Personalized Guidance of Edge-to-Edge Transcatheter Tricuspid Valve Repair by Multimodality Imaging
Abstract
1. Introduction
1.1. Status Quo
1.2. Why the Need for a Personalized Guidance Approach
1.3. Aim of the Study
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Study Endpoints
2.3. Studied Variables
2.4. Imaging Protocol
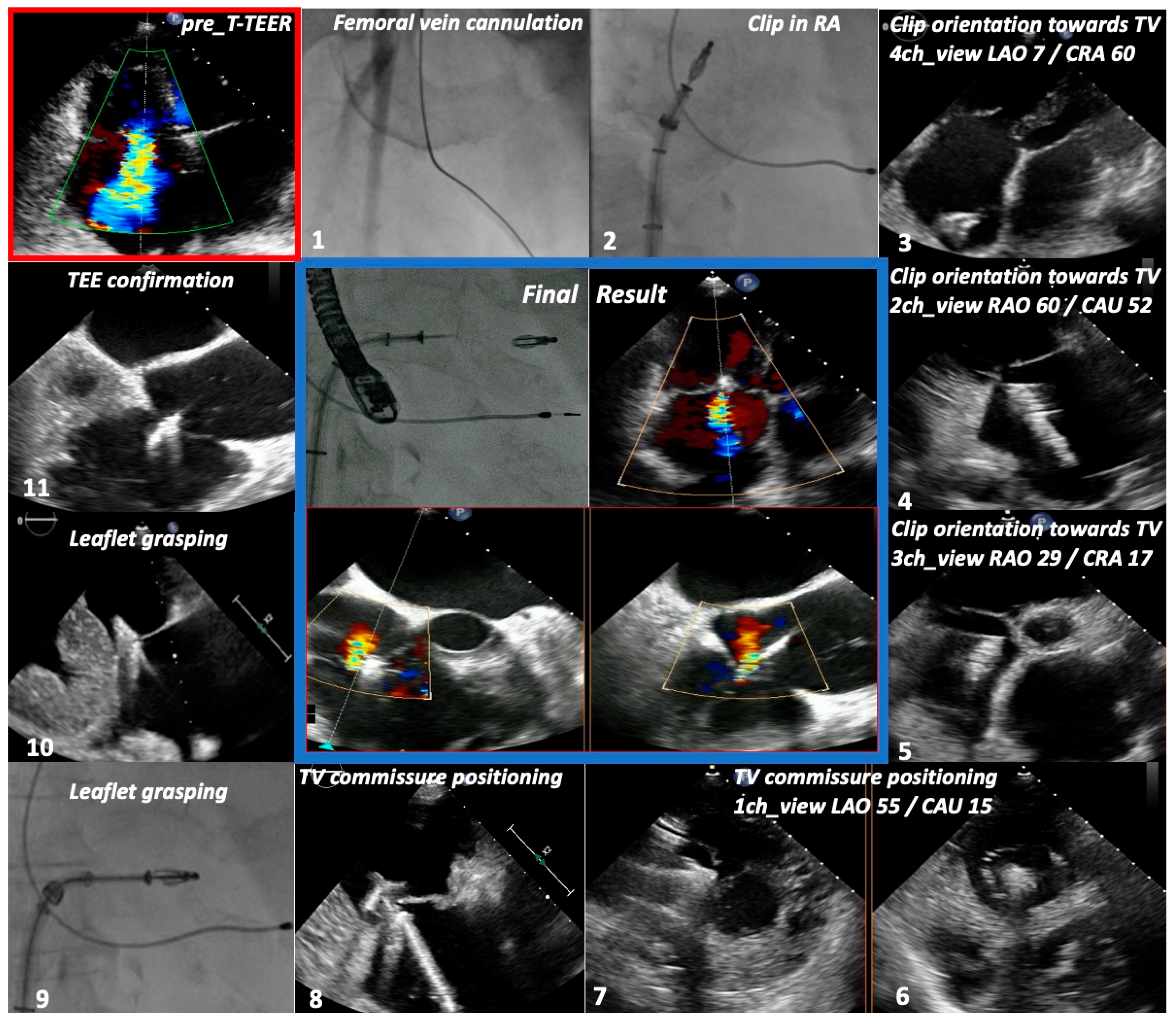
2.5. Procedural Protocol
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Procedural Characteristics
3.3. Safety Endpoint
3.4. Secondary Endpoints
4. Discussion
4.1. Summary of Main Findings
- T-TEER can be effectively and safely guided by a CT-aided, meticulously planned combination of TTE, intermittent TEE and fluoroscopy, tailored to the patient´s anatomy.
- The implementation of the concept of the four right-sided chamber views builds a bridge between imaging methods involved in procedural planning and interventional guidance. Furthermore, it allows cardiac imaging specialists and interventionalists to speak a common language.
- Irrespective of the chosen guiding method, successful TR reduction leads to improved quality of life and long-term outcomes.
4.2. Efficacy and Safety of the Multimodality Imaging Method
4.3. Advantages and Particularities of a Personalized Approach
4.4. Possible Future Development
4.5. Clinical Outcomes
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Topilsky, Y.; Maltais, S.; Medina-Inojosa, J.; Oguz, D.; Michelena, H.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovasc. Imaging 2019, 12, 433–442. [Google Scholar] [CrossRef]
- Singh, J.P.; Evans, J.C.; Levy, D.; Larson, M.G.; Freed, L.A.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am. J. Cardiol. 1999, 83, 897–902. [Google Scholar] [CrossRef]
- Kawsara, A.; Alqahtani, F.; Nkomo, V.T.; Eleid, M.F.; Pislaru, S.V.; Rihal, C.S.; Nishimura, R.A.; Schaff, H.V.; Crestanello, J.A.; Alkhouli, M. Determinants of Morbidity and Mortality Associated with Isolated Tricuspid Valve Surgery. J. Am. Hear. Assoc. 2021, 10, e018417. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Messika-Zeitoun, D.; Topilsky, Y.; Tribouilloy, C.; Benfari, G.; Michelena, H. Tricuspid regurgitation is a public health crisis. Prog. Cardiovasc. Dis. 2019, 62, 447–451. [Google Scholar] [CrossRef]
- Sorajja, P.; Whisenant, B.; Hamid, N.; Naik, H.; Makkar, R.; Tadros, P.; Price, M.J.; Singh, G.; Fam, N.; Kar, S.; et al. Transcatheter Repair for Patients with Tricuspid Regurgitation. N. Engl. J. Med. 2023, 388, 1833–1842. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Flint, N.; Price, M.J.; Little, S.H.; Mackensen, G.B.; Wunderlich, N.C.; Makar, M.; Siegel, R.J. State of the Art: Transcatheter Edge-to-Edge Repair for Complex Mitral Regurgitation. J. Am. Soc. Echocardiogr. 2021, 34, 1025–1037. [Google Scholar] [CrossRef]
- Addetia, K.; Yamat, M.; Mediratta, A.; Medvedofsky, D.; Patel, M.; Ferrara, P.; Mor-Avi, V.; Lang, R.M. Comprehensive Two-Dimensional Interrogation of the Tricuspid Valve Using Knowledge Derived from Three-Dimensional Echocardiography. J. Am. Soc. Echocardiogr. 2015, 29, 74–82. [Google Scholar] [CrossRef]
- Patrascu, A.; Binder, D.; Polleit, B.; Ott, I. Transthoracic guidance of percutaneous tricuspid valve repair: A case report. Eur. Hear. J. Case Rep. 2021, 5, ytab449. [Google Scholar] [CrossRef]
- Patrascu, I.; Binder, D.; Alashkar, I.; Weinmann, K.; Schneider, J.; Staehle, W.; Schnabel, P.; Ott, I. Transthoracic echocardiography guidance of transcatheter edge-to-edge percutaneous tricuspid valve repair: The TTE-TTVR pilot study and methodology proposal. Eur. Heart J. 2022, 43 (Suppl. S2), ehac544-106. [Google Scholar] [CrossRef]
- Kuecken, T.; Bannehr, M.; Lalou, E.; Lenz, J.; Zänker, M.; Edlinger, C.; Haase-Fielitz, A.; Neuss, M.; Butter, C. Oesophageal and gastric injuries caused by transoesophageal probe manipulation in patients undergoing transcatheter edge-to-edge repair for tricuspid regurgitation. EuroIntervention 2023, 19, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Pighi, M.; Thériault-Lauzier, P.; Alosaimi, H.; Spaziano, M.; Martucci, G.; Xiong, T.Y.; Buithieu, J.; Ybarra, L.F.; Afilalo, J.; Leipsic, J.; et al. Fluoroscopic Anatomy of Right-Sided Heart Structures for Transcatheter Interventions. JACC Cardiovasc Interv. 2018, 11, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.-Y.; Pighi, M.; Thériault-Lauzier, P.; Leipsic, J.A.; Spaziano, M.; Martucci, G.J.; Buithieu, J.; Mousavi, N.; Pilgrim, T.; Praz, F.; et al. Optimal fluoroscopic viewing angles of right-sided heart structures in patients with tricuspid regurgitation based on multislice computed tomography. EuroIntervention 2019, 15, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, G.; Weber, M.; Lurz, P.; von Bardeleben, R.S.; Sitges, M.; Sorajja, P.; Hausleiter, J.; Denti, P.; Trochu, J.N.; Naebauer, M.; et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month out-comes of the TRILUMINATE single-arm study. Lancet 2019, 394, 2002–2011. [Google Scholar] [PubMed]
- Hahn, R.T.; Lawlor, M.K.; Davidson, C.J.; Badhwar, V.; Sannino, A.; Spitzer, E.; Lurz, P.; Lindman, B.R.; Topilsky, Y.; Baron, S.J.; et al. TVARC Steering Committee. Tricuspid Valve Academic Research Consortium Definitions for Tricuspid Regurgitation and Trial Endpoints. J. Am. Coll. Cardiol. 2023, 82, 1711–1735. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Thomas, J.D.; Khalique, O.K.; Cavalcante, J.L.; Praz, F.; Zoghbi, W.A. Imaging Assessment of Tricuspid Regurgitation Se-verity. JACC Cardiovasc. Imaging 2019, 12, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, I.; Daraban, A.M.; Jasaityte, R.; Neskovic, A.N.; Claus, P.; Voigt, J.-U. Incremental Value of the En Face View of the Tricuspid Valve by Two-Dimensional and Three-Dimensional Echocardiography for Accurate Identification of Tricuspid Valve Leaflets. J. Am. Soc. Echocardiogr. 2014, 27, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Lurz, P.; von Bardeleben, R.S.; Weber, M.; Sitges, M.; Sorajja, P.; Hausleiter, J.; Denti, P.; Trochu, J.-N.; Nabauer, M.; Tang, G.H.; et al. Transcatheter Edge-to-Edge Repair for Treatment of Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2021, 77, 229–239. [Google Scholar] [CrossRef]
- Lurz, P.; Besler, C.; Schmitz, T.; Bekeredjian, R.; Nickenig, G.; Möllmann, H.; von Bardeleben, R.S.; Schmeisser, A.; Atmowihardjo, I.; Estevez-Loureiro, R.; et al. Short-Term Outcomes of Tricuspid Edge-to-Edge Repair in Clinical Practice. J. Am. Coll. Cardiol. 2023, 82, 281–291. [Google Scholar] [CrossRef]
- Agricola, E.; Meucci, F.; Ancona, F.; Sanz, A.P.; Zamorano, J.L. Echocardiographic guidance in transcatheter structural cardiac interventions. EuroIntervention 2022, 17, 1205–1226. [Google Scholar] [CrossRef] [PubMed]
- Patrascu, A.I.; Binder, D.; Alashkar, I.; Schnabel, P.; Stähle, W.; Weinmann, K.; Schneider, J.; Conzelmann, L.O.; Mehlhorn, U.; Ott, I. Transcatheter Tricuspid Valve Repair in Prohibitive Risk Patients: Impact on Quality of Life and Major Organ Systems. Can. J. Cardiol. 2022, 38, 1921–1931. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | MMI (n = 17) | TEE (n = 23) | p-Value | Characteristic | MMI (n = 17) | TEE (n = 23) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| Clinical | Comorbidities | ||||||||
| Age (years) | 83.1 ± 4.1 | 81 ± 5.3 | 0.182 | Atrial fibrillation | 16 (94%) | 23 (100%) | 1.000 | ||
| Female sex | 10 (59%) | 10 (44%) | 0.595 | Pulmonary hypertension | 16 (94%) | 22 (96%) | 1.000 | ||
| TTE acoustic window | Excellent | 17 (100%) | 0 (0%) | <0.001 | Type 2 diabetes | 6 (35%) | 10 (43%) | 0.773 | |
| Good | 0 (0%) | 2 (9%) | 0.506 | Arterial hypertension | 17 (100%) | 23 (100%) | 1.000 | ||
| Moderate | 0 (0%) | 13 (57%) | 0.004 | COPD | 3 (18%) | 6 (26%) | 0.719 | ||
| Poor | 0 (0%) | 8 (34%) | 0.037 | CKD stage 3–5 | 14 (82%) | 19 (83%) | 1.000 | ||
| BMI (kg/m2) | 22.9 ± 1.1 | 30.4 ± 3.7 | <0.001 | Prior stroke/TIA | 3 (18%) | 5 (22%) | 1.000 | ||
| EuroSCORE II (%) | 10.1 ± 8.2 | 8.6 ± 5.6 | 0.496 | Coronary artery disease | 8 (47%) | 15 (65%) | 0.339 | ||
| STS Score (%) | 11.1 ± 7.4 | 10.6 ± 5.9 | 0.813 | Pacemaker/ICD/CRT | 5 (29%) | 4 (17%) | 0.712 | ||
| NYHA class III–IV | 15 (88%) | 20 (87%) | 1.000 | Prior MV repair | percutaneous | 7 (41%) | 3 (13%) | 0.164 | |
| RHF hospitalizations | 2.8 ± 0.7 | 2.5 ± 0.7 | 0.188 | surgical | 1 (10%) | 1 (4%) | 1.000 | ||
| TTE View | Focus | ||
|---|---|---|---|
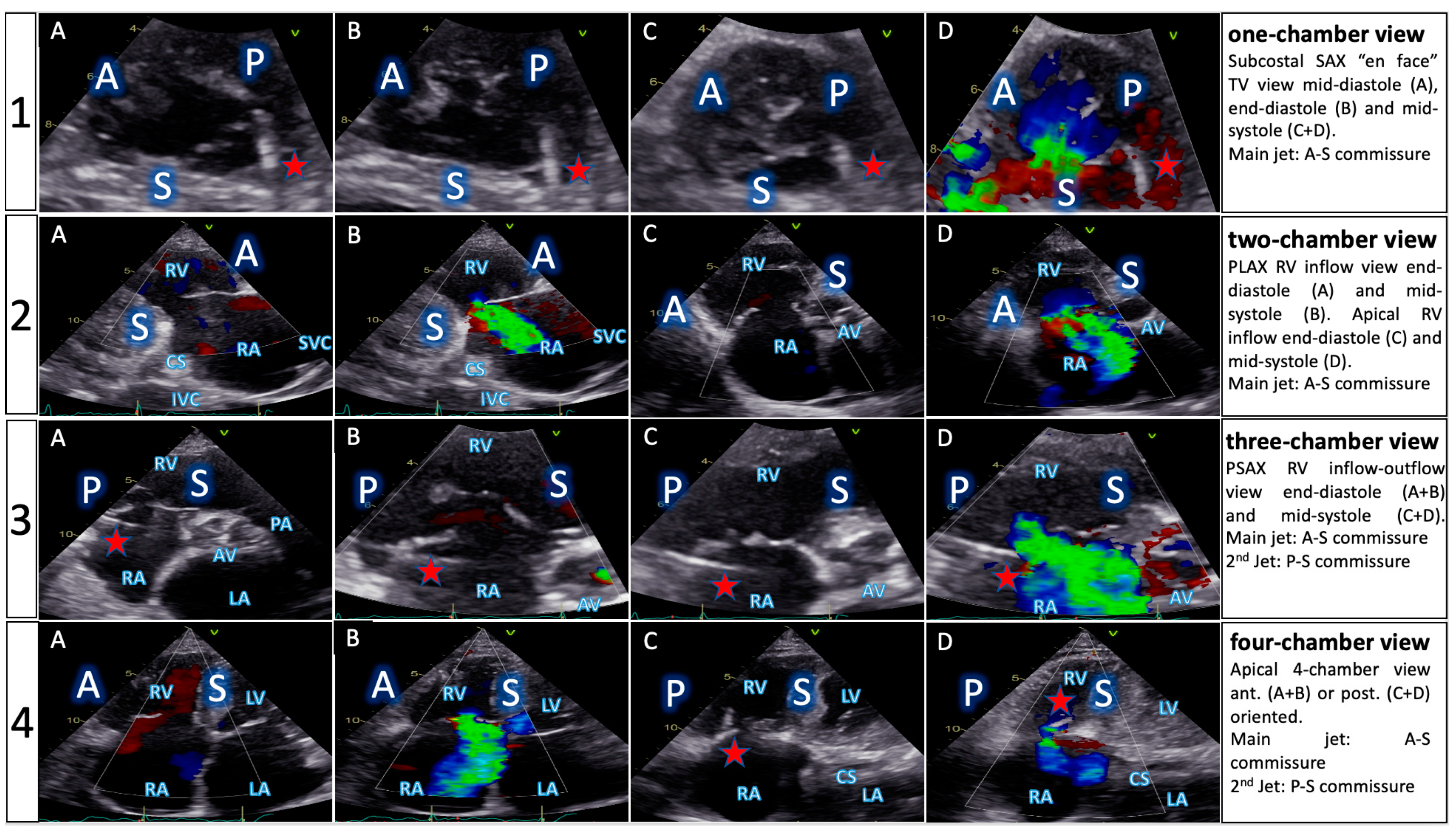
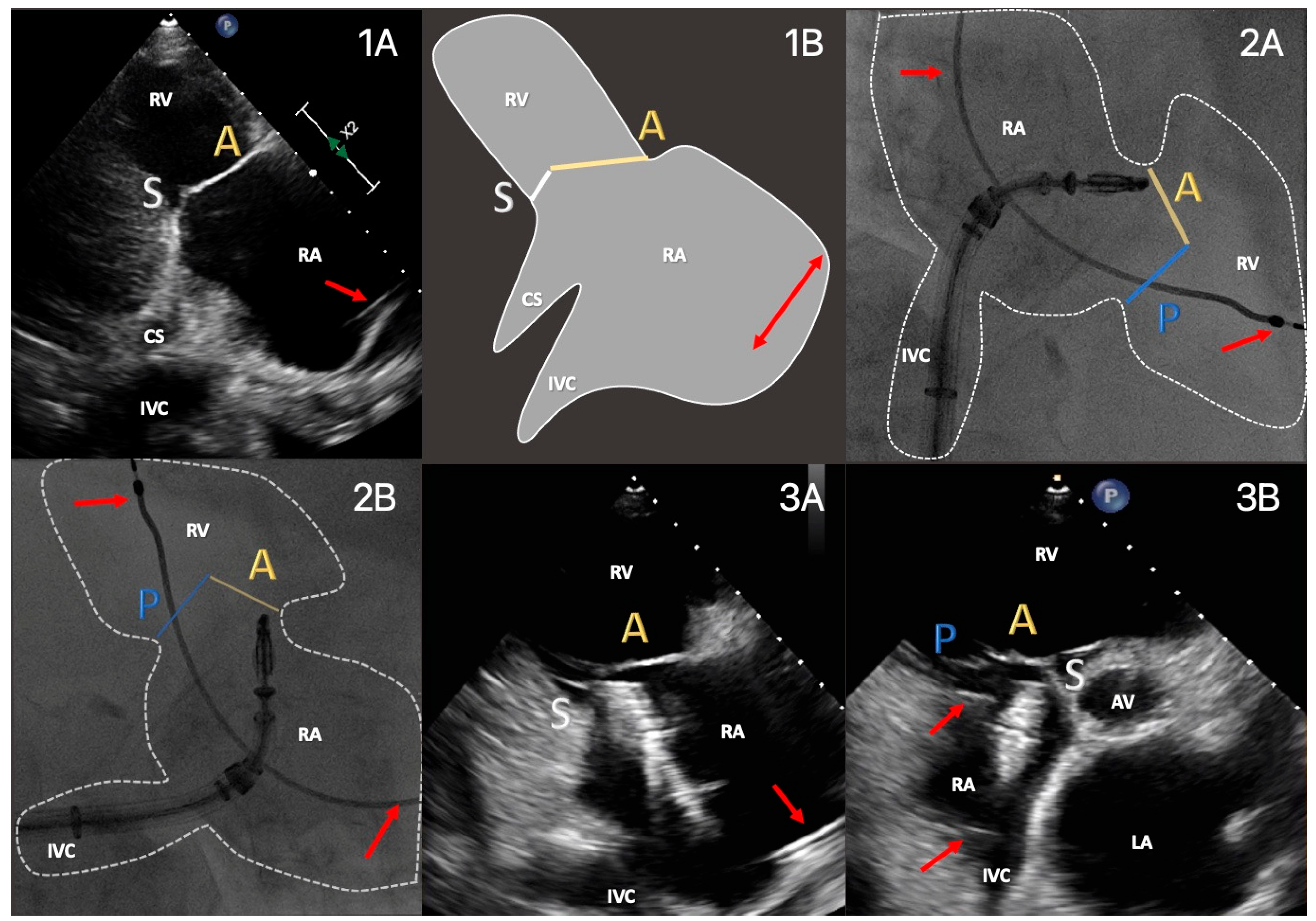
| Functional Parameters | Right Heart Morphology | TV Anatomy | |
| PLA standard | LVOT diameter (Qs/CO calculation) | RV function and size (eyeballing) | -- |
| PLA RV inflow RH two-chamber view | TR severity (eyeballing) TR Jet VC and PISA (optional) | RV function and size (eyeballing) | AL visualization SL vs. PL distinction |
| PSA standard RH three-chamber view | TR severity (eyeballing) RVOT VTI RVOT diameter | RV size PA size | Leaflet distinction, if possible |
| PSA-modified alternative RH one-chamber view | TR severity (eyeballing) | TV annulus size Coaptation gap | Simultaneous visualization of all leaflets |
| A4C RH four-chamber view | TR Jet area, VC and PISA TR VTI, RVSP TAPSE RV free wall TDI RV FAC RA volume RV diameters LVOT VTI (A5C/A3C) | RV function and size RA size TV annulus size Tenting height | SL visualization AL vs. PL distinction |
| A2C right alternative RH two-chamber view | TR Jet area, VC and PISA | RA size TV annulus size | AL visualization |
| Subcostal long axis | Hepatic systolic vein flow reversal Inferior vena cava size | RV function and size (eyeballing) | PL visualization AL vs. SL distinction |
| Subcostal short axis RH one-chamber view | TR severity (eyeballing) | Coaptation gap | Simultaneous visualization of all leaflets |
| Characteristic | MMI (n = 17) | TEE (n = 23) | p-Value | |
|---|---|---|---|---|
| Device success * | 100% (17/17) | 100% (23/23) | 1.000 | |
| TR reduction | 1-grade | 94% (16/17) | 91% (21/23) | 1.000 |
| ≥2 grades | 65% (11/17) | 52% (12/23) | 1.000 | |
| Mean no. clips/patient | 1.5 ± 0.6 | 1.5 ± 0.5 | 0.851 | |
| Procedural time (min) ** | 113.6 ± 72.2 | 110.7 ± 54.9 | 0.888 | |
| Device time (min) *** | 66.1 ± 35.1 | 58.7 ± 27.5 | 0.459 | |
| Fluoroscopy time (min) | 14.4 ± 8.8 | 13 ± 7.1 | 0.578 | |
| Radiation dose (cGy) | 4074.6 ± 2491.7 | 5125.1 ± 3827.6 | 0.330 | |
| TV mean gradient (mmHg) | 1.8 ± 0.6 | 1.8 ± 0.7 | 0.896 | |
| RA pressure decrease (∆) | 2.7 ± 1.3 | 3.2 ± 2.6 | 0.432 | |
| Length of hospital stay (days) | 5.1 ± 3.2 | 8 ± 4.9 | 0.045 | |
| Technique | Bicuspidalization | 18% (3/17) | 30% (7/23) | 0.719 |
| Clover | 35% (6/17) | 18% (4/23) | 0.480 | |
| “1-Clip” technique | 47% (8/17) | 52% (12/23) | 1.000 | |
| Clip position | ant.-sept. | 52% (14/27) | 59% (20/34) | 0.831 |
| post.-sept. | 48% (13/27) | 38% (13/34) | 0.645 | |
| ant.-post. | 0% | 3% (1/34) | 1.000 | |
| Event | MMI (n = 15) | TEE (n = 19) | p-Value |
|---|---|---|---|
| Cardiovascular mortality | 1 | 2 | 1.000 |
| All-cause mortality | 2 | 4 | 0.661 |
| Device related adverse events | 0 | 0 | --- |
| Myocardial infarction | 0 | 0 | --- |
| Major bleeding | 1 * | 1 ** | 1.000 |
| Vascular complications | 0 | 1 *** | 1.000 |
| Emergent cardiac surgery | 0 | 0 | --- |
| New onset renal failure | 0 | 0 | --- |
| New onset liver failure | 0 | 0 | --- |
| Tricuspid valve stenosis | 0 | 0 | --- |
| Stroke | 0 | 0 | --- |
| Rehospitalization for AHF | 4 | 8 | 0.734 |
| Non-cardiac rehospitalization **** | 7 | 13 | 0.575 |
| Gastrointestinal bleeding | 0 | 3 ***** | 0.237 |
| Variable | MMI | TEE | MMI vs. TEE (∆) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline (n = 17) | 12 Months (n = 15) | p-Value | Baseline (n = 23) | 12 Months (n = 20) | p-Value | p-Value | ||
| Quality of Life | ||||||||
| KCCQ Score (pts.) | 32.3 ± 18.7 | 61.9 ± 19.6 | <0.001 | 26.7 ± 16.7 | 48.6 ± 20.9 | <0.001 | 0.441 | |
| 6-minute walk test (m) | 183.2 ± 91.4 | 284.7 ± 114.7 | <0.001 | 162.1 ± 94.1 | 247.8 ± 114.2 | <0.001 | 0.541 | |
| NYHA class reduction | 1-grade | -- | 15/15 (100%) | -- | -- | 20/20 (100%) | -- | 1.000 |
| ≥2 grades | -- | 5/15 (33%) | -- | -- | 2/20 (10%) | -- | 0.112 | |
| Major organ systems | ||||||||
| GFR (mL/m2/1.73m2) | 55.3 ± 15.9 | 59.4 ± 16.3 | 0.152 | 52.4 ± 18.4 | 55.2 ± 20.6 | 0.326 | 0.762 | |
| BUN (mg/dL) | 56.1 ± 30.6 | 41.4 ± 15.3 | 0.014 | 62.7 ± 32.2 | 56.3 ± 26.1 | 0.181 | 0.244 | |
| AST (U/L) | 34.2 ± 15.7 | 30.6 ± 11.8 | 0.085 | 33.4 ± 24.1 | 24.3 ± 8.3 | 0.033 | 0.274 | |
| ALT (U/L) | 23.5 ± 18.5 | 20.5 ± 12.3 | 0.451 | 21 ± 14.6 | 16.8 ± 12.5 | 0.176 | 0.807 | |
| Bilirubin (mg/dL) | 1.17 ± 0.97 | 0.90 ± 0.59 | 0.163 | 0.86 ± 0.84 | 0.69 ± 0.44 | 0.082 | 0.636 | |
| NTproBNP (pg/mL) | 2594.5 ± 1756.6 | 2076.4 ± 1304.1 | 0.103 | 4103.5 ± 6018 | 3415.8 ± 5294 | 0.047 | 0.704 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patrascu, A.; Binder, D.; Alashkar, I.; Schnabel, P.; Stähle, W.; Risha, O.; Weinmann, K.; Ott, I. Personalized Guidance of Edge-to-Edge Transcatheter Tricuspid Valve Repair by Multimodality Imaging. J. Clin. Med. 2024, 13, 2833. https://doi.org/10.3390/jcm13102833
Patrascu A, Binder D, Alashkar I, Schnabel P, Stähle W, Risha O, Weinmann K, Ott I. Personalized Guidance of Edge-to-Edge Transcatheter Tricuspid Valve Repair by Multimodality Imaging. Journal of Clinical Medicine. 2024; 13(10):2833. https://doi.org/10.3390/jcm13102833
Chicago/Turabian StylePatrascu, Alexandru, Donat Binder, Ibrahim Alashkar, Peter Schnabel, Wilfried Stähle, Osama Risha, Kai Weinmann, and Ilka Ott. 2024. "Personalized Guidance of Edge-to-Edge Transcatheter Tricuspid Valve Repair by Multimodality Imaging" Journal of Clinical Medicine 13, no. 10: 2833. https://doi.org/10.3390/jcm13102833
APA StylePatrascu, A., Binder, D., Alashkar, I., Schnabel, P., Stähle, W., Risha, O., Weinmann, K., & Ott, I. (2024). Personalized Guidance of Edge-to-Edge Transcatheter Tricuspid Valve Repair by Multimodality Imaging. Journal of Clinical Medicine, 13(10), 2833. https://doi.org/10.3390/jcm13102833






