2.47 | 0.78 ± 0.96 | −1.15; 3.89 | ||
| Vertical | −0.07 ± 0.60 | −2.25; 1.39 | 0.02 ± 0.72 | −1.81; 3.14 | ||
| Transversal | 0.62 ± 1.70 | −3.53; 5.17 | 1.54 ± 1.95 | −3.75; 6.49 | ||
| En’ L | Overall | 1.01 ± 1.09 | −0.96; 3.54 | En’ R | 0.82 ± 0.91 | −0.92; 2.39 |
| Horizontal | 0.65 ± 0.72 | −1.79; 2.12 | 0.58 ± 0.67 | −0.68; 1.99 | ||
| Vertical | 0.15 ± 0.50 | −1.32; 1.30 | −0.16 ± 0.36 | −1.19; 0.44 | ||
| Transversal | 0.53 ± 0.85 | −1.60; 3.20 | 0.46 ± 0.60 | −0.74; 2.06 | ||
| Ch L | Overall | 1.56 ± 1.86 | −2.68; 7.29 | Ch R | 1.99 ± 1.61 | −1.35; 6.45 |
| Horizontal | 1.13 ± 1.37 | −2.53; 4.60 | 1.60 ± 1.31 | −1.04; 5.80 | ||
| Vertical | 0.00 ± 1.34 | −2.58; 5.47 | 0.31 ± 1.13 | −2.18; 3.13 | ||
| Transversal | 0.52 ± 0.82 | −1.00; 2.52 | 0.25 ± 0.94 | −1.67; 3.57 | ||
| Arc’ L | Overall | 1.24 ± 0.75 | −2.00; 3.05 | Arc’ R | 1.30 ± 0.54 | −1.70; 4.76 |
| Horizontal | 0.20 ± 1.7 | −0.23; 1.13 | 0.25 ± 0.19 | −0.41; 1.01 | ||
| Vertical | 0.17 ± 0.25 | −0.16; 0.42 | 0.13 ± 0.22 | −0.29; 0.85 | ||
| Transversal | 1.22 ± 0.67 | −1.98; 2.82 | 1.20 ± 0.82 | −1.64; 4.58 | ||
| Ex’ L | Overall | 1.14 ± 0.53 | −1.06; 3.87 | Ex’ R | 1.07 ± 0.65 | −1.78; 5.08 |
| Horizontal | 0.88 ± 0.46 | −0.76; 2.47 | 0.85 ± 0.53 | −1.70; 3.28 | ||
| Vertical | 0.15 ± 0.15 | −0.94; 1.39 | 0.12 ± 0.15 | −0.94; −0.98 | ||
| Transversal | 0.49 ± 0.56 | −0.62; 2.84 | 0.46 ± 0.57 | −0.87; 3.84 | ||
| Variable | T0 | T1 | T1-T0 Increment | ||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | 95% CI | p Value | |
| Transversal dimension (mm) | |||||
| Ex’L-Ex’R | 94.0 ± 6.7 | 95.2 ± 6.4 | 1.2 ±1.2 | −1.50; −0.84 | <0.001 |
| En’L-En’R | 34.0 ± 5.2 | 35.0 ± 5.0 | 1.0 ±1.2 | −1.33; −0.63 | <0.001 |
| AlL-AlR | 33.9 ± 3.2 | 36.1 ± 3.6 | 2.2 ± 1.8 | −2.65; −1.65 | <0.001 |
| Zyg’L-Zyg’R | 73.3 ± 8.8 | 74.5 ± 8.8 | 1.2 ± 0.8 | 1.41; −0.93 | <0.001 |
| ChL-ChR | 44.5 ± 5.1 | 45.2 ± 5.4 | 0.8 ± 1.3 | −1.16; −0.41 | <0.001 |
| Vertical dimension (mm) | |||||
| Na’-Sn’ | 50.7 ± 3.6 | 51.9 ± 3.6 | 1.1 ± 1.3 | −1.51; −0.78 | <0.001 |
| Na’-Ls | 63.9 ± 4.2 | 63.8 ± 4.3 | 0.0 ± 0.5 | −0.14; 0.16 | 0.915 |
| Na’-Li | 74.9 ± 4.8 | 74.6 ± 4.8 | −0.3 ± 0.7 | 0.11; 0.50 | 0.003 |
| Na’-B’ | 87.5 ± 5.5 | 87.6 ± 5.6 | 0.1 ± 0.9 | −0.32; 0.19 | 0.622 |
| Sn’-Ls | 13.1 ± 2.5 | 12.0 ± 3.1 | −1.2 ± 1.2 | 0.80; 1.51 | <0.001 |
| Sn’-Li | 24.2 ± 3.3 | 22.7 ± 3.6 | −1.4 ± 1.3 | 1.09; 1.82 | <0.001 |
| Sn’-B’ | 36.8 ± 5.2 | 35.7 ± 5.4 | −1.1 ± 1.4 | 0.67; 1.50 | <0.001 |
| Sagittal dimension (mm) | |||||
| Tr’-Na’ | 82.6 ± 6.3 | 83.9 ± 6.6 | 1.4 ± 1.5 | −3.92; −0.533 | 0.011 |
| Tr’-Ex’ | 61.9 ± 4.9 | 62.9 ± 4.9 | 1.0 ± 1.2 | −1.39; −0.69 | <0.001 |
| Tr’-Prn | 98.9 ± 6.7 | 102.0 ± 7.4 | 3.1 ± 1.7 | −3.63; −2.66 | <0.001 |
| Tr’-Sn’ | 88.0 ± 6.5 | 89.7 ± 6.7 | 1.6 ± 1.5 | −2.04; −1.21 | <0.001 |
| Tr’-A’ | 88.2 ± 8.1 | 90.9 ± 8.3 | 2.7 ± 1.7 | −3.20; −2.21 | <0.001 |
| Tr’-Ls | 89.6 ±6.0 | 92.3 ±6.1 | 2.7 ± 1.9 | −3.08; −2.04 | <0.001 |
| Tr’-Li | 91.8 ±5.9 | 93.9 ± 5.8 | 2.0 ± 2.0 | −2.72; −1.56 | <0.001 |
| Tr’-B’ | 86.7 ± 6.7 | 88.9 ± 6.9 | 2.3 ± 1.6 | −2.80; −1.85 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steegman, R.M.; Klein Meulekamp, A.F.; Renkema, A.-M.; Janssen, K.I.; Kuijpers-Jagtman, A.M.; Ren, Y. Three-Dimensional Cone Beam Computed Tomography (CBCT)-Derived Soft Tissue Changes in Patients with Unilateral Cleft Lip, Alveolus, and Palate with Midfacial Deficiency after 1.5 Years of Bone-Anchored Maxillary Protraction. J. Clin. Med. 2024, 13, 2890. https://doi.org/10.3390/jcm13102890
Steegman RM, Klein Meulekamp AF, Renkema A-M, Janssen KI, Kuijpers-Jagtman AM, Ren Y. Three-Dimensional Cone Beam Computed Tomography (CBCT)-Derived Soft Tissue Changes in Patients with Unilateral Cleft Lip, Alveolus, and Palate with Midfacial Deficiency after 1.5 Years of Bone-Anchored Maxillary Protraction. Journal of Clinical Medicine. 2024; 13(10):2890. https://doi.org/10.3390/jcm13102890
Chicago/Turabian StyleSteegman, Ralph M., Annemarlien F. Klein Meulekamp, Anne-Marie Renkema, Krista I. Janssen, Anne Marie Kuijpers-Jagtman, and Yijin Ren. 2024. "Three-Dimensional Cone Beam Computed Tomography (CBCT)-Derived Soft Tissue Changes in Patients with Unilateral Cleft Lip, Alveolus, and Palate with Midfacial Deficiency after 1.5 Years of Bone-Anchored Maxillary Protraction" Journal of Clinical Medicine 13, no. 10: 2890. https://doi.org/10.3390/jcm13102890
APA StyleSteegman, R. M., Klein Meulekamp, A. F., Renkema, A.-M., Janssen, K. I., Kuijpers-Jagtman, A. M., & Ren, Y. (2024). Three-Dimensional Cone Beam Computed Tomography (CBCT)-Derived Soft Tissue Changes in Patients with Unilateral Cleft Lip, Alveolus, and Palate with Midfacial Deficiency after 1.5 Years of Bone-Anchored Maxillary Protraction. Journal of Clinical Medicine, 13(10), 2890. https://doi.org/10.3390/jcm13102890







