Stroke in Patients with Atrial Fibrillation: Epidemiology, Screening, and Prognosis
Abstract
:1. Introduction
2. Epidemiology and Clinical Features
2.1. Burden of AF and Factors Predisposing to AF
2.2. Mechanism and Typical Clinical Picture of IS Due to AF
2.3. Risk Stratification Schemes
2.4. AF and Hemorrhagic Stroke
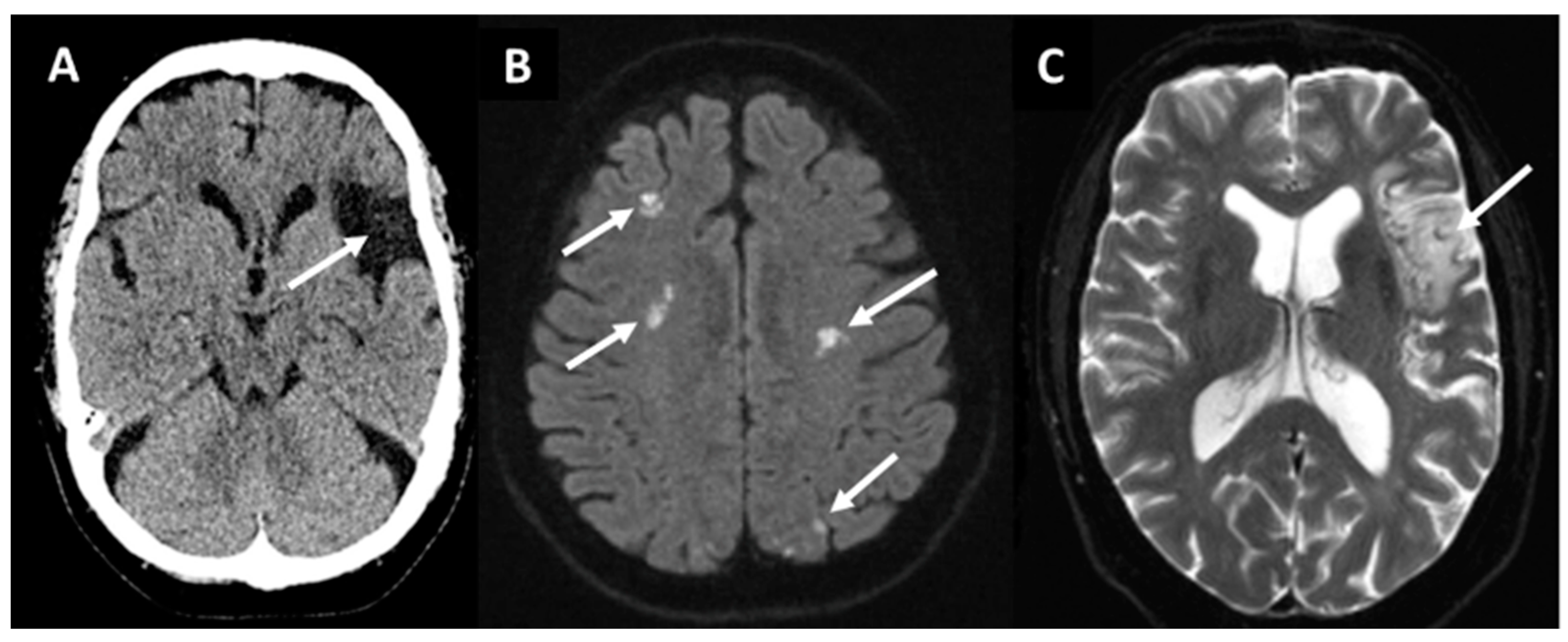
2.5. Cryptogenic IS and ESUS
3. Screening
3.1. AF Screening in the General Population to Prevent IS
3.2. AF Screening in Cryptogenic IS
3.3. Detection of Atrial Tachyarrhythmia’s with Pacing Devices
4. Primary and Secondary Prevention of IS in Patients with AF
4.1. Timing of OAC Initiation after IS and Its Impact on Short-Term Outcomes
4.2. LAA
5. Acute Recanalization Treatment and Its Outcomes after AF-Associated IS
6. AF and Hemorrhagic Stroke
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Teppo, K.; Airaksinen, K.E.J.; Jaakkola, J.; Halminen, O.; Linna, M.; Haukka, J.; Putaala, J.; Mustonen, P.; Kinnunen, J.; Hartikainen, J.; et al. Trends in treatment and outcomes of atrial fibrillation during 2007–17 in Finland. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 9, qcac086. [Google Scholar] [CrossRef]
- Choi, S.E.; Sagris, D.; Hill, A.; Lip, G.Y.H.; Abdul-Rahim, A.H. Atrial fibrillation and stroke. Expert Rev. Cardiovasc. Ther. 2023, 21, 35–56. [Google Scholar] [CrossRef]
- Kammersgaard, L.P.; Olsen, T.S. Cardiovascular risk factors and 5-year mortality in the Copenhagen Stroke Study. Cerebrovasc. Dis. 2006, 21, 187–193. [Google Scholar] [CrossRef]
- Lane, D.A.; Skjøth, F.; Lip, G.Y.H.; Larsen, T.B.; Kotecha, D. Temporal Trends in Incidence, Prevalence, and Mortality of Atrial Fibrillation in Primary Care. J. Am. Heart Assoc. 2017, 6, e005155. [Google Scholar] [CrossRef]
- Marini, C.; De Santis, F.; Sacco, S.; Russo, T.; Olivieri, L.; Totaro, R.; Carolei, A. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: Results from a population-based study. Stroke 2005, 36, 1115–1119. [Google Scholar] [CrossRef]
- Katsanos, A.H.; Kamel, H.; Healey, J.S.; Hart, R.G. Stroke Prevention in Atrial Fibrillation: Looking Forward. Circulation 2020, 142, 2371–2388. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Lane, D.A. Stroke Prevention in Atrial Fibrillation: A Systematic Review. JAMA 2015, 313, 1950–1962. [Google Scholar] [CrossRef]
- Li, L.; Yiin, G.S.; Geraghty, O.C.; Schulz, U.G.; Kuker, W.; Mehta, Z.; Rothwell, P.M.; Oxford Vascular Study. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: A population-based study. Lancet Neurol. 2015, 14, 903–913. [Google Scholar] [CrossRef]
- Hart, R.G.; Diener, H.C.; Coutts, S.B.; Easton, J.D.; Granger, C.B.; O’Donnell, M.J.; Sacco, R.L.; Connolly, S.J.; Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet Neurol. 2014, 13, 429–438. [Google Scholar] [CrossRef]
- Lehto, M.; Haukka, J.; Aro, A.; Halminen, O.; Putaala, J.; Linna, M.; Mustonen, P.; Kinnunen, J.; Kouki, E.; Niiranen, J.; et al. Comprehensive nationwide incidence and prevalence trends of atrial fibrillation in Finland. Open Heart 2022, 9, e002140. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Gheorghe-Andrei, D.; Polychronis, E.D.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Escudero-Martínez, I.; Morales-Caba, L.; Segura, T. Atrial fibrillation and stroke: A review and new insights. Trends Cardiovasc. Med. 2023, 33, 23–29. [Google Scholar] [CrossRef]
- Kamel, H.; Healey, J.S. Cardioembolic Stroke. Circ. Res. 2017, 120, 514–526. [Google Scholar] [CrossRef]
- Sharobeam, A.; Churilov, L.; Parsons, M.; Donnan, G.A.; Davis, S.M.; Yan, B. Patterns of Infarction on MRI in Patients with Acute Ischemic Stroke and Cardio-Embolism: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 606521. [Google Scholar] [CrossRef]
- Ntaios, G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 333–340. [Google Scholar] [CrossRef]
- Rubiera, M.; Aires, A.; Antonenko, K.; Lémeret, S.; Nolte, C.H.; Putaala, J.; Schabel, R.B.; Tuladhar, A.M.; Werring, D.J.; Zeraatkar, D.; et al. European Stroke Organisation (ESO) guideline on screening for subclinical atrial fibrillation after stroke or transient ischaemic attack of undetermined origin. Eur. Stroke J. 2022, 7, VI. [Google Scholar] [CrossRef]
- Sagris, D.; Harrison, S.L.; Buckley, B.J.R.; Ntaios, G.; Lip, G.Y.H. Long-Term Cardiac Monitoring After Embolic Stroke of Undetermined Source: Search Longer, Look Harder. Am. J. Med. 2022, 135, e311–e317. [Google Scholar] [CrossRef]
- Kalscheur, M.M.; Goldberger, Z.D. Screening for Atrial Fibrillation—Refining the Target. JAMA Netw. Open 2022, 5, e2139910. [Google Scholar] [CrossRef]
- Zhang, J.; Johnsen, S.P.; Guo, Y.; Lip, G.Y.H. Epidemiology of Atrial Fibrillation: Geographic/Ecological Risk Factors, Age, Sex, Genetics. Card. Electrophysiol. Clin. 2021, 13, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef]
- Staerk, L.; Wang, B.; Preis, S.R.; Larson, M.G.; Lubitz, S.A.; Ellinor, P.T.; McManus, D.D.; Ko, D.; Weng, L.C.; Lunetta, K.L.; et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: Cohort study based on longitudinal data from the Framingham Heart Study. BMJ 2018, 361, k1453. [Google Scholar] [CrossRef] [PubMed]
- Krijthe, B.P.; Kunst, A.; Benjamin, E.J.; Lip, G.Y.H.; Franco, O.H.; Hofman, A.; Witteman, J.C.M.; Stricker, B.H.; Heeringa, J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur. Heart J. 2013, 34, 2746–2751. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Marcus, G.M.; Alonso, A.; Peralta, C.A.; Lettre, G.; Vittinghoff, E.; Lubitz, S.A.; Fox, E.R.; Levitzky, Y.S.; Mehra, R.; Kerr, K.F.; et al. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation 2010, 122, 2009–2015. [Google Scholar] [CrossRef]
- Zoni-Berisso, M.; Filippi, A.; Landolina, M.; Brignoli, O.; D’Ambrosio, G.; Maglia, G.; Grimaldi, M.; Ermini, G. Frequency, patient characteristics, treatment strategies, and resource usage of atrial fibrillation (from the Italian Survey of Atrial Fibrillation Management [ISAF] study). Am. J. Cardiol. 2013, 111, 705–711. [Google Scholar] [CrossRef]
- Gómez-Doblas, J.J.; Muñiz, J.; Martin, J.J.A.; Rodríguez-Roca, G.; Lobos, J.M.; Awamleh, P.; Permanyer-Miralda, G.; Chorro, F.J.; Anguita, M.; Roig, F.; et al. Prevalence of atrial fibrillation in Spain. OFRECE study results. Rev. Esp. Cardiol. 2014, 67, 259–269. [Google Scholar] [CrossRef]
- Arboix, A.; Alió, J. Cardioembolic stroke: Clinical features, specific cardiac disorders and prognosis. Curr. Cardiol. Rev. 2010, 6, 150–161. [Google Scholar] [CrossRef]
- Dzeshka, M.S.; Lip, G.Y.H.; Snezhitskiy, V.; Shantsila, E. Cardiac Fibrosis in Patients with Atrial Fibrillation: Mechanisms and Clinical Implications. J. Am. Coll. Cardiol. 2015, 66, 943–959. [Google Scholar] [CrossRef]
- Kirchhof, P.; Breithardt, G.; Camm, A.J.; Crijns, H.J.; Kuck, K.H.; Vardas, P.; Wagscheider, K. Improving outcomes in patients with atrial fibrillation: Rationale and design of the Early treatment of Atrial fibrillation for Stroke prevention Trial. Am. Heart J. 2013, 166, 442–448. [Google Scholar] [CrossRef]
- Manolio, T.A.; Kronmal, R.A.; Burke, G.L.; O’Leary, D.H.; Price, T.R. Short-term Predictors of Incident Stroke in Older Adults. Stroke 1996, 27, 1479–1486. [Google Scholar] [CrossRef]
- Akar, J.G.; Marieb, M.A. Atrial Fibrillation and Thrombogenesis: Innocent Bystander or Guilty Accomplice? JACC Clin. Electrophysiol. 2015, 1, 218–219. [Google Scholar] [CrossRef]
- Watson, T.; Shantsila, E.; Lip, G.Y.H. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet 2009, 373, 155–166. [Google Scholar] [CrossRef]
- Shen, M.J.; Arora, R.; Jalife, J. Atrial Myopathy. JACC Basic Transl. Sci. 2019, 4, 640–654. [Google Scholar] [CrossRef]
- Lin, H.J.; Wolf, P.A.; Kelly-Hayes, M.; Beiser, A.S.; Kase, C.S.; Benjamin, E.J.; D’Agostino, B.R.B. Stroke Severity in Atrial Fibrillation. Stroke 1996, 27, 1760–1764. [Google Scholar] [CrossRef]
- Jørgensen, H.S.; Nakayama, H.; Reith, J.; Raaschou, H.O.; Olsen, T.S. Acute stroke with atrial fibrillation. The Copenhagen Stroke Study. Stroke 1996, 27, 1765–1769. [Google Scholar] [CrossRef]
- Vinding, N.E.; Kristensen, S.L.; Rørth, R.; Butt, J.H.; Østergaard, L.; Olesen, J.B.; Torp-Pedersen, C.; Gislason, G.H.; Kober, L.; Kruuse, C.; et al. Ischemic Stroke Severity and Mortality in Patients with and without Atrial Fibrillation. J. Am. Heart Assoc. 2022, 11, e022638. [Google Scholar] [CrossRef]
- Sun, J.; Lam, C.; Christie, L.; Blair, C.; Li, X.; Werdiger, F.; Yang, Q.; Bivard, A.; Lin, L.; Parsons, M. Risk factors of hemorrhagic transformation in acute ischaemic stroke: A systematic review and meta-analysis. Front. Neurol. 2023, 14, 1079205. [Google Scholar] [CrossRef]
- Olesen, J.B.; Lip, G.Y.H.; Hansen, M.L.; Hansen, P.R.; Tolstrup, J.S.; Lindhardsen, J.; Selmer, C.; Ahlehoff, O.; Schernjing Olsen, A.M.; Gislason, G.H.; et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: Nationwide cohort study. BMJ 2011, 342, d124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.G.; Xiong, Q.M.; Hong, K. Meta-analysis of CHADS2 versus CHA2DS2-VASc for predicting stroke and thromboembolism in atrial fibrillation patients independent of anticoagulation. Tex. Heart Inst. J. 2015, 42, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Joundi, R.A.; Cipriano, L.E.; Sposato, L.A.; Saposnik, G. Ischemic Stroke Risk in Patients with Atrial Fibrillation and CHA2DS2-VASc Score of 1: Systematic Review and Meta-Analysis. Stroke 2016, 47, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Kolominsky-Rabas, P.L.; Weber, M.; Gefeller, O.; Neundoerfer, B.; Heuschmann, P.U. Epidemiology of ischemic stroke subtypes according to TOAST criteria: Incidence, recurrence, and long-term survival in ischemic stroke subtypes: A population-based study. Stroke 2001, 32, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Phil, D.; Weitz, J.I.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Benavente, O.; McBride, R.; Pearce, L.A. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: A meta-analysis. Ann. Intern. Med. 1999, 131, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeless, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Ganesan, A.N.; Chew, D.P.; Hartshorne, T.; Selvanayagam, J.B.; Aylward, P.E.; Sanders, P.; MaGavigan, A.D. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: A systematic review and meta-analysis. Eur. Heart J. 2016, 37, 1591–1602. [Google Scholar] [CrossRef]
- Friberg, L.; Rosenqvist, M.; Lip, G.Y.H. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: The Swedish Atrial Fibrillation cohort study. Eur. Heart J. 2012, 33, 1500–1510. [Google Scholar] [CrossRef]
- Curtze, S.; Strbian, D.; Meretoja, A.; Putaala, J.; Eriksson, H.; Haapaniemi, E.; Mustanoja, S.; Sairanen, T.; Satopää, J.; Silvennoinen, H.; et al. Higher baseline international normalized ratio value correlates with higher mortality in intracerebral hemorrhage during warfarin use. Eur. J. Neurol. 2014, 21, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Borre, E.D.; Goode, A.; Raitz, G.; Shah, B.; Lowenstern, A.; Chatterjee, R.; Sharan, L.; Lapointe, N.M.A.; Yapa, R.; Davis, J.K.; et al. Predicting Thromboembolic and Bleeding Event Risk in Patients with Non-Valvular Atrial Fibrillation: A Systematic Review. Thromb. Haemost. 2018, 118, 2171–2187. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Ge, W.Q.; Zhou, C.F.; Zhou, C.Y. The Correlation between Atrial Fibrillation and Prognosis and Hemorrhagic Transformation. Eur. Neurol. 2019, 82, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Paciaroni, M.; Agnelli, G.; Corea, F.; Ageno, W.; Alberti, A.; Lanari, A.; Caso, V.; Michelli, S.; Bertolani, L.; Venti, M.; et al. Early Hemorrhagic Transformation of Brain Infarction: Rate, Predictive Factors, and Influence on Clinical Outcome. Stroke 2008, 39, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.E.; Plumber, N.; Venkatapathappa, P.; Gorantla, V. A Review of Risk Factors and Predictors for Hemorrhagic Transformation in Patients with Acute Ischemic Stroke. Int. J. Vasc. Med. 2021, 2021, 4244267. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.M.; Kim, D.S.; Kim, M. Hemorrhagic Transformation After Ischemic Stroke: Mechanisms and Management. Front. Neurol. 2021, 12, 703258. [Google Scholar] [CrossRef] [PubMed]
- Paciaroni, M.; Bandini, F.; Agnelli, G.; Tsivgoulis, G.; Yaghi, S.; Furie, K.L.; Tadi, P.; Becattini, C.; Zedde, M.; Abdul-Rahim, A.H.; et al. Hemorrhagic Transformation in Patients with Acute Ischemic Stroke and Atrial Fibrillation: Time to Initiation of Oral Anticoagulant Therapy and Outcomes. J. Am. Heart Assoc. 2018, 7, e010133. [Google Scholar] [CrossRef]
- Gokcal, E.; Pasi, M.; Fisher, M.; Gurol, M.E. Atrial Fibrillation for the Neurologist: Preventing both Ischemic and Hemorrhagic Strokes. Curr. Neurol. Neurosci. Rep. 2018, 18, 6. [Google Scholar] [CrossRef]
- Rosand, J.; Eckman, M.H.; Knudsen, K.A.; Singer, D.E.; Greenberg, S.M. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch. Intern. Med. 2004, 164, 880–884. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, J.; Qian, J.; Ma, F.; Lv, M.; Zhang, J. Risk Factors for Anticoagulant-Associated Intracranial Hemorrhage: A Systematic Review and Meta-analysis. Neurocrit. Care 2023, 38, 812–820. [Google Scholar] [CrossRef]
- Wilson, D.; Seiffge, D.J.; Traenka, C.; Basir, G.; Purrucker, J.C.; Rizos, T.; Sobowale, O.A.; Sallinen, H.; Yeh, S.J.; Wu, T.Y.; et al. Outcome of intracerebral hemorrhage associated with different oral anticoagulants. Neurology 2017, 88, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L. Cryptogenic Stroke. N. Engl. J. Med. 2016, 375, e26. [Google Scholar] [CrossRef] [PubMed]
- Tayal, A.H.; Tian, M.; Kelly, K.M.; Jones, S.C.; Wright, D.G.; Singh, D.; Jarouse, J.; Brillman, J.; Murali, S.; Gupta, R. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008, 71, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, D.J.; Spring, M.; Dorian, P.; Panzov, V.; Thorpe, K.E.; Hall, J.; Vaid, H.; O’Donnell, M.; Laupacis, A.; Lote, R.; et al. Atrial Fibrillation in Patients with Cryptogenic Stroke. N. Engl. J. Med. 2014, 370, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Favilla, C.G.; Ingala, E.; Jara, J.; Fessler, E.; Cucchiara, B.; Messé, S.R.; Mullen, M.T.; Prasad, A.; Siegler, J.; Hutchinson, M.D.; et al. Predictors of finding occult atrial fibrillation after cryptogenic stroke. Stroke 2015, 46, 1210–1215. [Google Scholar] [CrossRef]
- Ntaios, G.; Perlepe, K.; Lambrou, D.; Sirimarco, G.; Strambo, D.; Eskandari, A.; Karagkiozi, E.; Vemmou, A.; Koroboki, E.; Manios, E.; et al. Prevalence and Overlap of Potential Embolic Sources in Patients with Embolic Stroke of Undetermined Source. J. Am. Heart Assoc. 2019, 8, e012858. [Google Scholar] [CrossRef]
- Camen, S.; Ojeda, F.M.; Niiranen, T.; Gianfagna, F.; Vishram-Nielsen, J.K.; Costanzo, S.; Söderberg, S.; Vartiainen, E.; Donati, M.B.; Lochen, M.J.; et al. Temporal relations between atrial fibrillation and ischaemic stroke and their prognostic impact on mortality. EP Eur. 2020, 22, 522–529. [Google Scholar] [CrossRef]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Willems, S.; Borof, K.; Brandes, A.; Breithardt, G.; Camm, A.J.; Crijns, H.J.G.M.; Eckardt, L.; Gessler, N.; Goette, A.; Haegeli, L.M.; et al. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: The EAST-AFNET 4 trial. Eur. Heart J. 2022, 43, 1219–1230. [Google Scholar] [CrossRef]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. New Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef]
- Faranesh, A.; Selvaggi, A.Z.; Atlas, C.; McManus, S.J.; Singer, D.D.; Pagoto, S.; McConnell, M.V.; Pantelopoulos, A.; Foulkes, A.S. Detection of Atrial Fibrillation in a Large Population Using Wearable Devices: The Fitbit Heart Study. Circulation 2022, 146, 1415–1424. [Google Scholar]
- Schnabel, R.B.; Marinelli, E.A.; Arbelo, E.; Boriani, G.; Boveda, S.; Buckley, C.M.; Camm, A.J.; Casadei, B.; Chua, W.; Dagres, N.; et al. Early diagnosis and better rhythm management to improve outcomes in patients with atrial fibrillation: The 8th AFNET/EHRA consensus conference. EP Eur. 2023, 25, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Palaiodimou, L.; Triantafyllou, S.; Köhrmann, M.; Dilaveris, P.; Tsioufis, K.; Magiorkinis, G.; Krogias, C.; Schellinger, P.D.; Caso, V.; et al. Prolonged cardiac monitoring for stroke prevention: A systematic review and meta-analysis of randomized-controlled clinical trials. Eur. Stroke J. 2023, 8, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Svennberg, E.; Friberg, L.; Frykman, V.; Al-Khalili, F.; Engdahl, J.; Rosenqvist, M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): A multicentre, parallel group, unmasked, randomised controlled trial. Lancet 2021, 398, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Kemp Gudmundsdottir, K.; Fredriksson, T.; Svennberg, E.; Al-Khalili, F.; Friberg, L.; Frykman, V.; Hijazi, Z.; Rosenqvist, M.; Engdahl, J. Stepwise mass screening for atrial fibrillation using N-terminal B-type natriuretic peptide: The STROKESTOP II study. EP Eur. 2020, 22, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, J.H.; Diederichsen, S.Z.; Højberg, S.; Krieger, D.W.; Graff, C.; Kronborg, C.; Olesen, M.S.; Nielsen, J.B.; Holst, A.G.; Brandes, A.; et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): A randomised controlled trial. Lancet 2021, 398, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Toennis, T.; Goette, A.; Camm, A.J.; Diener, H.C.; Becher, N.; Bertaglia, E.; Blomstrom Lundqvist, C.; Borlich, M.; Brandes, A.; et al. Anticoagulation with Edoxaban in Patients with Atrial High-Rate Episodes. N. Engl. J. Med. 2023, 389, 1167–1179. [Google Scholar] [CrossRef]
- Hart, R.G.; Sharma, M.; Mundl, H.; Kasner, S.E.; Bangdiwala, S.I.; Berkowitz, S.D.; Swaminathan, B.; Lavados, P.; Wang, Y.; Davalos, A.; et al. Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source. N. Engl. J. Med. 2018, 378, 2191–2201. [Google Scholar] [CrossRef]
- Diener, H.C.; Sacco, R.L.; Easton, J.D.; Granger, C.B.; Bernstein, R.A.; Uchiyama, S.; Kreuzer, J.; Cronin, L.; Cotton, D.; Grauer, C.; et al. Dabigatran for Prevention of Stroke after Embolic Stroke of Undetermined Source. N. Engl. J. Med. 2019, 380, 1906–1917. [Google Scholar] [CrossRef]
- Kim, A.S.; Kamel, H.; Bernstein, R.A.; Manchanda, M.; Caprio, F.Z. Controversies in Stroke: Should Patients with Embolic Stroke of Undetermined Source Undergo Intensive Heart Rhythm Monitoring with an Implantable Loop Recorder? Stroke 2022, 53, 3243–3247. [Google Scholar] [CrossRef]
- Pikija, S.; Rösler, C.; Leitner, U.; Zellner, T.; Bubel, N.; Ganser, B.; Hacker, C.; Mutzenbach, J.S. Neurologist-Led Management of Implantable Loop-Recorders After Embolic Stroke of Undetermined Source. Front. Neurol. 2021, 12, 816511. [Google Scholar] [CrossRef] [PubMed]
- Sposato, L.A.; Cipriano, L.E.; Saposnik, G.; Vargas, E.R.; Riccio, P.M.; Hachinski, V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Mahajan, V.; Chen, H.H.L.; Gan, G.C.H.; Pontes-Neto, O.M.; Tan, T.C. Embolic Stroke of Undetermined Source: Approaches in Risk Stratification for Cardioembolism. Stroke 2021, 52, e820–e836. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Tan, S.Y.; Wang, J.K.; Li, J.; Tu, T.M.; Tan, V.H.; Yeo, C. A meta-analysis of extended ECG monitoring in detection of atrial fibrillation in patients with cryptogenic stroke. Open Heart 2022, 9, e002081. [Google Scholar] [CrossRef] [PubMed]
- Dilaveris, P.E.; Antoniou, C.K.; Caiani, E.G.; Casado-Arroyo, R.; Climent, A.Μ.; Cluitmans, M.; Cowie, M.R.; Doehner, W.; Guerra, F.; Jensen, M.T.; et al. ESC Working Group on e-Cardiology Position Paper: Accuracy and reliability of electrocardiogram monitoring in the detection of atrial fibrillation in cryptogenic stroke patients: In collaboration with the Council on Stroke, the European Heart Rhythm Association, and the Digital Health Committee. Eur. Heart J. Digit Health 2022, 3, 341–358. [Google Scholar] [PubMed]
- Lumikari, T.J.; Putaala, J.; Kerola, A.; Sibolt, G.; Pirinen, J.; Pakarinen, S.; Lehto, M.; Nieminen, T. Continuous 4-week ECG monitoring with adhesive electrodes reveals AF in patients with recent embolic stroke of undetermined source. Ann. Noninvasive Electrocardiol. 2019, 24, e12649. [Google Scholar] [CrossRef] [PubMed]
- Lumikari, T.J.; Pirinen, J.; Putaala, J.; Sibolt, G.; Kerola, A.; Pakarinen, S.; Lehto, M.; Nieminen, T. Prolonged ECG with a novel recorder utilizing electrode belt and mobile device in patients with recent embolic stroke of undetermined source: A pilot study. Ann. Noninvasive Electrocardiol. 2020, 25, e12802. [Google Scholar] [CrossRef] [PubMed]
- De Voogt, W.G.; van Hemel, N.M.; van de Bos, A.A.; Koïstinen, J.; Fast, J.H. Verification of pacemaker automatic mode switching for the detection of atrial fibrillation and atrial tachycardia with Holter recording. EP Eur. 2006, 8, 950–961. [Google Scholar] [CrossRef]
- Silberbauer, J.; Arya, A.; Veasey, R.A.; Boodhoo, L.; Kamalvand, K.; O’Nunain, S.; Hildick-Smith, D.; Paul, V.; Patel, N.R.; Lloyd, G.W.; et al. The effect of bipole tip-to-ring distance in atrial electrodes upon atrial tachyarrhythmia sensing capability in modern dual-chamber pacemakers. Pacing Clin. Electrophysiol. PACE 2010, 33, 85–93. [Google Scholar] [CrossRef]
- Chen-Scarabelli, C.; Scarabelli, T.M.; Ellenbogen, K.A.; Halperin, J.L. Device-detected atrial fibrillation: What to do with asymptomatic patients? J. Am. Coll. Cardiol. 2015, 65, 281–294. [Google Scholar] [CrossRef]
- De Voogt, W.G.; van Hemel, N.M. Diagnostic tools for atrial tachyarrhythmias in implantable pacemakers: A review of technical options and pitfalls. Neth. Heart J. 2008, 16, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Pakarinen, S.; Toivonen, L. Performance of atrial tachyarrhythmia-sensing algorithms in dual-chamber pacing using a fixed long AV delay in patients with sinus node dysfunction. J. Interv. Card. Electrophysiol. 2012, 35, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kolb, C.; Wille, B.; Maurer, D.; Schuchert, A.; Weber, R.; Schibgilla, V.; Klein, N.; Hummer, A.; Schmitt, C.; Zrenner, B.; et al. Management of far-field R wave sensing for the avoidance of inappropriate mode switch in dual chamber pacemakers: Results of the FFS-test study. J. Cardiovasc. Electrophysiol. 2006, 17, 992–997. [Google Scholar] [CrossRef]
- Liu, F.; Yang, Y.; Cheng, W.; Ma, J.; Zhu, W. Reappraisal of Non-vitamin K Antagonist Oral Anticoagulants in Atrial Fibrillation Patients: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 757188. [Google Scholar] [CrossRef] [PubMed]
- Barold, S.S.; Ilercil, A.; Leonelli, F.; Herweg, B. First-degree atrioventricular block. Clinical manifestations, indications for pacing, pacemaker management & consequences during cardiac resynchronization. J. Interv. Card. Electrophysiol. 2006, 17, 139–152. [Google Scholar] [PubMed]
- Glotzer, T.V.; Hellkamp, A.S.; Zimmerman, J.; Sweeney, M.O.; Yee, R.; Marinchak, R.; Cook, J.; Paraschos, A.; Love, J.; Radoslovich, G.; et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: Report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003, 107, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- Capucci, A.; Santini, M.; Padeletti, L.; Gulizia, M.; Botto, G.; Boriani, G.; Ricci, R.; Favale, S.; Zolezzi, F.; Di Belardinho, N.; et al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J. Am. Coll. Cardiol. 2005, 46, 1913–1920. [Google Scholar] [CrossRef]
- Glotzer, T.V.; Daoud, E.G.; Wyse, D.G.; Singer, D.E.; Ezekowitz, M.D.; Hilker, C.; Miller, C.; Qi, D.; Ziegler, P.D. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: The TRENDS study. Circ. Arrhythmia Electrophysiol. 2009, 2, 474–480. [Google Scholar] [CrossRef]
- Daoud, E.G.; Glotzer, T.V.; Wyse, D.G.; Ezekowitz, M.D.; Hilker, C.; Koehler, J.; Ziegler, P.D.; TRENDS Investigators. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: A subgroup analysis of TRENDS. Heart Rhythm 2011, 8, 1416–1423. [Google Scholar] [CrossRef]
- Brambatti, M.; Connolly, S.J.; Gold, M.R.; Morillo, C.A.; Capucci, A.; Muto, C.; Lau, C.P.; Van Gelder, I.C.; Hohnloser, S.H.; Carlson, M.; et al. Temporal Relationship Between Subclinical Atrial Fibrillation and Embolic Events. Circulation 2014, 129, 2094–2099. [Google Scholar] [CrossRef]
- Turakhia, M.P.; Ziegler, P.D.; Schmitt, S.K.; Chang, Y.; Fan, J.; Than, C.T.; Keung, E.K.; Singer, D.E. Atrial Fibrillation Burden and Short-Term Risk of Stroke: Case-Crossover Analysis of Continuously Recorded Heart Rhythm From Cardiac Electronic Implanted Devices. Circ. Arrhythmia Electrophysiol. 2015, 8, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.S.; Lopes, R.D.; Granger, C.B.; Alings, M.; Rivard, L. Apixaban for Stroke Prevention in Subclinical Atrial Fibrillation. N. Engl. J. Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, W.F.; Benz, A.P.; Becher, N.; Healey, J.S.; Granger, C.B.; Rivard, L.; Camm, J.; Goette, A.; Zapf, A.; Alings, M.; et al. Direct Oral Anticoagulants for Stroke Prevention in Patients with Device-Detected Atrial Fibrillation: A Study-Level Meta-Analysis of the NOAH-AFNET 6 and ARTESiA Trials. Circulation 2023. [Google Scholar] [CrossRef] [PubMed]
- Hannon, N.; Daly, L.; Murphy, S.; Smith, S.; Hayden, D.; Ní Chróinín, D.; Callaly, E.; Horgan, G.; Sheehan, O.; Honari, B.; et al. Acute hospital, community, and indirect costs of stroke associated with atrial fibrillation: Population-based study. Stroke 2014, 45, 3670–3674. [Google Scholar] [CrossRef] [PubMed]
- Hannon, N.; Sheehan, O.; Kelly, L.; Marnane, M.; Merwick, A.; Moore, A.; Kyne, L.; Duggan, J.; Moroney, J.; McCormack, P.M.E.; et al. Stroke associated with atrial fibrillation—Incidence and early outcomes in the north Dublin population stroke study. Cerebrovasc. Dis. 2010, 29, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Minematsu, K.; Yamaguchi, T. Atrial fibrillation as a predictive factor for severe stroke and early death in 15,831 patients with acute ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2005, 76, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.C.; Go, A.S.; Chang, Y.; Borowsky, L.H.; Pomernacki, N.K.; Udaltsova, N.; Singer, D.E. Long-term survival after ischemic stroke in patients with atrial fibrillation. Neurology 2014, 82, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Frost, L.; Andersen, L.V.; Vestergaard, P.; Husted, S.; Mortensen, L.S. Trend in mortality after stroke with atrial fibrillation. Am. J. Med. 2007, 120, 47–53. [Google Scholar] [CrossRef]
- Gao, X.; Passman, R. Stroke Prevention in Atrial Fibrillation. Curr. Cardiol. Rep. 2022, 24, 1765–1774. [Google Scholar] [CrossRef]
- Hindsholm, M.F.; Damgaard, D.; Gurol, M.E.; Gaist, D.; Simonsen, C.Z. Management and Prognosis of Acute Stroke in Atrial Fibrillation. J. Clin. Med. 2023, 12, 5752. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.J.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, 104–132. [Google Scholar] [PubMed]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; John Camm, A.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Van de Werf, F.; Brueckmann, M.; Connolly, S.J.; Friedman, J.; Granger, C.B.; Härtter, S.; Harper, R.; Kappetein, A.P.; Lehr, T.; Mack, M.J.; et al. A comparison of dabigatran etexilate with warfarin in patients with mechanical heart valves: THE Randomized, phase II study to evaluate the safety and pharmacokinetics of oral dabigatran etexilate in patients after heart valve replacement (RE-ALIGN). Am. Heart J. 2012, 163, 931–937.e1. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Caso, V.; Connolly, S.J.; Fox, K.A.A.; Oldgren, J.; Jones, W.S.; Gorog, D.A.; Durdil, V.; Viethen, T.; Neumann, C.; et al. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): A multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet 2022, 399, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Klijn, C.J.; Paciaroni, M.; Berge, E.; Korompoki, E.; Kõrv, J.; Lal, A.; Putaala, J.; Werring, D.J. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: A European Stroke Organisation guideline. Eur. Stroke J. 2019, 4, 198–223. [Google Scholar] [CrossRef]
- Verheugt, F.W.A.; Ambrosio, G.; Atar, D.; Bassand, J.P.; Camm, A.J.; Costabel, J.P.; Fitzmaurice, D.A.; Illingworth, L.; Goldhaber, S.Z.; Goto, S.; et al. Outcomes in Newly Diagnosed Atrial Fibrillation and History of Acute Coronary Syndromes: Insights from GARFIELD-AF. Am. J. Med. 2019, 132, 1431–1440.e7. [Google Scholar] [CrossRef]
- Heidbuchel, H.; Verhamme, P.; Alings, M.; Antz, M.; Hacke, W.; Oldgren, J.; Sinnaeve, P.; John Camm, A.; Kirchhof, P. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: Executive summary. Eur. Heart J. 2013, 34, 2094–2106. [Google Scholar] [CrossRef]
- Oldgren, J.; Åsberg, S.; Hijazi, Z.; Wester, P.; Bertilsson, M.; Norrving, B. Early Versus Delayed Non-Vitamin K Antagonist Oral Anticoagulant Therapy After Acute Ischemic Stroke in Atrial Fibrillation (TIMING): A Registry-Based Randomized Controlled Noninferiority Study. Circulation 2022, 146, 1056–1066. [Google Scholar] [CrossRef]
- Fischer, U.; Koga, M.; Strbian, D.; Branca, M.; Abend, S.; Trelle, S.; Paciaroni, M.; Thomalla, G.; Michel, P.; Nedeltchev, K.; et al. Early versus Later Anticoagulation for Stroke with Atrial Fibrillation. N. Engl. J. Med. 2023, 388, 2411–2421. [Google Scholar] [CrossRef]
- Altavilla, R.; Caso, V.; Bandini, F.; Agnelli, G.; Tsivgoulis, G.; Yaghi, S.; Furie, K.L.; Tadi, P.; Becattini, C.; Zedde, M.; et al. Anticoagulation After Stroke in Patients with Atrial Fibrillation. Stroke 2019, 50, 2093–2100. [Google Scholar] [CrossRef]
- Paciaroni, M.; Caso, V.; Agnelli, G.; Mosconi, M.G.; Giustozzi, M.; Seiffge, D.J.; Engelter, S.T.; Lyrer, P.; Polymeris, A.A.; Kriemler, L.; et al. Recurrent Ischemic Stroke and Bleeding in Patients with Atrial Fibrillation Who Suffered an Acute Stroke While on Treatment with Nonvitamin K Antagonist Oral Anticoagulants: The RENO-EXTEND Study. Stroke 2022, 53, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Galea, R.; Seiffge, D.; Räber, L. Atrial Fibrillation and Ischemic Stroke despite Oral Anticoagulation. J. Clin. Med. 2023, 12, 5784. [Google Scholar] [CrossRef] [PubMed]
- Seiffge, D.J.; De Marchis, G.M.; Koga, M.; Paciaroni, M.; Wilson, D.; Cappellari, M.; Macha, M.D.K.; Tsivgoulis, G.; Ambler, G.; Arihiro, S.; et al. Ischemic Stroke despite Oral Anticoagulant Therapy in Patients with Atrial Fibrillation. Ann. Neurol. 2020, 87, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, S.; Henninger, N.; Giles, J.A.; Leon Guerrero, C.; Mistry, E.; Liberman, A.L.; Asad, D.; Liu, A.; Nagy, M.; Kaushai, A.; et al. Ischaemic stroke on anticoagulation therapy and early recurrence in acute cardioembolic stroke: The IAC study. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Kobeissi, H.; Ghozy, S.; Seymour, T.; Gupta, R.; Bilgin, C.; Kadirvel, R.; Rabinstein, A.A.; Kallmes, D.F. Outcomes of Patients with Atrial Fibrillation Following Thrombectomy for Stroke: A Systematic Review and Meta-analysis. JAMA Netw. Open 2023, 6, e2249993. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.D., 3rd; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients with Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar] [CrossRef] [PubMed]
- Dowlatshahi, D.; Butcher, K.S.; Asdaghi, N.; Nahirniak, S.; Bernbaum, M.L.; Giulivi, A.; Wasserman, J.K.; Poon, M.C.; Coutts, S.B.; Canadian PCC Registry (CanPro) Investigstors. Poor prognosis in warfarin-associated intracranial hemorrhage despite anticoagulation reversal. Stroke 2012, 43, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Himmelreich, J.C.L.; Lucassen, W.A.M.; Heugen, M.; Bossuyt, P.M.M.; Tan, H.L.; Harskamp, R.E.; van Etten-Jamaludin, F.S.; van Meert, H.C.P.M. Frequent premature atrial contractions are associated with atrial fibrillation, brain ischaemia, and mortality: A systematic review and meta-analysis. EP Eur. 2019, 21, 698–707. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Kaufman, E.S.; Lin, Y.C.; Chung, M.K.; Elkind, M.S.V.; Joglar, J.A.; Leal, M.A.; McCabe, P.J.; Pokorney, S.D.; Yao, X.; et al. Subclinical and Device-Detected Atrial Fibrillation: Pondering the Knowledge Gap: A Scientific Statement From the American Heart Association. Circulation 2019, 140, e944–e963. [Google Scholar] [CrossRef]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Attia, Z.I.; Behnken, E.M.; Giblon, R.E.; Bews, K.A.; Liu, S.; Gosse, T.A.; Linn, Z.D.; Deng, Y.; Yin, J.; et al. Artificial intelligence-guided screening for atrial fibrillation using electrocardiogram during sinus rhythm: A prospective non-randomised interventional trial. Lancet 2022, 400, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Kartoun, U.; Ashburner, J.M.; Trinquart, L.; Philippakis, A.; Khera, A.V.; Ellinor, P.T.; Ng, K.; Lubitz, S.A.l. Performance of Atrial Fibrillation Risk Prediction Models in Over 4 Million Individuals. Circ. Arrhythmic Electrophysiol. 2021, 14, e008997. [Google Scholar] [CrossRef] [PubMed]

| Clinical Variables | |
|---|---|
| Strong prediction | Previous stroke or TIA, hypertension ageing, structural heart disease, diabetes mellitus, vascular disease, CHF/LV dysfunction, sex category (female) CHA2DS2-VASc-score (points): CHF (1), hypertension (1), age 75 years old or older (2), diabetes mellitus (1), Stroke (2), vascular disease (1), age 65–74 (1), sex category, female (1) |
| Weak prediction | Impaired renal function/CKD, OSA, HCM, amyloidosis in degenerative cerebral and heart diseases, hyperlipidemia, smoking, metabolic syndrome, malignancy, genetic predisposition |
| Laboratory biomarkers | |
| Blood tests | Cardiac troponin T and I, natriuretic peptides, cystatin c, CrCl/eGFR, CRP, IL-6, GDF-15, von Willebrand factor, D-dimer |
| Imaging and ECG biomarkers | |
| Echocardiogram | Left atrial enlargement or dilatation, spontaneous contrast or thrombus in LA, low LAA velocities, decreased left atrial strain, reduced left ventricular ejection fraction, complex aortic plaque |
| MRI or NCCT | SVD Prior or acute cortical or cerebellar infarction, multi-territory brain infarction, LVO, CMB, and white matter changes |
| ECG markers | Premature atrial contractions, left ventricular hypertrophy, atrioventricular block, as well as more prolonged PR interval, P-wave duration, P-wave dispersion, P-wave index, and QTc interval |
| ESUS-type ischemic stroke | Non-lacunar, cryptogenic ischemic strokes with proximal embolism as a probable mechanism |
| Early vs. Late Initiation of Anticoagulation after Ischemic Stroke | |||||
|---|---|---|---|---|---|
| Trial | Intervention | Primary Outcome | Time Frame | Study Sites | Sample Size |
| OPTIMAS (NCT03759938) | Early (within 4 days) vs. standard (7 to 14 days) initiation of DOAC | Stroke (ischemic or hemorrhagic) or systemic embolism. | 3 months | United Kingdom | 3478 |
| Left atrial appendage closure after ischemic stroke | |||||
| Occlusion-AF (NCT03642509) | LAAC vs. DOAC within 180 after ischemic stroke | Stroke (ischemic or hemorrhagic), systemic embolism, major bleeding, or all-cause mortality. | 5 years | Scandinavia | 750 |
| ELAPSE | LAAC and DOAC vs. DOAC alone | Ischemic stroke, systemic embolism, and cardiovascular death. | 4 years | Not yet provided | 482 |
| LAAOS-4 (NCT05963698) | LAAC and OAC vs. OAC alone | Ischemic stroke or systemic embolism. | 4 years | Not yet provided | 4000 |
| Other secondary prevention after ischemic stroke | |||||
| INTERCEPT (NCT05723926) | Bilateral carotid filter implants and OAC vs. OAC alone | Ischemic stroke. | UNK | Not yet provided | 200 |
| STABLED (NCT03777631) | Catheter ablation and OAC vs. OAC alone 1 to 6 months after ischemic stroke | Ischemic stroke, systemic embolism, all-cause death, and hospitalization for heart failure. | 3 years | Japan | 250 |
| OCEANIC-AF (NCT05643573) | FXIa inhibitor (asundexian) vs. apixaban mainly as primary prevention but also includes patients with prior ischemic stroke or TIA | Stroke (ischemic or hemorrhagic) or systemic embolism. | 3 years | America, Europe, Asia, Australia | 18,000 |
| LIBREXIA-AF (NCT05757869) | FXIa inhibitor (milvexian) vs. apixaban mainly as primary prevention but also includes patients with prior ischemic stroke or TIA | Stroke (ischemic or hemorrhagic) or systemic embolism. | 4 years | America, Europe, Asia, Australasia, Africa | 15,500 |
| Oral anticoagulation resumption intracerebral hemorrhage | |||||
| ASPIRE (NCT03907046) | Apixaban vs. aspirin 15 to 180 days after ICH | Stroke (ischemic or hemorrhagic) or all-cause mortality. Time frame 3 years. | 3 years | United States | 700 |
| ENRICH-AF (NCT03950076) | Edoxaban vs. either no antithrombotic therapy or antiplatelet monotherapy | Stroke (ischemic or hemorrhagic) or major hemorrhage. | 2 years | America, Europe, Asia, Africa | 1200 |
| PRESTIGE-AF (NCT03996772) | DOAC vs. no anticoagulation 15 to 180 days after ICH | Stroke (ischemic or hemorrhagic). | 3 years | Europe | 350 |
| STATICH (NCT03186729) | Anticoagulant treatment vs. no anticoagulant treatment 1 to 180 days after ICH | Fatal or non-fatal symptomatic recurrent ICH. | 2 years | Scandinavia | 500 |
| Left atrial appendage closure after intracerebral hemorrhage | |||||
| A3ICH (NCT03243175) | Apixaban vs. LAAC vs. no intervention at least 14 days from ICH | Fatal or non-fatal major cardiovascular/cerebrovascular ischemic or hemorrhagic events. | 2 years | France | 300 |
| STROKECLOSE (NCT02830152) | LAAO vs. medical therapy after 4 to 52 weeks after ICH | Stroke (ischemic or hemorrhagic), systemic embolism, life-threatening or major bleeding, or all-cause mortality. | 5 years | Europe | 750 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suomalainen, O.P.; Martinez-Majander, N.; Broman, J.; Mannismäki, L.; Aro, A.; Curtze, S.; Pakarinen, S.; Lehto, M.; Putaala, J. Stroke in Patients with Atrial Fibrillation: Epidemiology, Screening, and Prognosis. J. Clin. Med. 2024, 13, 30. https://doi.org/10.3390/jcm13010030
Suomalainen OP, Martinez-Majander N, Broman J, Mannismäki L, Aro A, Curtze S, Pakarinen S, Lehto M, Putaala J. Stroke in Patients with Atrial Fibrillation: Epidemiology, Screening, and Prognosis. Journal of Clinical Medicine. 2024; 13(1):30. https://doi.org/10.3390/jcm13010030
Chicago/Turabian StyleSuomalainen, Olli Pekka, Nicolas Martinez-Majander, Jenna Broman, Laura Mannismäki, Aapo Aro, Sami Curtze, Sami Pakarinen, Mika Lehto, and Jukka Putaala. 2024. "Stroke in Patients with Atrial Fibrillation: Epidemiology, Screening, and Prognosis" Journal of Clinical Medicine 13, no. 1: 30. https://doi.org/10.3390/jcm13010030
APA StyleSuomalainen, O. P., Martinez-Majander, N., Broman, J., Mannismäki, L., Aro, A., Curtze, S., Pakarinen, S., Lehto, M., & Putaala, J. (2024). Stroke in Patients with Atrial Fibrillation: Epidemiology, Screening, and Prognosis. Journal of Clinical Medicine, 13(1), 30. https://doi.org/10.3390/jcm13010030






