Hyperbaric Oxygen Therapy in Ophthalmology: A Narrative Review
Abstract
:1. Introduction
Search Methodology
2. Impact of HBOT on Healthy Retinas
3. HBOT in Retinal Diseases
3.1. DR and DME
3.2. HBOT for the Treatment of CRAO/BRAO
3.3. Impact of HBOT on Central Corneal Thickness
3.4. The Treatment of Optic Neuropathy with HBOT
3.5. Choroidal Neovascularization
3.6. Case Reports of HBOT Use
4. Disadvantages of HBOT
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bagli, B.S.; Çevik, S.G.; Çevik, M.T. Effect of hyperbaric oxygen treatment in central retinal artery occlusion. Undersea Hyperb. Med. 2018, 45, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, H.; Kauert, A.; Martiano, D.; Chiabo, J.; Di Vincenzo, D.; Sozonoff, I.; Baillif, S.; Martel, A. Efficacy and safety of a standardized hyperbaric oxygen therapy protocol for retinal artery occlusion. Undersea Hyperb. Med. 2022, 49, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, J.; Xie, J.; Kang, Z.; Sun, X.; Chen, N.; Liu, L.; Xu, J. Hyperbaric oxygen preconditioning promotes survival of retinal ganglion cells in a rat model of optic nerve crush. J. Neurotrauma 2010, 27, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Dollery, C.T.; Bulpitt, C.J.; Kohner, E.M. Oxygen supply to the retina from the retinal and choroidal circulations at normal and increased arterial oxygen tensions. Investig. Ophthalmol. 1969, 8, 588–594. [Google Scholar]
- Butler, F.K., Jr.; Hagan, C.; Murphy-Lavoie, H. Hyperbaric oxygen therapy and the eye. Undersea Hyperb. Med. 2008, 35, 333–387. [Google Scholar] [PubMed]
- Sayin, O.; Altinkaynak, H. The effects of hyperbaric oxygen therapy on retinal layers in healthy eyes. Undersea Hyperb. Med. 2022, 49, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, P.H.; Nevill, C.R.; Stratton, I.M.; Maruti, S.S.; Massó-González, E.L.; Sivaprasad, S.; Bailey, C.; Ehrlich, M.; Chong, V. Prevalence and incidence of diabetic retinopathy (DR) in the UK population of Gloucestershire. Acta Ophthalmol. 2022, 100, e560–e570. [Google Scholar] [CrossRef]
- Schmidt, I.; Walter, P.; Siekmann, U.; Plange, N.; Koutsonas, A.; Mazinani, B.E.; Kuerten, D. Development of visual acuity under hyperbaric oxygen treatment (HBO) in non arteritic retinal branch artery occlusion. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 303–310. [Google Scholar] [CrossRef]
- Sellman, A.; Katzman, P.; Andreasson, S.; Lõndahl, M. Long-term effects of hyperbaric oxygen therapy on visual acuity and retinopathy. Undersea Hyperb. Med. 2020, 47, 423–430. [Google Scholar] [CrossRef]
- Senol, M.G.; Yildiz, S.; Ersanli, D.; Uzun, G.; Gumus, T.; Narin, Y.; Ozkan, S.; Ayata, A. Carbon monoxide-induced cortical visual loss: Treatment with hyperbaric oxygen four years later. Med. Princ. Pract. 2009, 18, 67–69. [Google Scholar] [CrossRef]
- Shroff, D.; Kothari, A.; Gupta, P.; Sahni, T.K.; Narain, S. Hyperbaric oxygen therapy combined with immunosuppression for acute macular neuroretinopathy in systemic lupus erythematosus. Ocul. Immunol. Inflamm. 2023, 31, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.C.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.K.; et al. Guidelines on diabetic eye care: The international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology 2018, 125, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Pakola, S.J.; Grunwald, J.E. Effects of oxygen and carbon dioxide on human retinal circulation. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2866–2870. [Google Scholar]
- Al-Rawahi, A. Role of hyperbaric oxygen therapy on microvascular diabetic complications and metabolic profile among patients with type 2 diabetes mellitus. Oman Med. J. 2020, 35, e129. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Carneiro, Â.; Neri, G.; Fradinho, A.C.; Quenderra, B.; Barata, M.J.; Tenreiro, S.; Seabra, M.C. Choroidal vascular impairment in intermediate age-related macular degeneration. Diagnostics 2022, 12, 1290. [Google Scholar] [CrossRef] [PubMed]
- Sidorczuk, P.; Pieklarz, B.; Konopinska, J.; Saeed, E.; Mariak, Z.; Dmuchowska, D. Foveal avascular zone does not correspond to choroidal characteristics in patients with diabetic retinopathy: A Singlesingle-Center center cross-sectional analysis. Diabetes Metab. Syndr. Obes. 2021, 14, 2893–2903. [Google Scholar] [CrossRef] [PubMed]
- Stoica, S.I.; Bleotu, C.; Ciobanu, V.; Ionescu, A.M.; Albadi, I.; Onose, G.; Munteanu, C. Considerations about hypoxic changes in neuraxis tissue injuries and recovery. Biomedicines 2022, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Çevik, S.G.; Bağlı, B.S. Change in the foveal avascular zone and macular capillary network density after hyperbaric oxygen therapy in healthy retina. J. Ophthalmic Vis. Res. 2021, 16, 393–399. [Google Scholar] [CrossRef]
- Tukenmez Dikmen, N.; Akyol, U.C.; Comerter, D.; Sadik, M.T.; Demir, N.; Sumen, S.G.; Sonmez, M. The effect of hyperbaric oxygen therapy on retina, choroidal thickness, and choroidal vascularity index. Photodiagn Photodyn. Ther. 2022, 38, 102854. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chen, P.L.; Tai, M.C.; Chen, C.H.; Lu, D.W.; Chen, J.T. Hyperbaric oxygen therapy ameliorates the blood-retinal barrier breakdown in diabetic retinopathy. Clin. Exp. Ophthalmol. 2006, 34, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Kiryu, J.; Takahashi, K.; Honda, Y. Visual improvement in diabetic macular edema by hyperbaric oxygen treatment. Nippon. Ganka Gakkai Zasshi 1988, 92, 1456–1460. [Google Scholar] [PubMed]
- Pfoff, D.S.; Thom, S.R. Preliminary report on the effect of hyperbaric oxygen on cystoid macular edema. J. Cataract. Refract. Surg. 1987, 13, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Maalej, A.; Khallouli, A.; Choura, R.; Ben Sassi, R.; Rannen, R.; Gharsallah, H. The effects of hyperbaric oxygen therapy on diabetic retinopathy: A preliminary study. J. Fr. Ophtalmol. 2020, 43, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Kaldırım, H.; Yazgan, S.; Ceylan, B.; Atalay, K. The effect of hyperbaric oxygen therapy on retinal thickness and progression of retinopathy in patients with Type 2 diabetes: A prospective cohort study. Cutan. Ocul. Toxicol. 2019, 38, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Kiryu, J.; Ogura, Y. Hyperbaric oxygen treatment for macular edema in retinal vein occlusion: Relation to severity of retinal leakage. Ophthalmologica 1996, 210, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Gün, R.D.; Gümüş, T.; Kardaş, A.S.Y.; Kardaş, G. Acute effect of hyperbaric oxygen therapy on macular and choroidal thickness in patients with type 2 diabetes and diabetic foot ulcers: Optical coherence tomography based study. Photodiagn Photodyn. Ther. 2022, 39, 102926. [Google Scholar] [CrossRef]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef]
- Hopf, H.W.; Gibson, J.J.; Angeles, A.P.; Constant, J.S.; Feng, J.J.; Rollins, M.D.; Zamirul Hussain, M.; Hunt, T.K. Hyperoxia and angiogenesis. Wound Repair. Regen. 2005, 13, 558–564. [Google Scholar] [CrossRef]
- Sheikh, A.Y.; Gibson, J.J.; Rollins, M.D.; Hopf, H.W.; Hussain, Z.; Hunt, T.K. Effect of hyperoxia on vascular endothelial growth factor levels in a wound model. Arch. Surg. 2000, 135, 1293–1297. [Google Scholar] [CrossRef]
- Liu, R.; Li, L.; Yang, M.; Boden, G.; Yang, G. Systematic review of the effectiveness of hyperbaric oxygenation therapy in the management of chronic diabetic foot ulcers. Mayo Clin. Proc. 2013, 88, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Stoekenbroek, R.M.; Santema, T.B.; Legemate, D.A.; Ubbink, D.T.; van den Brink, A.; Koelemay, M.J. Hyperbaric oxygen for the treatment of diabetic foot ulcers: A systematic review. Eur. J. Vasc. Endovasc. Surg. 2014, 47, 647–655. [Google Scholar] [CrossRef] [PubMed]
- McCartney, P.J.; McCartney, P.W. Vitreous haemorrhage after hyberbaric oxygen therapy. Eye 1994, 8, 705–706. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.; Smart, D. Proliferative retinopathy during hyperbaric oxygen treatment. Diving Hyperb. Med. 2017, 47, 203. [Google Scholar] [CrossRef] [PubMed]
- Lifson, N.; Salloum, G.; Kurochkin, P.; Bivona, M.; Yin, H.Y.; Alpert, S. Treatment outcomes on neovascularization after CRAO treated with hyperbaric oxygen. Undersea Hyperb. Med. 2021, 48, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.J.; Lin, Y.C.; Chang, H.Y.; Chen, M.J. Risk factors for ocular neovascularization after central retinal artery occlusion. J. Chin. Med. Assoc. 2022, 85, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Savas, I.; Erdinc, E. Long-Term Evaluation of Retinal Artery Occlusion Patients Who Applied Hyperbaric Oxygen Treatment. J. Health Sci. 2018, 6, 148–152. [Google Scholar] [CrossRef]
- Chiabo, J.; Kauert, A.; Casolla, B.; Contenti, J.; Nahon-Esteve, S.; Bailiff, S.; Arnaud, M. Efficacy and safety of hyperbaric oxygen therapy monitored by fluorescein angiography in patients with retinal artery occlusion. Br. J. Ophthalmol. 2023. [Google Scholar] [CrossRef]
- Wu, X.; Chen, S.; Li, S.; Zhang, J.; Luan, D.; Zhao, S.; Chu, Z.; Xu, Y. Oxygen therapy in patients with retinal artery occlusion: A meta-analysis. PLoS ONE 2018, 13, e0202154. [Google Scholar] [CrossRef]
- Li, H.K.; Dejean, B.J.; Tang, R.A. Reversal of visual loss with hyperbaric oxygen treatment in a patient with Susac syndrome. Ophthalmology 1996, 103, 2091–2098. [Google Scholar] [CrossRef]
- Mangat, H.S. Retinal artery occlusion. Surv. Ophthalmol. 1995, 40, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Gaydar, V.; Ezrachi, D.; Dratviman-Storobinsky, O.; Hofstetter, S.; Avraham-Lubin, B.C.; Goldenberg-Cohen, N. Reduction of apoptosis in ischemic retinas of two mouse models using hyperbaric oxygen treatment. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7514–7522. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Zimmerman, M.B. Central retinal artery occlusion: Visual outcome. Am. J. Ophthalmol. 2005, 140, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, A.; Hadad, A.; Peled, A.; Dubinsky-Pertzov, B.; Or, L.; Eting, E.; Efrati, S.; Pras, E.; Einan-Lifshitz, A. Hyperbaric oxygen treatment for non-arteritic central retinal artery occlusion retrospective comparative analysis from two tertiary medical centres. Eye 2022, 36, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Masters, T.C.; Westgard, B.C.; Hendriksen, S.M.; Decanini, A.; Abel, A.S.; Logue, C.J.; Walter, J.W.; Linduska, J.; Engel, K.C. Case series of hyperbaric oxygen therapy for central retinal artery occlusion. Retin. Cases Brief. Rep. 2021, 15, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Elder, M.J.; Rawstron, J.A.; Davis, M. Hyperbaric oxygen in the treatment of acute retinal artery occlusion. Diving Hyperb. Med. 2017, 47, 233–238. [Google Scholar] [CrossRef]
- Kim, Y.S.; Nam, M.S.; Park, E.J.; Lee, Y.; Kim, H.; Kim, S.H.; Cha, Y.S. The effect of adjunctive hyperbaric oxygen therapy in patients with central retinal artery occlusion. Undersea Hyperb. Med. 2020, 47, 57–64. [Google Scholar] [CrossRef]
- Khallouli, A.; Khelifi, K.; Saidane, R.; Choura, R.; Maalej, A.; Sassi, R.B. Hyperbaric oxygen treatment of central retinal vein occlusion with cilioretinal artery occlusion secondary to hormonal treatment: Case report and review. Diving Hyperb. Med. 2020, 50, 431–436. [Google Scholar] [CrossRef]
- Lopes, A.S.; Basto, R.; Henriques, S.; Colaço, L.; Costa e Silva, F.; Prieto, I.; Guerreiro, F. Hyperbaric oxygen therapy in retinal arterial occlusion: Epidemiology, clinical approach, and visual outcomes. Case Rep. Ophthalmol. Med. 2019, 2019, 9765938. [Google Scholar] [CrossRef]
- Menzel-Severing, J.; Siekmann, U.; Weinberger, A.; Roessler, G.; Walter, P.; Mazinani, B. Early hyperbaric oxygen treatment for nonarteritic central retinal artery obstruction. Am. J. Ophthalmol. 2012, 153, 454–459. [Google Scholar] [CrossRef]
- Kim, S.H.; Cha, Y.S.; Lee, Y.; Kim, H.; Yoon, I.N. Successful treatment of central retinal artery occlusion using hyperbaric oxygen therapy. Clin. Exp. Emerg. Med. 2018, 5, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Butler, F.K.; Hagan, C.; Van Hoesen, K.; Murphy-Lavoie, H. Management of central retinal artery occlusion following successful hyperbaric oxygen therapy: Case report. Undersea Hyperb. Med. 2018, 45, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Gokce, G.; Metin, S.; Erdem, U.; Sobaci, G.; Durukan, A.H.; Cagatay, H.H.; Ekinci, M. Late hyperbaric oxygen treatment of cilioretinal artery occlusion with nonischemic central retinal vein occlusion secondary to high altitude. High. Alt. Med. Biol. 2014, 15, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Youn, T.S.; Lavin, P.; Patrylo, M.; Schindler, J.; Kirshner, H.; Greer, D.M.; Schrag, M. Current treatment of central retinal artery occlusion: A national survey. J. Neurol. 2018, 265, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Oguz, H.; Sobaci, G. The use of hyperbaric oxygen therapy in ophthalmology. Surv. Ophthalmol. 2008, 53, 112–120. [Google Scholar] [CrossRef]
- Ayata, A.; Uzun, G.; Mutluoglu, M.; Unal, M.; Yildiz, S.; Ersanli, D. Influence of hyperbaric oxygen therapy on central corneal thickness. Ophthal Res. 2012, 47, 19–22. [Google Scholar] [CrossRef]
- Herse, P.; Adams, L. Effect of hyperglycemia duration on rabbit corneal thickness and endothelial ATPase activity. Acta Ophthalmol. Scand. 1995, 73, 158–161. [Google Scholar] [CrossRef]
- Malik, A.; Golnik, K. Hyperbaric oxygen therapy in the treatment of radiation optic neuropathy. J. Neuroophthalmol. 2012, 32, 128–131. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Liang, C.M.; Tai, M.C.; Chen, Y.J. Benefit of hyperbaric oxygen therapy treatment in direct traumatic optic neuropathy: Case report. Undersea Hyperb. Med. 2018, 45, 463–471. [Google Scholar] [CrossRef]
- Allashem, H.M.; Sward, D.G.; Sethuraman, K.; Matthews, M.K. Hyperbaric oxygen therapy for perioperative posterior ischemic optic neuropathy: A case report. Undersea Hyperb. Med. 2019, 46, 701–707. [Google Scholar] [CrossRef]
- Alexander, J.L.; Shulman, M.D.; Sethuraman, K. Acute direct traumatic optic neuropathy treated with steroids, minocycline and hyperbaric oxygen: A case report. Undersea Hyperb. Med. 2019, 46, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Malerbi, F.K.; Novais, E.A.; Emmerson, B.; Bonomo, P.P.; Pereira, A.J.; Lottenberg, C.L.; Maia, A. Hyperbaric oxygen therapy for choroidal neovascularization: A pilot study. Undersea Hyperb. Med. 2015, 42, 125–131. [Google Scholar] [PubMed]
- Sayadi, J.; Ksiaa, I.; Malek, I.; Ben Sassi, R.; Essaddam, L.; Khairallah, M.; Nacef, L. Hyperbaric oxygen therapy for mumps-associated outer retinitis with frosted branch angiitis. Ocul. Immunol. Inflamm. 2022, 30, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Iniesta-Sanchez, D.L.; Romero-Caballero, F.; Aguirre-Alvarado, A.; Rebollo-Hurtado, V.; Velez-Montoya, R. Management of orbital emphysema secondary to rhegmatogenous retinal detachment repair with hyperbaric oxygen therapy. Am. J. Ophthalmol. Case Rep. 2016, 1, 26–30. [Google Scholar] [CrossRef] [PubMed]
- McMonnies, C.W. Hyperbaric oxygen therapy and the possibility of ocular complications or contraindications. Clin. Exp. Optom. 2015, 98, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Lyne, A.J. Ocular effects of hyperbaric oxygen. Trans. Ophthalmol. Soc. UK 1978, 98, 66–68. [Google Scholar] [PubMed]
- Riedl, P.; Škiljić, D.; Arnell, P.; Wannholt, R.; Zetterberg, M.; Andersson Grönlund, M. Myopic shift and lens turbidity following hyperbaric oxygen therapy–A prospective, longitudinal, observational cohort study. Acta Ophthalmol. 2019, 97, 596–602. [Google Scholar] [CrossRef]
- McMonnies, C. Reactive oxygen species, oxidative stress, glaucoma and hyperbaric oxygen therapy. J. Optom. 2018, 11, 3–9. [Google Scholar] [CrossRef]
- Siegfried, C.J.; Shui, Y.B.; Holekamp, N.M.; Bai, F.; Beebe, D.C. Oxygen distribution in the human eye: Relevance to the etiology of open-angle glaucoma after vitrectomy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5731–5738. [Google Scholar] [CrossRef]
- Yonekawa, Y.; Hypes, S.M.; Abbey, A.M.; Williams, G.A.; Wolfe, J.D. Exacerbation of macular oedema associated with hyperbaric oxygen therapy. Clin. Exp. Ophthalmol. 2016, 44, 625–626. [Google Scholar] [CrossRef]
- Neubauer, A.S.; Mueller, A.J.; Schriever, S.; Grüterich, M.; Ulbig, M.; Kampik, A. Minimal invasive Therapie bei klinisch komplettem Zentralarterienverschluss–Eigene Ergebnisse und Literaturvergleich [Minimally invasive therapy for clinically complete central retinal artery occlusion—Results and meta-analysis of literature]. Klin. Monbl. Augenheilkd. 2000, 217, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Duker, J.S.; Brown, G.C. Recovery following acute obstruction of the retinal and choroidal circulations. A case history. Retina 1988, 8, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Mac Grory, B.; Schrag, M.; Biousse, V.; Furie, K.L.; Gerhard-Herman, M.; Lavin, P.J.; Sobrin, L.; Tjoumakaris, S.I.; Weyand, C.M.; Yaghi, S.; et al. Management of central retinal artery occlusion: A scientific statement from the American Heart Association. Stroke 2021, 52, e282–e294. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Callejón-Peláez, E.; Sáez, M.A.; Álvarez-Mon, M.A.; García-Honduvilla, N.; Monserrat, J.; Álvarez-Mon, M.; Bujan, J.; et al. A general overview on the hyperbaric oxygen therapy: Applications, mechanisms and translational opportunities. Medicina 2021, 57, 864. [Google Scholar] [CrossRef]
- Li, S.; Ding, G.; Sun, Y.; Zhang, C. Impact of different oxygen supply methods on the healing of corneal epithelial wound and the level of acetylcholine. J. Ophthalmol. 2021, 2021, 4737479. [Google Scholar] [CrossRef]
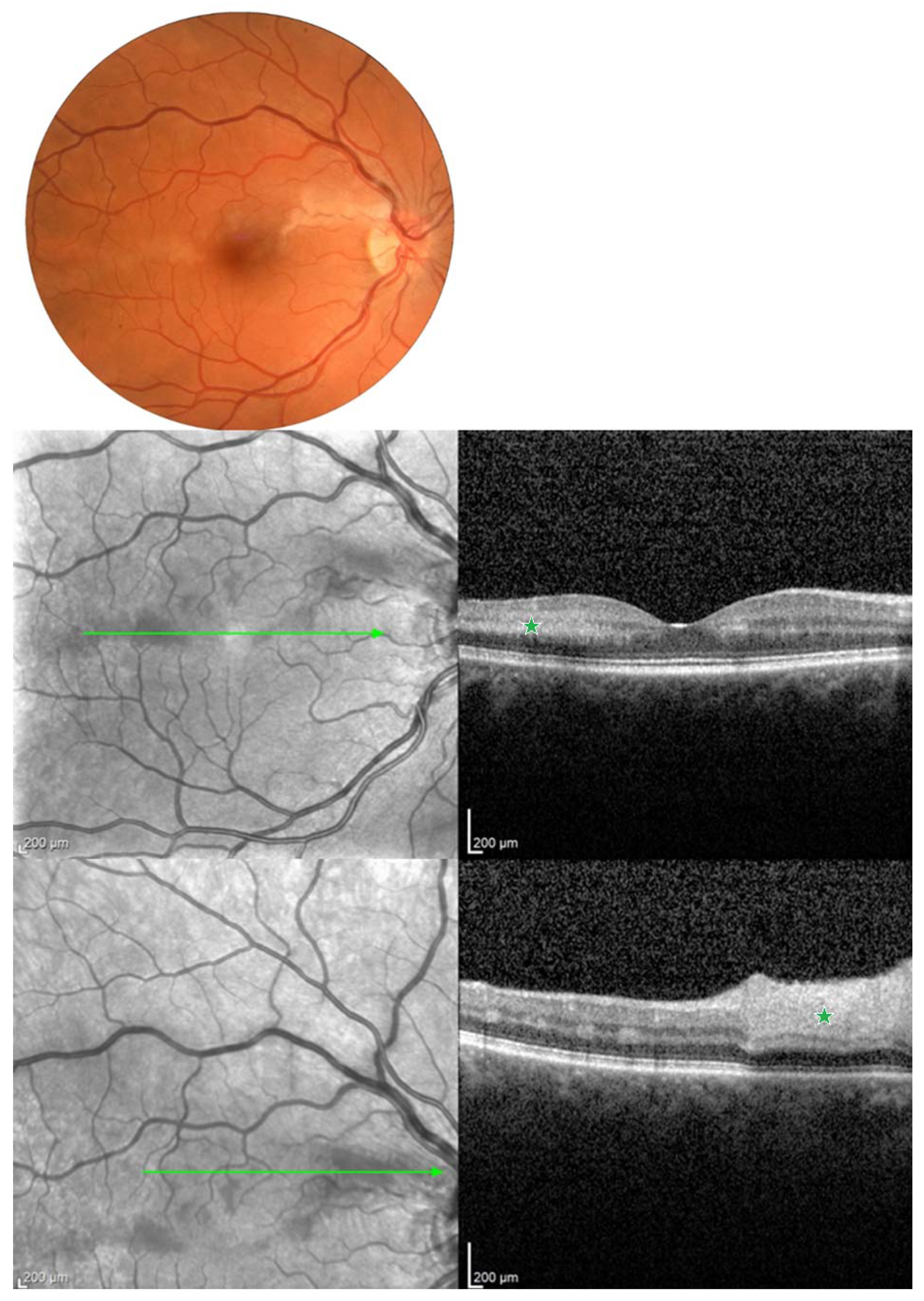
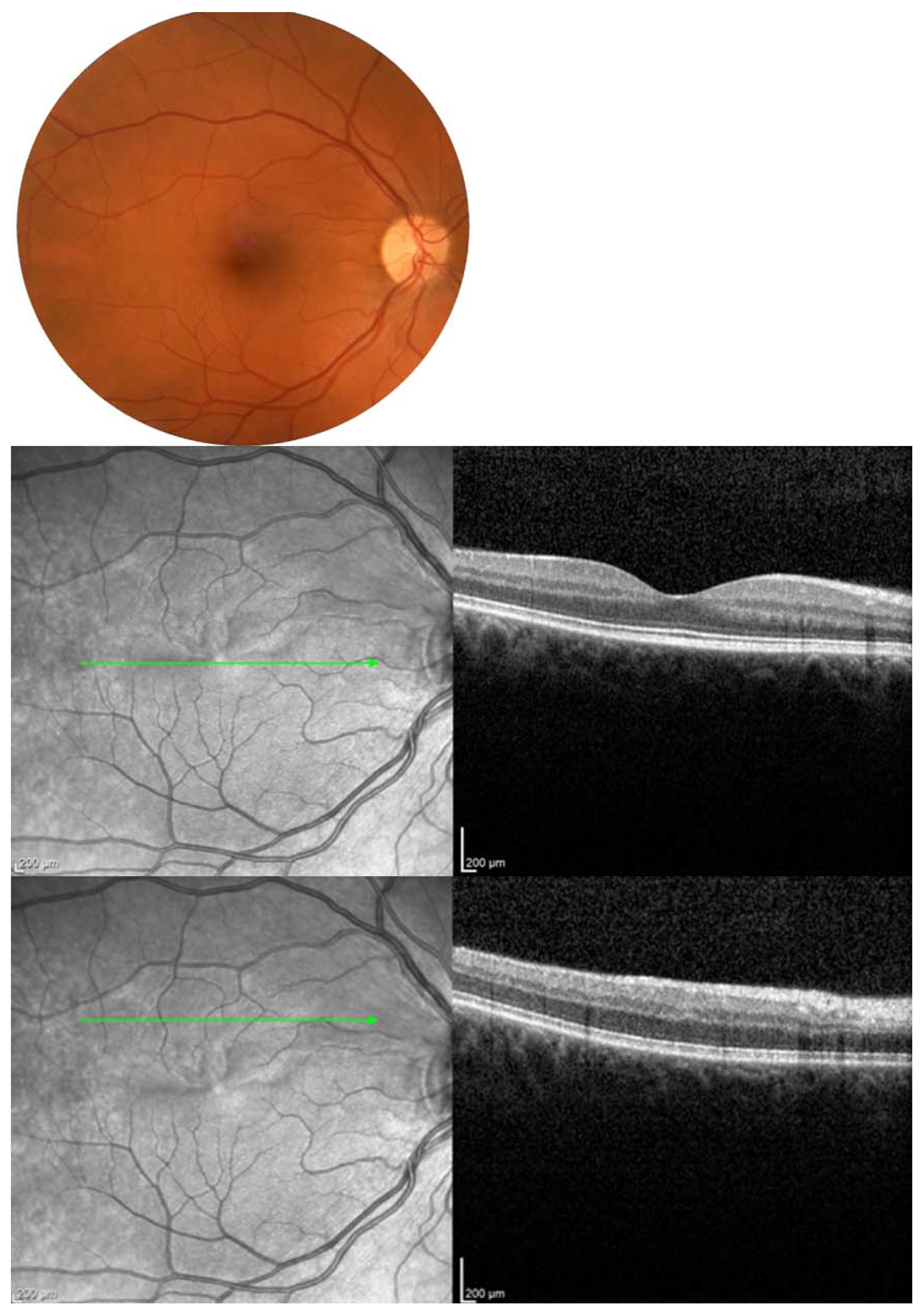
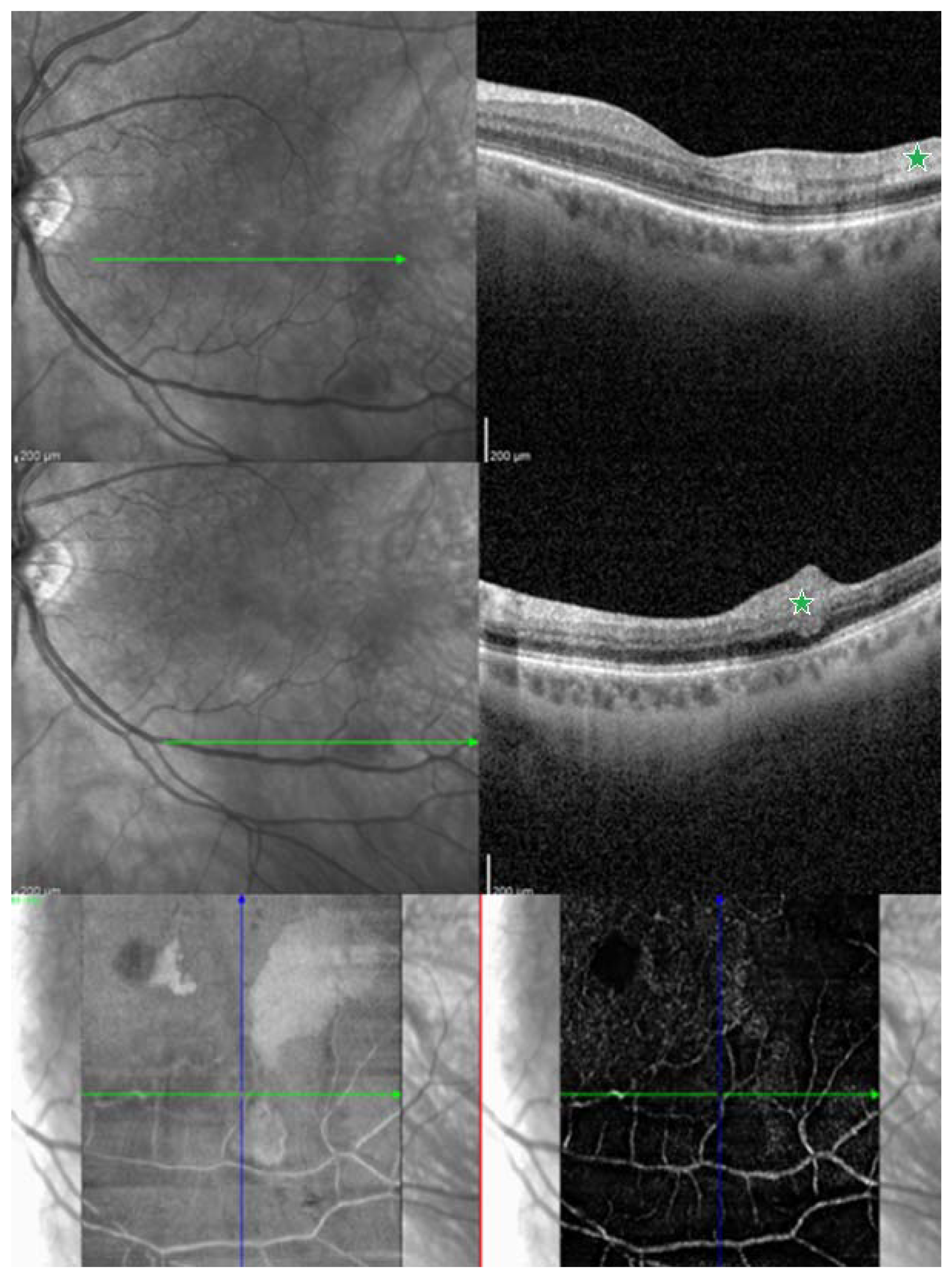
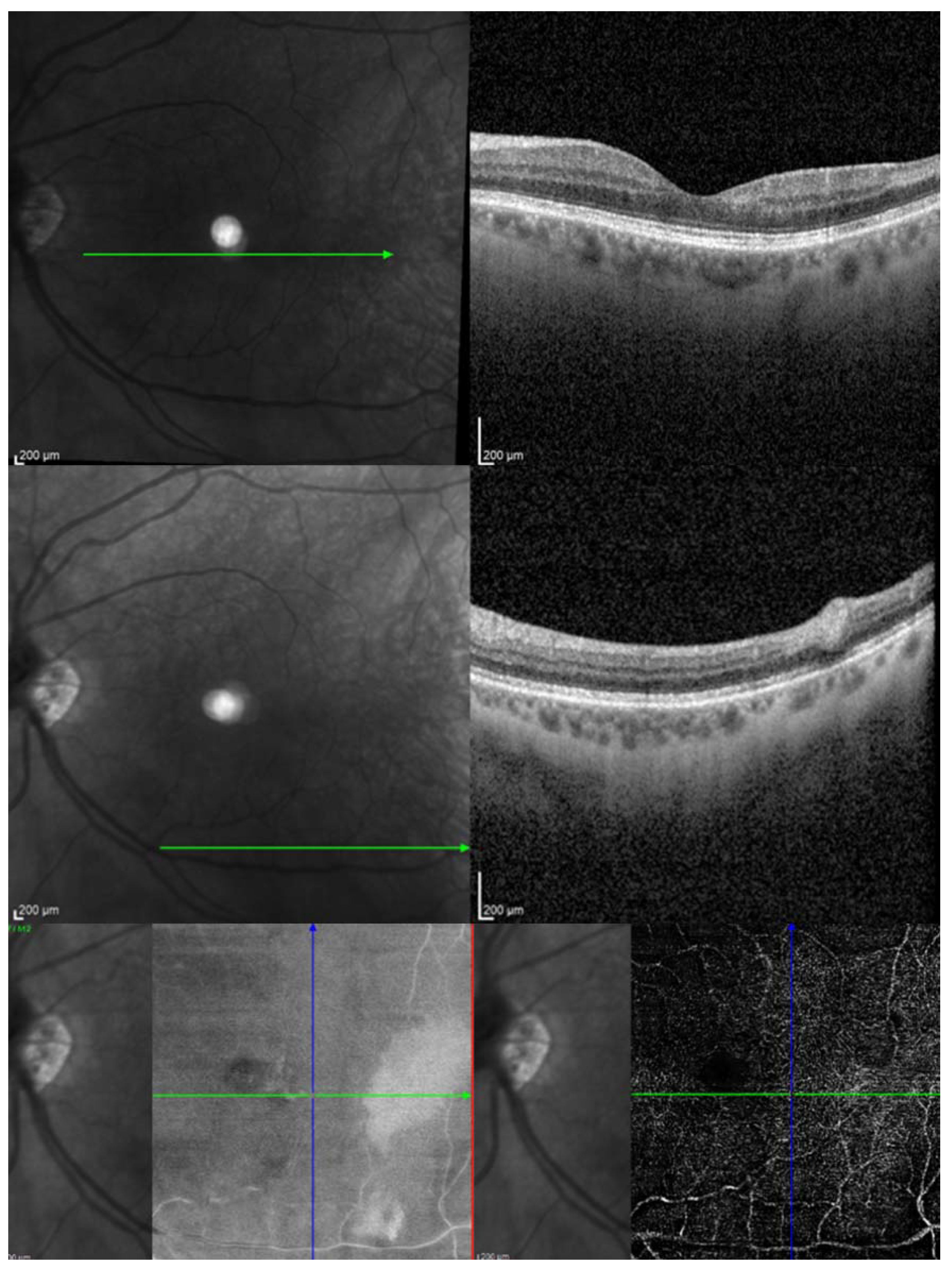
| Report | CRAO/BRAO | Study Objective | Number of HBOT Sessions | Conclusions/Findings |
|---|---|---|---|---|
| Rozenberg et al. [44] | CRAO | Comparing HBOT vs. standard care (SC) outcomes. | Three sessions within 24 h, then daily until max VA reached | HBOT group had markedly better final VA than that of controls |
| Masters et al. [45] | CRAO | Reporting outcomes from patients treated with HBOT. | Total of 10 sessions | 72% showed improved vision up to 30 months post-treatment, notably higher in those treated within 12 h of symptom onset |
| Bagli et al. [1] | CRAO | Presenting visual outcomes in HBOT-treated patients | Total of 20 sessions | Initial average logMAR 3.0; Improved to logMAR 1.8 post-treatment |
| Lifson et al. [35] | CRAO | To assess enhancement in VA and reduction in neovascularization post-HBOT | 20% of patients developed neovascularization after HBOT compared to 29.8% of those who did not undergo HBOT (p < 0.05). | HBOT exhibits significant protection against neovascularization, potentially enhancing long-term visual acuity |
| Elder et al. [46] | CRAO/BRAO | Utilizing HBOT for acute retinal artery occlusion treatment | At least one session | Early HBOT intervention benefits ARAO patients, fostering potential visual recovery. |
| Vincenzo et al. [2] | CRAO/BRAO | To evaluate the effectiveness and safety of a standardized HBOT protocol | Two sessions daily for at least 15 days | 50% of patients achieved an improvement in VA of at least 0.3 logMAR |
| Kim et al. [47] | CRAO | To examine the effect of adjunctive HBOT on VA on adult patients | 19 were included in the study, of which, 10 patients (52.6%) were treated with adjunctive HBOT | HBOT group showed a significantly greater improvement in VA compared to that of the control group |
| Lopes et al. [49] | CRAO/BRAO | To evaluate the efficacy and safety of HBOT | Two sessions daily for 3 days, then based on BCVA improvement till stability | Early HBOT improved BCVA outcomes post-symptom onset |
| Schmidt et al. [8] | BRAO | Comparing HBOT vs. standard care (SC) outcomes. | 5 times within 48 h, with 3 treatments within the first 24 h | HBOT patients showed significant VA improvement versus control |
| Menzel-Severing et al. [50] | CRAO/BRAO | Comparing HBOT + hemodilution vs. hemodilution alone effects. | Five treatments in 48 h, three within the first 24 | HBOT saw a 3-line VA improvement versus 1 line in hemodilution alone; not statistically significant |
| Wu et al. [39] | CRAO/BRAO | To examine seven randomized controlled trials using various types of oxygen therapy | - | Oxygen therapy, especially HBOT, may offer visual advantages when paired with other treatments. |
| Report | CRAO/BRAO/CRVO/CLRAO | Therapy | No. of HBOT Sessions | Delay to Tx | Initial VA | Final VA |
|---|---|---|---|---|---|---|
| Kim et al. [51] | CRAO | HBOT, ocular massage, topical brimonidine and dorzolamide/timolol | 3 over 3 days | 10 h | hand motion | 0.4 (OD) for far vision; 0.5 (OD) for near vision |
| Khallouli et al. [48] | CLRAO CRVO | HBOT | total of 30 sessions | 2 days | 7/10 | 10/10 |
| Butler et al. [52] | CRAO | HBOT, ocular massage, timolol drops, and acetazolamide | Total of 7 sessions | 9.5 h | hand motion | Count fingers |
| Gokce et al. [53] | CRVO | HBOT | Total of 11 sessions | 2 weeks | 10/20 | 20/20 |
| Drawbacks of existing studies of HBOT |
| Primarily comprises case reports and observational studies |
| Paucity of high-quality randomized controlled trials |
| Limited methodological rigor inherent in these study designs |
| Small sample groups |
| Absence of sex- and age-matched control groups |
| Disparity in the time elapsed from onset to treatment among the studied groups |
| Lack of standardized HBOT protocol |
| Lack of consensus on the duration of HBOT and the ideal number of daily sessions |
| Oversight of comorbidities |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micun, Z.; Dobrzyńska, W.; Sieśkiewicz, M.; Zawadzka, I.; Dmuchowska, D.A.; Wojewodzka-Zelezniakowicz, M.; Konopińska, J. Hyperbaric Oxygen Therapy in Ophthalmology: A Narrative Review. J. Clin. Med. 2024, 13, 29. https://doi.org/10.3390/jcm13010029
Micun Z, Dobrzyńska W, Sieśkiewicz M, Zawadzka I, Dmuchowska DA, Wojewodzka-Zelezniakowicz M, Konopińska J. Hyperbaric Oxygen Therapy in Ophthalmology: A Narrative Review. Journal of Clinical Medicine. 2024; 13(1):29. https://doi.org/10.3390/jcm13010029
Chicago/Turabian StyleMicun, Zuzanna, Weronika Dobrzyńska, Michał Sieśkiewicz, Izabela Zawadzka, Diana Anna Dmuchowska, Marzena Wojewodzka-Zelezniakowicz, and Joanna Konopińska. 2024. "Hyperbaric Oxygen Therapy in Ophthalmology: A Narrative Review" Journal of Clinical Medicine 13, no. 1: 29. https://doi.org/10.3390/jcm13010029
APA StyleMicun, Z., Dobrzyńska, W., Sieśkiewicz, M., Zawadzka, I., Dmuchowska, D. A., Wojewodzka-Zelezniakowicz, M., & Konopińska, J. (2024). Hyperbaric Oxygen Therapy in Ophthalmology: A Narrative Review. Journal of Clinical Medicine, 13(1), 29. https://doi.org/10.3390/jcm13010029









