Cardiac Mechanics Evaluation in Preschool-Aged Children with Preterm Birth History: A Speckle Tracking and 4D Echocardiography Study
Abstract
1. Introduction
2. Aim of the Study
3. Materials and Methods
Statistical Analysis
4. Results
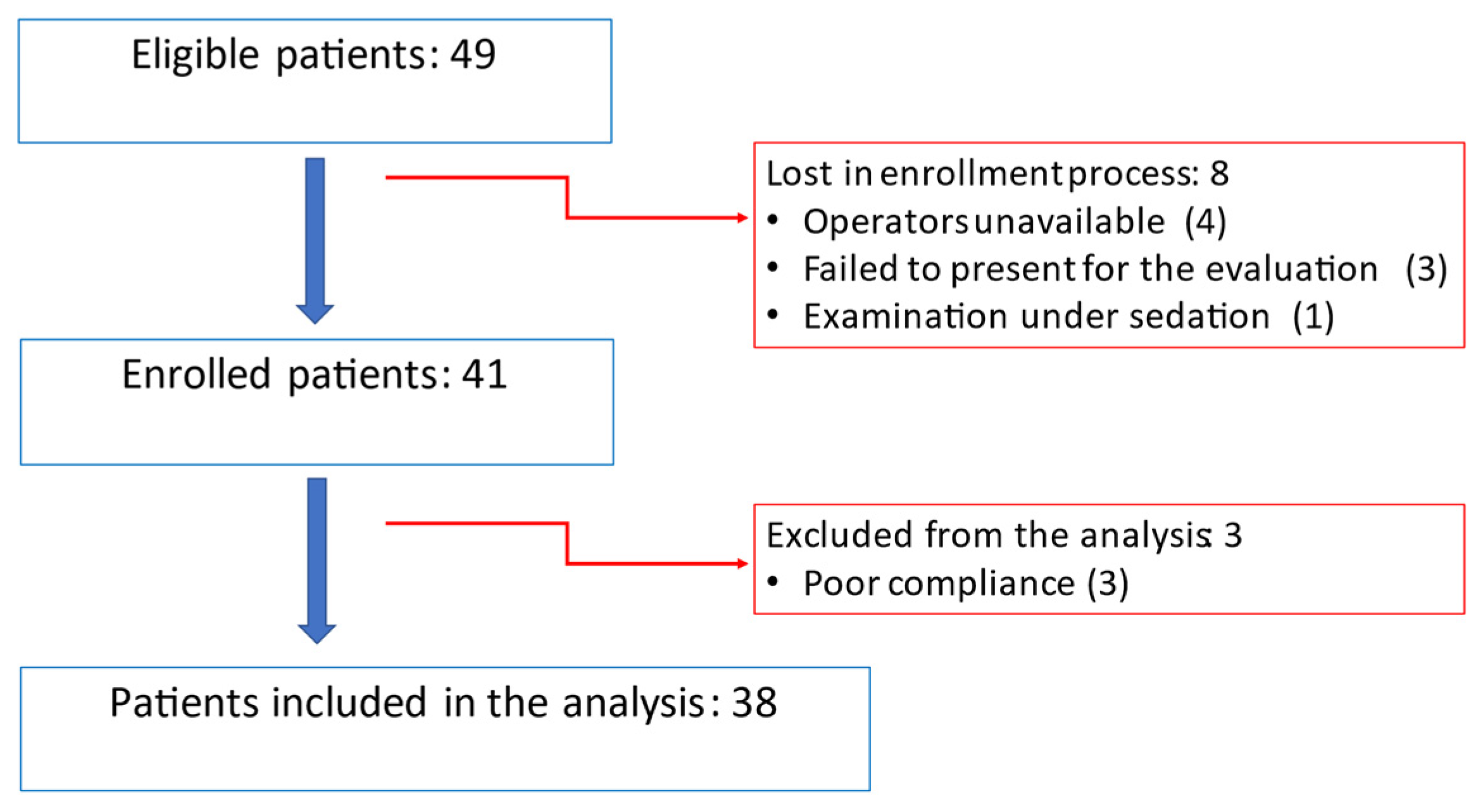
4.1. Population
4.2. Echocardiographic Assesment
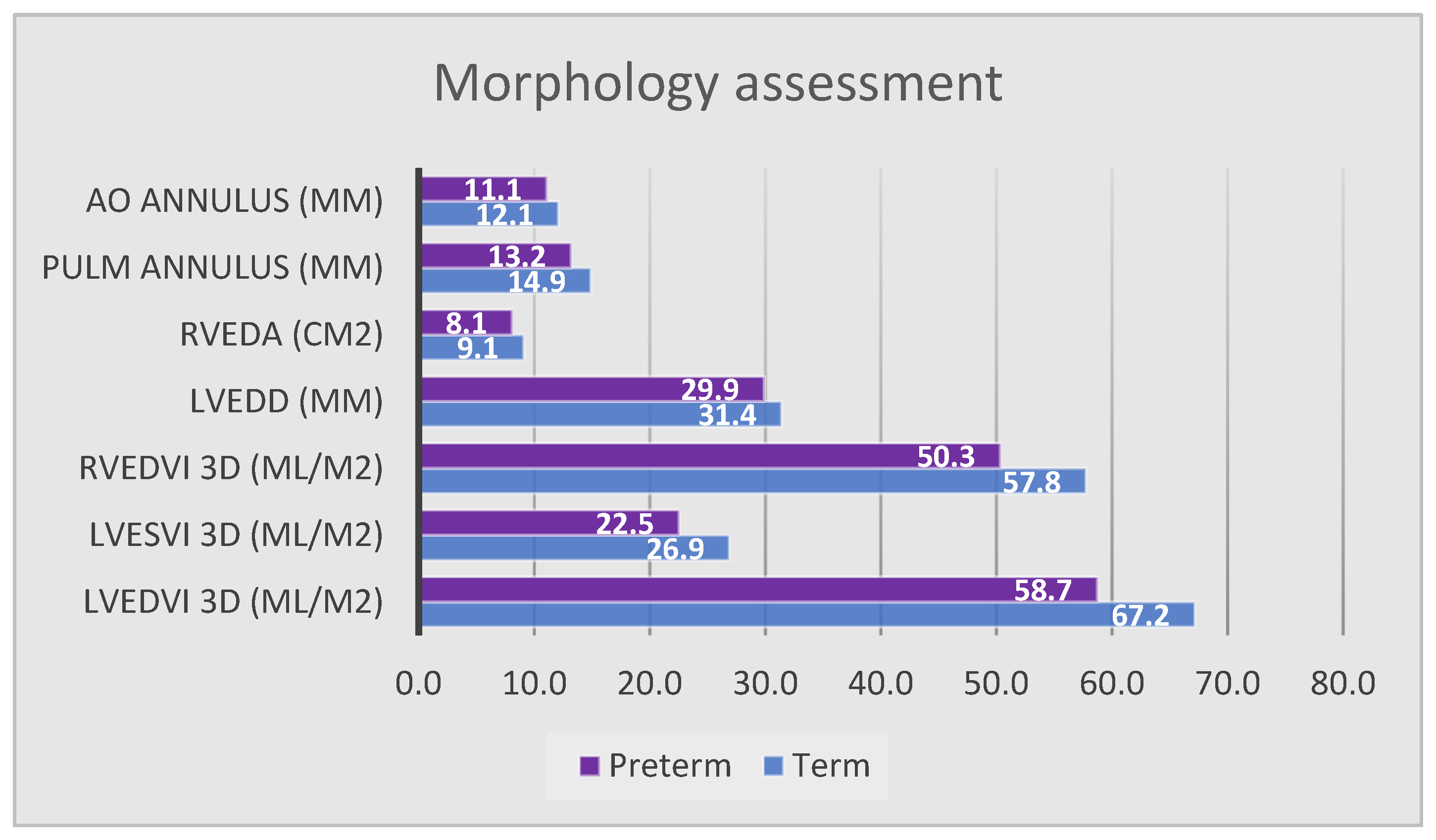
4.2.1. Morphologic Assessment
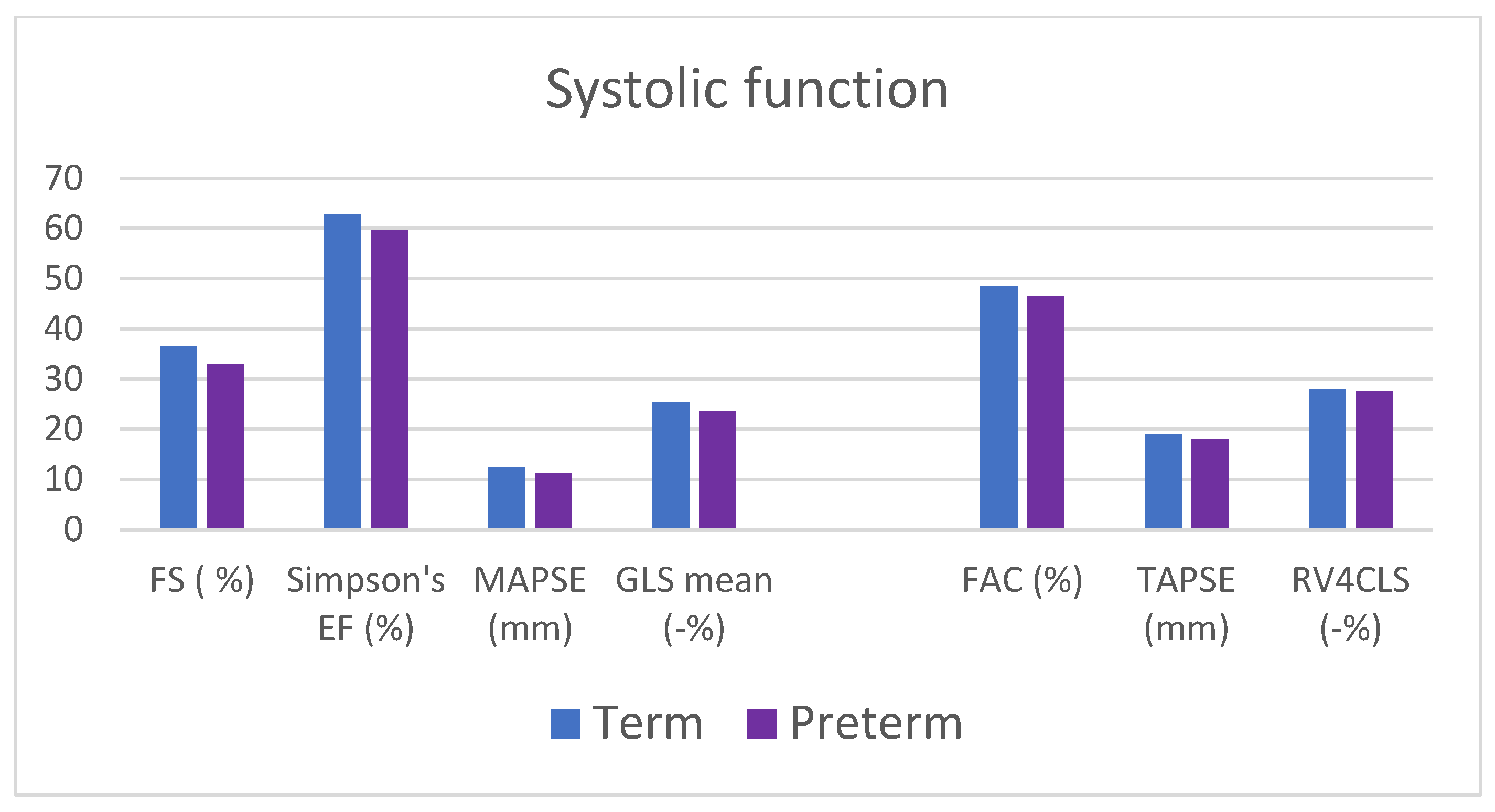
4.2.2. Functional Assessment
4.3. IUGR and SGA
4.4. Previous NICU Stay
4.5. BPD
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, J.; Szamotulska, K.; Drewniak, N.; Mohangoo, A.; Chalmers, J.; Sakkeus, L.; Irgens, L.; Gatt, M.; Gissler, M.; Blondel, B.; et al. Preterm birth time trends in Europe: A study of 19 countries. BJOG 2013, 120, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Stats of the States—Preterm Births. Available online: https://www.cdc.gov/nchs/pressroom/sosmap/preterm_births/preterm.htm (accessed on 9 September 2022).
- Harrison, M.S.; Goldenberg, R.L. Global burden of prematurity. Semin. Fetal Neonatal Med. 2016, 21, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Platt, M.J. Outcomes in preterm infants. Public Health 2014, 128, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Crump, C.; Sundquist, K.; Sundquist, J.; Winkleby, M.A. Gestational Age at Birth and Mortality in Young Adulthood. JAMA 2011, 306, 1233. [Google Scholar] [CrossRef] [PubMed]
- de Jong, F.; Monuteaux, M.C.; van Elburg, R.M.; Gillman, M.W.; Belfort, M.B. Systematic Review and Meta-Analysis of Preterm Birth and Later Systolic Blood Pressure. Hypertension 2012, 59, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Crump, C.; Winkleby, M.A.; Sundquist, K.; Sundquist, J. Risk of hypertension among young adults who were born preterm: A Swedish national study of 636,000 births. Am. J. Epidemiol. 2011, 173, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Carr, H.; Cnattingius, S.; Granath, F.; Ludvigsson, J.F.; Edstedt Bonamy, A.-K. Preterm Birth and Risk of Heart Failure Up to Early Adulthood. J. Am. Coll. Cardiol. 2017, 69, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Ueda, P.; Cnattingius, S.; Stephansson, O.; Ingelsson, E.; Ludvigsson, J.F.; Bonamy, A.-K.E. Cerebrovascular and ischemic heart disease in young adults born preterm: A population-based Swedish cohort study. Eur. J. Epidemiol. 2014, 29, 253–260. [Google Scholar] [CrossRef]
- Lewandowski, A.J. The preterm heart: A unique cardiomyopathy? Pediatr. Res. 2019, 85, 738–739. [Google Scholar] [CrossRef]
- Telles, F.; Mcnamara, N.; Nanayakkara, S.; Doyle, M.P.; Williams, M.; Yaeger, L.; Marwick, T.H.; Leeson, P.; Levy, P.T.; Lewandowski, A.J. Changes in the Preterm Heart From Birth to Young Adulthood: A Meta-analysis. Pediatrics 2020, 146, e20200146. [Google Scholar] [CrossRef]
- Rudolph, A.M. Myocardial growth before and after birth: Clinical implications. Acta Paediatr. 2000, 89, 129–133. [Google Scholar] [PubMed]
- Lewandowski, A.J.; Augustine, D.; Lamata, P.; Davis, E.F.; Lazdam, M.; Francis, J.; McCormick, K.; Wilkinson, A.R.; Singhal, A.; Lucas, A.; et al. Preterm Heart in Adult Life. Circulation 2013, 127, 197–206. [Google Scholar] [CrossRef]
- Sarvari, S.I.; Rodriguez-Lopez, M.; Nuñez-Garcia, M.; Sitges, M.; Sepulveda-Martinez, A.; Camara, O.; Butakoff, C.; Gratacos, E.; Bijnens, B.; Crispi, F.; et al. Persistence of Cardiac Remodeling in Preadolescents With Fetal Growth Restriction. Circ. Cardiovasc. Imaging 2017, 10, e005270. [Google Scholar] [CrossRef]
- Crispi, F.; Bijnens, B.; Figueras, F.; Bartrons, J.; Eixarch, E.; Le Noble, F.; Ahmed, A.; Gratacos, E. Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation 2010, 121, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Le, N.; Phad, N.; de Waal, K. Cardiac remodeling during the neonatal intensive care period; a window of opportunity for early prevention of heart failure? Early Hum. Dev. 2020, 151, 105168. [Google Scholar] [CrossRef]
- Aye, C.Y.L.; Lewandowski, A.J.; Lamata, P.; Upton, R.; Davis, E.; Ohuma, E.O.; Kenworthy, Y.; Boardman, H.; Wopperer, S.; Packham, A.; et al. Disproportionate cardiac hypertrophy during early postnatal development in infants born preterm. Pediatr. Res. 2017, 82, 36. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.J.; Bai, W.; Price, A.N.; Edwards, A.D.; Rueckert, D.; Groves, A.M. Ventricular remodeling in preterm infants: Computational cardiac magnetic resonance atlasing shows significant early remodeling of the left ventricle. Pediatr. Res. 2019, 85, 807–815. [Google Scholar] [CrossRef]
- Korhonen, P.; Hyödynmaa, E.; Lautamatti, V.; Iivainen, T.; Tammela, O. Cardiovascular findings in very low birthweight schoolchildren with and without bronchopulmonary dysplasia. Early Human. Development 2005, 81, 497–505. [Google Scholar] [CrossRef]
- Erickson, C.T.; Patel, M.D.; Choudhry, S.; Bisselou, K.S.; Sekarski, T.; Craft, M.; Li, L.; El Khuffash, A.; Hamvas, A.; Kutty, S.; et al. Persistence of right ventricular dysfunction and altered morphometry in asymptomatic preterm Infants through one year of age: Cardiac phenotype of prematurity. Cardiol. Young 2019, 29, 945–953. [Google Scholar] [CrossRef]
- Levy, P.T.; EL-Khuffash, A.; Patel, M.D.; Breatnach, C.R.; James, A.T.; Sanchez, A.A.; Abuchabe, C.; Rogal, S.R.; Holland, M.R.; McNamara, P.J.; et al. Maturational Patterns of Systolic Ventricular Deformation Mechanics by Two-Dimensional Speckle-Tracking Echocardiography in Preterm Infants over the First Year of Age. J. Am. Soc. Echocardiogr. 2017, 30, 685–698.e1. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Kim, M.; Hwang, S.J.; Kim, H.J. Progression of right ventricular systolic dysfunction detected by myocardial deformation imaging in asymptomatic preterm children. J. Cardiovasc. Ultrasound 2017, 25, 98–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joshi, S.; Wilson, D.G.; Kotecha, S.; Pickerd, N.; Fraser, A.G.; Kotecha, S. Cardiovascular function in children who had chronic lung disease of prematurity. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F373-9. [Google Scholar] [CrossRef]
- Mohlkert, L.A.; Hallberg, J.; Broberg, O.; Rydberg, A.; Halvorsen, C.P.; Liuba, P.; Fellman, V.; Domellöf, M.; Sjöberg, G.; Norman, M. The preterm heart in childhood: Left ventricular structure, geometry, and function assessed by echocardiography in 6-year-old survivors of periviable births. J. Am. Heart Assoc. 2018, 7, e007742. [Google Scholar] [CrossRef]
- Kwinta, P.; Jagła, M.; Grudzień, A.; Klimek, M.; Zasada, M.; Pietrzyk, J.J. From a regional cohort of extremely low birth weight infants: Cardiac function at the age of 7 years. Neonatology 2013, 103, 287–292. [Google Scholar] [CrossRef]
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The global epidemiology of preterm birth. Best. Pr. Res. Clin. Obs. Gynaecol. 2018, 52, 3–12. [Google Scholar] [CrossRef]
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.-B.; Narwal, R.; Adler, A.; Garcia, C.V.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Walani, S.R. Global burden of preterm birth. Int. J. Gynecol. Obstet. 2020, 150, 31–33. [Google Scholar] [CrossRef]
- Lai, W.W.; Mertens, L.L.; Cohen, M.S.; Geva, T. Echocardiography in Pediatric and Congenital Heart Disease; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Mondillo, S.; Galderisi, M.; Mele, D.; Cameli, M.; Lomoriello, V.S.; Zacà, V.; Ballo, P.; D’Andrea, A.; Muraru, D.; Losi, M.; et al. Speckle-tracking echocardiography: A new technique for assessing myocardial function. J. Ultrasound Med. 2011, 30, 71–83. [Google Scholar] [CrossRef]
- Medvedofsky, D.; Addetia, K.; Patel, A.R.; Sedlmeier, A.; Baumann, R.; Mor-Avi, V.; Lang, R.M. Novel Approach to Three-Dimensional Echocardiographic Quantification of Right Ventricular Volumes and Function from Focused Views. J. Am. Soc. Echocardiogr. 2015, 28, 1222–1231. [Google Scholar] [CrossRef]
- Lakatos, B.K.; Tokodi, M.; Kispál, E.; Merkely, B.; Kovács, A. Morphological and functional assessment of the right ventricle using 3d echocardiography. J. Vis. Exp. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jone, P.N.; Le, L.; Pan, Z.; Goot, B.H.; Parthiban, A.; Harrild, D.; Ferraro, A.M.; Marx, G.; Colen, T.; Khoo, N.S. Three-Dimensional Echocardiography Right Ventricular Volumes and Ejection Fraction Reference Values in Children: A North American Multicentre Study. Can. J. Cardiol. 2022, 38, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- DeVore, G.R. Three-dimensional and four-dimensional fetal echocardiography: A new frontier. Curr. Opin. Pediatr. 2005, 17, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, G.; Pergola, V.; Fadel, B.; Al Bulbul, Z.; Caso, P. Strain Echocardiography and Myocardial Mechanics: From Basics to Clinical Applications. J. Cardiovasc. Echogr. 2015, 25, 1. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, Z.; Issa, Z.; Siblini, G.; Moiduddin, N.; Di Salvo, G. Normal Range of Left Ventricular Strain, Dimensions and Ejection Fraction Using Three-dimensional Speckle-Tracking Echocardiography in Neonates. J. Cardiovasc. Echogr. 2015, 25, 67. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants An Evidence-based Approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.; Di Salvo, G.; Prota, C.; Bucciarelli, V.; Josen, M.; Paredes, J.; Borrelli, N.; Sirico, D.; Prasad, S.; Indolfi, C.; et al. Left Atrial Strain to Identify Diastolic Dysfunction in Children with Cardiomyopathies. J. Clin. Med. 2019, 8, 1243. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Addetia, K.; Maffessanti, F.; Mor-Avi, V.; Lang, R.M. LA Strain for Categorization of, L.V. Diastolic Dysfunction. JACC Cardiovasc. Imaging 2017, 10, 735–743. [Google Scholar] [CrossRef]
- Takahashi, N.; Nishida, H.; Arai, T.; Kaneda, Y. Abnormal cardiac histology in severe intrauterine growth retardation infants. Acta Paediatr. Jpn. 1995, 37, 341–346. [Google Scholar] [CrossRef]
- Rodríguez-López, M.; Cruz-Lemini, M.; Valenzuela-Alcaraz, B.; Garcia-Otero, L.; Sitges, M.; Bijnens, B.; Gratacós, E.; Crispi, F. Descriptive analysis of different phenotypes of cardiac remodeling in fetal growth restriction. Ultrasound Obs. Gynecol. 2017, 50, 207–214. [Google Scholar] [CrossRef]
- Noori, S.; McCoy, M.; Friedlich, P.; Bright, B.; Gottipati, V.; Seri, I.; Sekar, K. Failure of Ductus Arteriosus Closure Is Associated With Increased Mortality in Preterm Infants. Pediatrics 2009, 123, e138–e144. [Google Scholar] [CrossRef] [PubMed]
- Kluckow, M.; Evans, N. Ductal shunting, high pulmonary blood flow, and pulmonary hemorrhage. J. Pediatr. 2000, 137, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Fadel, B.M.; Mohty, D.; Husain, A.; Dahdouh, Z.; Al-Admawi, M.; Pergola, V.; Di Salvo, G. The Various Hemodynamic Profiles of the Patent Ductus Arteriosus in Adults. Echocardiography 2015, 32, 1172–1178. [Google Scholar] [CrossRef]
- Regan, W.; Benbrik, N.; Sharma, S.-R.; Auriau, J.; Bouvaist, H.; Bautista-Rodriguez, C.; Sirico, D.; Aw, T.-C.; di Salvo, G.; Foldvari, S.; et al. Improved ventilation in premature babies after transcatheter versus surgical closure of patent ductus arteriosus. Int. J. Cardiol. 2020, 311, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Koroglu, O.A.; Yalaz, M.; Levent, E.; Akisu, M.; Kültürsay, N. Cardiovascular consequences of bronchopulmonary dysplasia in prematurely born preschool children. Neonatology 2013, 104, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Wan, J.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Mohajerani, E.; Seeger, W.; Herberg, U.; et al. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2019, 12, e009047. [Google Scholar] [CrossRef] [PubMed]
- Negishi, T.; Negishi, K.; Thavendiranathan, P.; Cho, G.-Y.; Popescu, B.A.; Vinereanu, D.; Kurosawa, K.; Penicka, M.; Marwick, T.H.; Aakhus, S.; et al. Effect of Experience and Training on the Concordance and Precision of Strain Measurements. JACC Cardiovasc. Imaging 2017, 10, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Rösner, A.; Barbosa, D.; Aarsæther, E.; Kjønås, D.; Schirmer, H.; D’Hooge, J. The influence of frame rate on two-dimensional speckle-tracking strain measurements: A study on silico-simulated models and images recorded in patients. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1137–1147. [Google Scholar] [CrossRef]
- Mirea, O.; Pagourelias, E.D.; Duchenne, J.; Bogaert, J.; Thomas, J.D.; Badano, L.P.; Voigt, J.-U. Intervendor Differences in the Accuracy of Detecting Regional Functional Abnormalities a Report from the, E.A.CVI-ASE Strain Standardization Task Force. JACC Cardiovasc. Imaging 2018, 11, 25–34. [Google Scholar] [CrossRef]
| Preterms | Controls | p Value | |
|---|---|---|---|
| Number of patients | 38 | 19 | |
| Female | 12 (32%) | 6 (32%) | 1 |
| Gestational age at birth (GW) | 26.7 + 1.87 | 39.9 + 1.3 | |
| Birth weight (g) | 815 + 232 | 3359 + 413 | |
| SGA | 11 (29%) | 3 (18%) | 0.28 |
| IUGR | 12 (32%) | 1 (6%) | 0.026 * |
| Chronological age (months) | 34.9 + 9.4 | 32.2 + 7.1 | 0.26 |
| Corrected age (months) | 31.9 + 9.1 | 32.2₊7.1 | 0.9 |
| Weight (kg) | 12.2 + 2.2 | 13.6 + 1.8 | 0.029 * |
| Height (cm) | 90.1 + 6.5 | 93.6+ 6.5 | 0.074 |
| BSA (cm2) | 0.56 + 0.1 | 0.59 + 0.1 | 0.085 |
| Heart rate (bpm) | 107 + 20 | 107 + 11 | 0.96 |
| SBP (mmHg) | 103 + 11 | 99 5 | 0.24 |
| DBP (mmHg) | 65 + 10 | 66 + 9 | 0.71 |
| Categorical Variables | n° | % | |
|---|---|---|---|
| Respiratory | Need for mechanical ventilation | 38 | 100 |
| Invasive mechanical ventilation | 28 | 74 | |
| Need for surfactant administration | 32 | 84 | |
| Technique: LISA | 9 | 28 | |
| InSurE | 1 | 3 | |
| Infant intubated | 21 | 66 | |
| Mixed | 1 | 3 | |
| BPD at 36 weeks (PMA) | 23 | 61 | |
| Discharged with O2 supplementation | 5 | 13 | |
| Diuretic therapy at discharge | 10 | 26 | |
| Broncho-reactivity | 19 | 50 | |
| Admissions for respiratory disease | 13 | 33 | |
| Cardiovascular | Persistent pulmonary hypertension in newborn | 2 | 5 |
| Inotropic support | 15 | 39 | |
| Hemodynamically significant DA Surgical legation | 25 3 | 66 8 | |
| Other comorbidities | IVH > I grade | 7 | 18 |
| Chronic renal failure | 2 | 5 | |
| NEC Surgery needed | 9 5 | 24 13 | |
| Continuous variables | mean ± SD | ||
| Respiratory | Invasive mechanical ventilation (days) | 14.4 ± 6 | |
| Non-invasive mechanical ventilation (days) | 51.3 ± 19.8 | ||
| Number of surfactant administrations (n°) | 1.3 ± 0.9 | ||
| Cardiovascular | Inotropic support (days) | 2.1 ± 3.5 | |
| Medical cycles to treat ductus arteriosus (n°) | 1.7 ± 1.4 | ||
| Echocardiography Parameters | Cases Mean ± SD | Controls Mean ± SD | p-Value |
|---|---|---|---|
| Left-sided heart: | |||
| IVSd (mm) | 4.0 ± 0.4 | 3.9 ± 0.4 | 0.56 |
| LVEDD (mm) | 29.9 ± 2.2 | 31.4 ± 2.6 | 0.024 * |
| PWd (mm) | 5.1 ± 0.8 | 5.0 ± 0.8 | 0.63 |
| IVSs (mm) | 5.7 ± 1.1 | 5.6 ± 0.7 | 0.95 |
| LVESD | 20.0 ± 2.5 | 19.9 ± 1.4 | 0.75 |
| PWs (mm) | 6.7 ± 1 | 8.2 ± 1.2 | <0.001 * |
| Mid-cavity diameter (mm) | 29.7 ± 2.9 | 31.4 ± 2.2 | 0.025 * |
| Sphericity index | 1.64 ± 0.24 | 1.58 ± 0.1 | 0.26 |
| LVEDV 3D (mL) | 33.5 ± 7.8 | 39.8 ± 5.2 | 0.011 * |
| LVEDVi 3D (mL/m2) | 58.7 ± 11.2 | 67.2 ± 8.5 | 0.021 * |
| LESV 3D (mL) | 12.9 ± 4.3 | 15.9 ± 3.1 | 0.024 * |
| LVESVi 3D (mL/m2) | 22.5 ± 7.2 | 26.9 ± 4.8 | 0.049 * |
| Aortic annulus diameter (mm) | 11 ± 1 | 12.1 ± 0.9 | <0.001 * |
| Right-sided heart: | |||
| RVEDA (cm2) | 8.1 ± 1.5 | 9.1 ± 1.1 | 0.007 * |
| RVESA (cm2) | 27.5 ± 3.6 | 28 ± 2.4 | 0.59 |
| RVEDV 3D (mL) | 28.1 ± 8 | 34.3 ± 7.9 | 0.011 * |
| RVEDVi 3D (mL/m2) | 50.3 ± 10.4 | 57.7 ± 11 | 0.022 * |
| RVESV 3D (mL) | 11.8 ± 4.6 | 13.0 ± 3.0 | 0.35 |
| RVESVi 3D (mL/m2) | 21.1 ± 6.7 | 21.8 ± 4.3 | 0.68 |
| Pulmonary annulus diameter (mm) | 13.2 ± 1.7 | 14.9 ± 1.7 | <0.001 * |
| Echocardiography Parameters | Cases Mean ± SD | Controls Mean ± SD | p-Value |
|---|---|---|---|
| Left ventricle systolic function: | |||
| Fraction shortening (%) | 32.9 ± 6.8 | 36.5 ± 5.4 | 0.049 * |
| Ejection fraction (Simpson single plane) (%) | 59.2 ± 4.3 | 62.3 ± 3.7 | 0.003 * |
| Stroke volume (mL) | 17.2 ± 3.8 | 20.1 ± 4 | 0.015 * |
| MAPSE (mm) | 11.3 ± 2.1 | 12.6 ± 1.4 | 0.021 * |
| 4-chamber GLS (%) | −23.7 ± 2.8 | −26 ± 2.5 | 0.004 * |
| 3-chamber GLS (%) | −23.3 ± 3.3 | −25.4 ± 3.1 | 0.027 * |
| 2-chamber GLS (%) | −23.8 ± 3.3 | −25.0 ± 2.6 | 0.16 |
| GLS mean (%) | −23.6 ± 2.4 | −25.5 ± 1.7 | 0.003 * |
| Left ventricle diastolic function: | |||
| Atrial Strain (%) | 47.4 ± 9.7 | 54.9 ± 6.8 | 0.004 * |
| E/A | 1.7 ± 0.4 | 1.9 ± 0.5 | 0.25 |
| E/e’ lateral | 6.6 ± 1.3 | 6.3 ± 1.4 | 0.38 |
| E/e’ septal | 8.4 ± 1.7 | 8.4 ± 1.2 | 0.99 |
| RV Systolic function: | |||
| TAPSE (%) | 18.0 ± 2.6 | 19.1 ± 2.4 | 0.13 |
| TAPSE auto 3D (%) | 15.8 ± 3 | 16.9 ± 1.8 | 0.17 |
| FAC (%) | 46.6 ± 7.4 | 48.4 ± 6.1 | 0.36 |
| FAC auto 3D (%) | 49.5 ± 8.6 | 50.9 ± 5.1 | 0.55 |
| RVFWLS (%) | −32.8 ± 5 | −33.5 ± 3.4 | 0.61 |
| RV4CLS (%) | −27.5 ± 3.6 | 28.0 ± 2.4 | 0.59 |
| Auto septal RVLS (%) | −21.9 ± 5.8 | −22.8 ± 4.3 | 0.53 |
| Auto free wall RVLS (%) | −29.4 ± 6 | −32.3 ± 3.6 | 0.08 |
| Preterm vs. Term Children | Erickson 80 pt 1 y < 29 GWs | Kang 24 pt 2 y < 33 GWs | Mohlkert 176 pt 6 y < 26 GWs | Korhonen 34 pt 7.5 y < 1500 g | Kwinta 81 pt 7 y ELBW | Savio 38 pt 2–5 y < 30 GWs | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Right sections | Areas and diameters ↑ | RVEDD ↓ | RVEDA RVEDV 3D Pulmonary annulus | ↓ | |||||||
| MMODE | |||||||||||
| Areas | |||||||||||
| Volume 3D | |||||||||||
| RV function | TAPSE, FAC, FWLS ↓ | FAC = strain ↓ | TAPSE, FAC, strains, EF 3D | = | |||||||
| TAPSE, FAC | |||||||||||
| strain | |||||||||||
| EF 3D | |||||||||||
| RV afterload | PAAT; PAAT/ET | ↓ | RVSP ↑ PAAT, mPAP = | PAAT; PAAT/ET | = | PAAT; PAAT/ET RVSP | = | ||||
| PAAT, PAAT/ET | |||||||||||
| RVSP | |||||||||||
| Left sections | LV lenght, mid cavity, Ao Annuls, mass Stroke volume | ↓ | PWs, LVEDD Annulus Ao Simpson volumes Stroke volume | ↓ | PWs, LVEDD, mid cavity, Ao annulus, 3D volumes stroke volume | ↓ | |||||
| MMODE | |||||||||||
| Simpson | |||||||||||
| 3D | |||||||||||
| LV function | MAPSE, FS, s’ ↓ GLS = E/e’ lat ↑ | Diastolic function = | GLS, EF, FS Left atrial strain | ↓ | |||||||
| GLS, FS, EF | |||||||||||
| MAPSE, s’ | |||||||||||
| diastolic | |||||||||||
| Left atrium | Dimensions = | Diameter ↓ | Dimensions = | ||||||||
| Preterm with BPD vs. Preterm without BPD | Erickson 80 pt 1 y < 29 GWs | Levy 239 pt 1 y < 29 GWs (BPD e/o PH) | Kang 24 pt 2 y < 33 GW | Koroglu 41 pt 2–4 y | Joshi 60 pt 8–12 y < 32 GWs | Korhonen 34 pt 7–8 y < 1500 g | Savio 38 pt 2–45 y < 30 GWs | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LV function | GLS = | FS = | = | |||||||||
| GLS | ||||||||||||
| EF; FS | ||||||||||||
| RV function | TAPSE, FAC, FWLS | ↓ | Strain ↓ | Strain ↓ | TAPSE ↓ | |||||||
| TAPSE | ||||||||||||
| FAC | ||||||||||||
| strain | ||||||||||||
| RV afterload | PAAT; PAAT/ET | ↓ | PAAT; PAAT/ET | ↓ | PAAT; PAAT/ET | = | PAAT; PAAT/ET | = | PAAT; | = | ||
| PAAT | PAAT/ET | |||||||||||
| PAAT/ET | TAPSE/PASP | ↓ | ||||||||||
| RV dimension | Areas Diameters | ↑ | = | |||||||||
| Areas | ||||||||||||
| 3D volumes | ||||||||||||
| LV dimension | IVSd LVESD | ↓ | = | |||||||||
| Volumes | ||||||||||||
| MMODE | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savio, F.; Sirico, D.; Mazzon, G.; Bonadies, L.; Guiducci, S.; Nardo, D.; Salvadori, S.; Avesani, M.; Castaldi, B.; Baraldi, E.; et al. Cardiac Mechanics Evaluation in Preschool-Aged Children with Preterm Birth History: A Speckle Tracking and 4D Echocardiography Study. J. Clin. Med. 2024, 13, 2762. https://doi.org/10.3390/jcm13102762
Savio F, Sirico D, Mazzon G, Bonadies L, Guiducci S, Nardo D, Salvadori S, Avesani M, Castaldi B, Baraldi E, et al. Cardiac Mechanics Evaluation in Preschool-Aged Children with Preterm Birth History: A Speckle Tracking and 4D Echocardiography Study. Journal of Clinical Medicine. 2024; 13(10):2762. https://doi.org/10.3390/jcm13102762
Chicago/Turabian StyleSavio, Federica, Domenico Sirico, Giada Mazzon, Luca Bonadies, Silvia Guiducci, Daniel Nardo, Sabrina Salvadori, Martina Avesani, Biagio Castaldi, Eugenio Baraldi, and et al. 2024. "Cardiac Mechanics Evaluation in Preschool-Aged Children with Preterm Birth History: A Speckle Tracking and 4D Echocardiography Study" Journal of Clinical Medicine 13, no. 10: 2762. https://doi.org/10.3390/jcm13102762
APA StyleSavio, F., Sirico, D., Mazzon, G., Bonadies, L., Guiducci, S., Nardo, D., Salvadori, S., Avesani, M., Castaldi, B., Baraldi, E., & Di Salvo, G. (2024). Cardiac Mechanics Evaluation in Preschool-Aged Children with Preterm Birth History: A Speckle Tracking and 4D Echocardiography Study. Journal of Clinical Medicine, 13(10), 2762. https://doi.org/10.3390/jcm13102762







