Follow-up for More than 10 Years of Patients with Peritoneal Metastases Treated with Cytoreductive Surgery + Hyperthermic Intraperitoneal Chemotherapy in a Specialized Unit
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Selection Criteria
2.3. Patient Data
2.4. Procedure
2.5. Follow-up
2.6. Statistical Analysis
3. Results
3.1. Study Population and Surgical Outcomes
3.2. Postoperative Outcomes
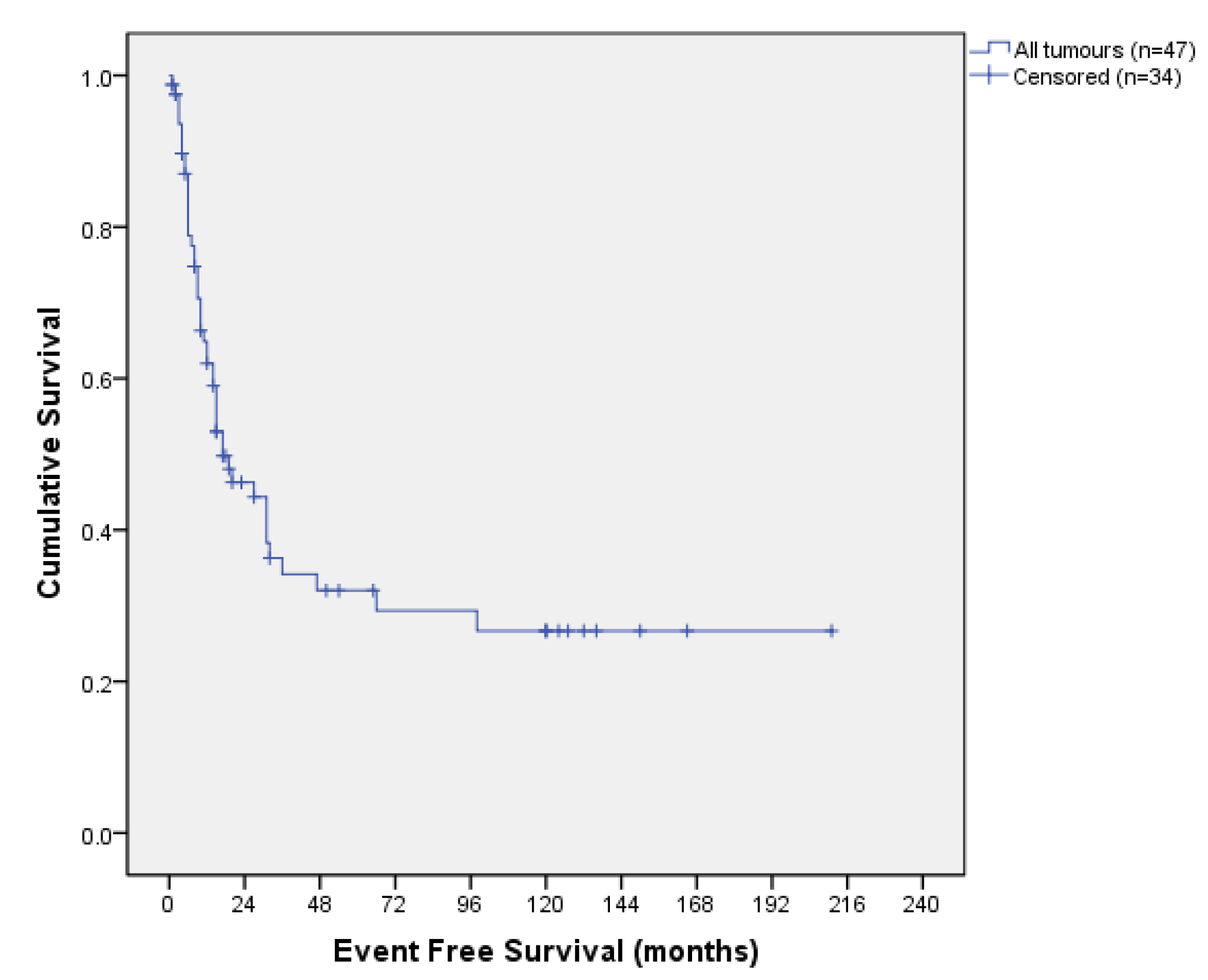
3.3. Survival Outcomes
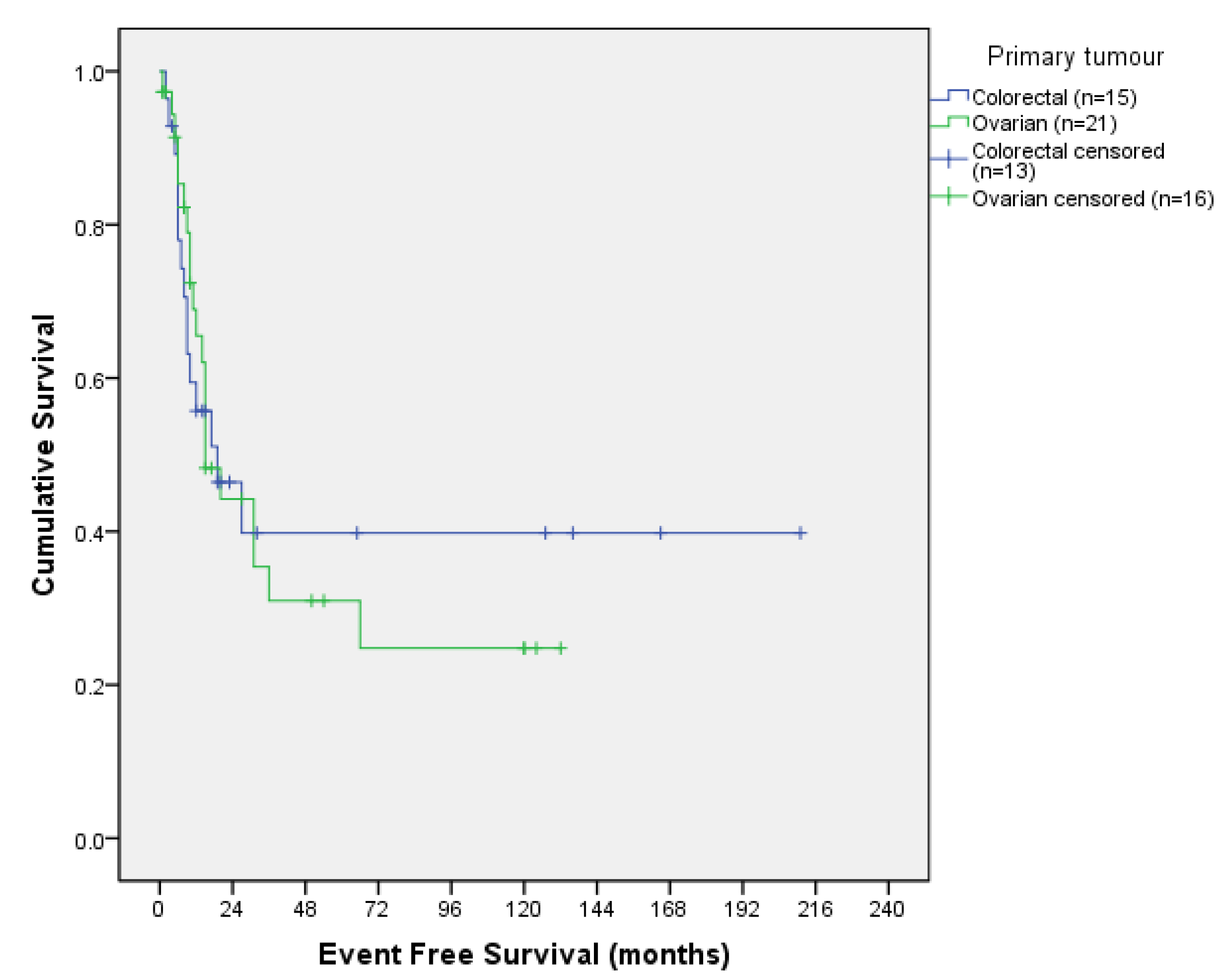
3.3.1. Survival Outcomes According to Tumour Origin
3.3.2. Survival Outcomes According to PCI
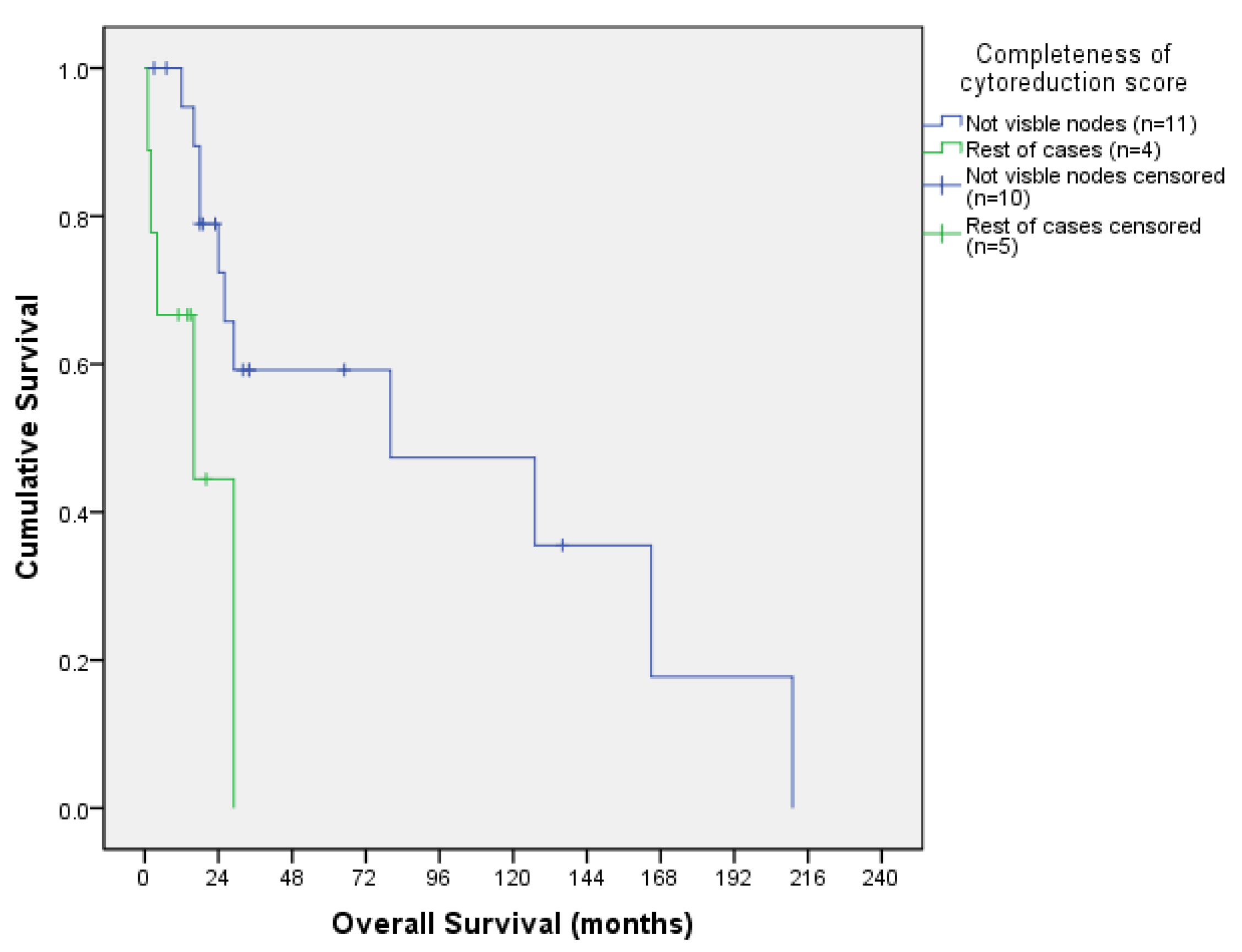
3.3.3. Survival Outcomes According to CCS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, D.Z.J.; Lang, N.P.; Thompson, C.; Osteen, P.K.; Westbrook, K.C. Peritoneal carcinomatosis in nongynecological malignancy: A prospective study of prognostic factors. Cancer 1989, 63, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Kober, F.; Heiss, A.; Roka, R. Diffuse and gross peritoneal carcinomatosis treated by intraperitoneal hyperthermic chemoperfusion. In Peritoneal Carcinomatosis: Principles of Management; Sugarbaker, P.H., Ed.; Kluwer Academic Publishers: Boston, MA, USA, 1996; pp. 211–219. [Google Scholar]
- Sadeghi, B.; Arvieux, C.; Glehen, O.; Beaujard, A.C.; Rivoire, M.; Baulieux, J.; Fontaumard, E.; Brachet, A.; Caillot, J.L.; Faure, J.L.; et al. Peritoneal carcinomatosis from nongynecological malignancies: Results of the EVOCAPE 1 multicentric prospective study. Cancer 2000, 88, 358–363. [Google Scholar] [CrossRef]
- Jayne, D.G.; Fook, S.; Loi, C.; Seow-Choen, F. Peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2002, 89, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Sloothen, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Kwiatkowski, F.; Sugarbaker, P.H.; Elias, D.; Levine, E.A.; De Simone, M.; Barone, R.; Yonemura, Y.; Cavaliere, F.; Quenet, F.; et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J. Clin. Oncol. 2004, 22, 3284–3292. [Google Scholar] [CrossRef] [PubMed]
- Raspagliesi, F.; Kusamura, S.; Campos Torres, J.C.; de Souza, G.A.; Ditto, A.; Zanaboni, F.; Younan, R.; Baratti, D.; Mariani, L.; Laterza, B.; et al. Cytoreduction combined with intraperitoneal hyperthermic perfusion chemotherapy in advanced/recurrent ovarian cancer patients: The experience of National Cancer Institute of Milan. Eur. J. Surg. Oncol. 2006, 32, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Schreiber, V.; Cotte, E.; Sayag-Beaujard, A.C.; Osinsky, D.; Freyer, G.; François, Y.; Vignal, J.; Gilly, F.N. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch. Surg. 2004, 139, 20–26. [Google Scholar] [CrossRef]
- Marchettini, P.; Sugarbaker, P.H. Mucinous adenocarcinoma of the small bowel with peritoneal seeding. Eur. J. Surg. Oncol. 2002, 28, 19–23. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Cytoreduction including total gastrectomy for pseudomyxoma peritonei. Br. J. Surg. 2002, 89, 208–212. [Google Scholar] [CrossRef]
- Gómez Portilla, A.; Barrios, P.; Rufian, S.; Camps, B.; Bretcha, P.; Gonzalez Bayon, L.; Torres Melero, J.; García Polavieja, M.; Gonzalez Moreno, S. Management of peritoneal surface malignancy with cytoreductive surgery and perioperative intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2006, 32, 628–631. [Google Scholar] [CrossRef]
- Kyang, L.S.; Alzahrani, N.A.; Valle, S.J.; Rahman, M.K.; Arrowaili, A.; Liauw, W.; Morris, D.L. Long-term survival outcomes of cytoreductive surgery and perioperative intraperitoneal chemotherapy: Single-institutional experience with 1225 cases. J. Surg. Oncol. 2019, 120, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Ntatsis, K.; Papantoni, E.; Kyziridis, D.; Kalakonas, A.; Hristakis, C.; Tzavara, C.; Tentes, A.A. Ovarian cancer: 20-year experience with cytoreductive surgery and perioperative intraperitoneal chemotherapy. J. BUON 2021, 26, 1754–1761. [Google Scholar] [PubMed]
- Kim, S.R.; Kotsopoulos, J.; Sun, P.; Bernardini, M.Q.; Laframboise, S.; Ferguson, S.E.; Rosen, B.; Narod, S.A.; May, T. The impacts of neoadjuvant chemotherapy and of cytoreductive surgery on 10-year survival from advanced ovarian cancer. Int. J. Gynaecol. Obstet. 2021, 153, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J. Exp. Clin. Cancer Res. 1996, 15, 49–58. [Google Scholar]
- Elias, D.; Pocard, M.; Goere, D. HIPEC with oxaliplatin in the treatment of peritoneal carcinomatosis of colorectal origin. Cancer Treat. Res. 2007, 134, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Carmigani, C.P.; Esquivel, J.; Sugarbaker, P.H. Cytoreductive surgery and intraperitoneal chemotherapy for the treatment of peritoneal surface malignancy. Rev. Oncol. 2003, 5, 192–198. [Google Scholar] [CrossRef]
- Cascales Campos, P.; Gil, J.; Parrilla, P. Morbidity and mortality outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with primary and recurrent advanced ovarian cancer. Eur. J. Surg. Oncol. 2014, 40, 970–975. [Google Scholar] [CrossRef]
- van Stein, R.M.; Engbersen, M.P.; Stolk, T.; Lopez-Yurda, M.; Lahaye, M.J.; Beets-Tan, R.G.H.; Lok, C.A.R.; Sonke, G.S.; Van Driel, W.J. Peroperative extent of peritoneal metastases affects the surgical outcome and survival in advanced ovarian cancer. Gynecol. Oncol. 2022, 167, 269–276. [Google Scholar] [CrossRef]
- Narasimhan, V.; Tan, S.; Kong, J.; Pham, T.; Michael, M.; Ramsay, R.; Warrier, S.; Heriot, A. Prognostic factors influencing survival in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for isolated colorectal peritoneal metastases: A systematic review and meta-analysis. Colorectal Dis. 2020, 22, 1482–1495. [Google Scholar] [CrossRef]
- Goéré, D.; Glehen, O.; Quenet, F.; Guilloit, J.M.; Bereder, J.M.; Lorimier, G.; Thibaudeau, E.; Ghouti, L.; Pinto, A.; Tuech, J.J.; et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): A randomised, phase 3 study. Lancet Oncol. 2020, 21, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Goéré, D.; Dumont, F.; Honoré, C.; Dartigues, P.; Stoclin, A.; Malka, D.; Boige, V.; Ducreux, M. Role of hyperthermic intraoperative peritoneal chemotherapy in the management of peritoneal metastases. Eur. J. Cancer 2014, 50, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.C.; Saxena, A.; Schellekens, J.F.; Liauw, W.; Yan, T.D.; Fransi, S.; Zhao, J.; Morris, D.L. Morbidity and mortality outcomes of cytoreductive surgery and perioperative intraperitoneal chemotherapy at a single tertiary institution: Towards a new perspective of this treatment. Ann. Surg. 2010, 251, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.M.; Sleightholm, R.; Patel, A.; Shostrom, V.; Hall, B.; Neilsen, B.; Bartlett, D.; Smith, L. Morbidity and mortality rates following cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy compared with other high-risk surgical oncology procedures. JAMA Netw. Open 2019, 2, e186847. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H.; Graves, T.; DeBruijn, E.A.; Cunliffe, W.J.; Mullins, R.E.; Hull, W.E.; Oliff, L.; Schlag, P. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: Pharmacological studies. Cancer Res. 1990, 50, 5790–5794. [Google Scholar] [PubMed]
- Elias, D.; Benizri, E.; Di Pietrantonio, D.; Menegon, P.; Malka, D.; Raynard, B. Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Ann. Surg. Oncol. 2007, 14, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Bretcha-Boix, P.; Farré-Alegre, J.; Sureda, M.; Dussan, C.; Pérez Ruixo, J.J.; Brugarolas Masllorens, A. Cytoreductive surgery and perioperative intraperitoneal chemotherapy in patients with peritoneal carcinomatosis of colonic origin: Outcomes after 7 years’ experience of a new center for peritoneal surface malignancies. Clin. Transl. Oncol. 2010, 12, 437–442. [Google Scholar] [CrossRef]





| n = 86 | |
|---|---|
| Age | 56 (24–78) |
| Sex | |
| Men | 23 (26.7%) |
| Women | 63 (73.3%) |
| Neoadjuvant therapy | |
| No | 12 (14%) |
| Yes | 74 (86%) |
| Origin | |
| Ovarian | 38 (44.2%) |
| Colorectal | 31 (36%) |
| Gastric | 9 (10.5%) |
| Appendicular | 5 (5.8%) |
| Mesothelioma | 3 (3.5%) |
| PCI (mean) | 9 (0–39) |
| PCI | |
| 0 | 22 (26.2%) |
| 1–9 | 33 (39.3%) |
| 10–19 | 15 (17.9%) |
| ≥20 | 14 (16.7%) |
| PCI 0 | |
| Ovarian | 10 (45%) |
| Colorectal | 7 (31.81%) |
| Gastric | 4 (18.18%) |
| Mesothelioma | 1 (4.55%) |
| Cytoreduction | |
| Complete (CC-0) | 68 (80%) |
| Optimal (CC-1) | 9 (10.6%) |
| Incomplete (CC-2) | 7 (8.2%) |
| Non-resectable (CC-3) | 1 (1.2%) |
| Type of surgery | |
| Primary tumor | 61 (70.9%) |
| Persistence | 2 (2.3%) |
| Recurrence | 15 (17.4%) |
| Second look | 8 (9.3%) |
| HIPEC | |
| Oxaliplatin | 55 (64.7%) |
| Mytomicin C | 14 (16.5%) |
| Taxol | 11 (12.9%) |
| Carboplatin | 2 (2.4%) |
| Carboplatin + taxol | 1 (1.2%) |
| Cisplatin + Doxorubicin | 1 (1.2%) |
| Oxaliplatin + Doxorubicin | 1 (1.2%) |
| EPIC | |
| No | 14 (16.3%) |
| Yes | 72 (83.7%) |
| n = 86 | |
|---|---|
| Clavien–Dindo classification | |
| Grade I | 40 (46.5%) |
| Grade II | 20 (23.3%) |
| Grade IIIa | 7 (8.1%) |
| Grade IIIb | 9 (10.5%) |
| Grade IVa | 6 (7%) |
| Grade IVb | 2 (2.3%) |
| Grade V | 2 (2.3%) |
| Clavien–Dindo categories | |
| No morbidity | 40 (46.5%) |
| Minor morbidity | 20 (23.3%) |
| Major morbidity | 26 (27.9%) |
| In hospital stay | 17 (4–63) |
| 30-day mortality | 2.3% |
| Reintervention | |
| No | 72 (83.7%) |
| Yes | 13 (15.1%) |
| PCI | n | Median OS | 12 Months OS | 36 Months OS | 120 Months OS |
|---|---|---|---|---|---|
| 0 | 6 | 127 months | 100% | 55.6% | 55.6% |
| 1–9 | 12 | 80 months | 91.7% | 56.2% | 37.5% |
| 10–19 | 2 | 16 months | 66.7% | 33.3% | 33.3% |
| ≥20 | 6 | 29 months | 66.7% | 0% | 0% |
| PCI | n | Median OS | 12 Months OS | 36 Months OS | 120 Months OS |
|---|---|---|---|---|---|
| 0 | 10 | 20 months | 90% | 48% | 16% |
| 1–9 | 13 | 50 months | 85.1% | 61.9% | 24.8% |
| 10–19 | 8 | 44 months | 87.5% | 54.7% | 18.2% |
| ≥20 | 5 | 22 months | 60% | 30% | 30% |
| CCS | n | Median OS | 12 Months OS | 36 Months OS | 120 Months OS |
|---|---|---|---|---|---|
| CC-0 | 21 | 80 months | 94.7% | 59.2% | 47.5% |
| CC-1 | 4 | 29 months | 75% | 0% | 0% |
| CC-2 | 5 | 16 months | 60% | 30% | 30% |
| CC-3 | 0 | - | - | - | - |
| CCS | n | Median OS | 12 Months OS | 36 Months OS | 120 Months OS |
|---|---|---|---|---|---|
| CC-0 | 32 | 50 months | 84.7% | 51.3% | 23.1% |
| CC-1 | 3 | 44 months | 100% | 100% | 0% |
| CC-2 | 1 | 8 months * | 0% * | 0% * | 0% * |
| CC-3 | 1 | 120 months * | 100% * | 100% * | 0% * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Candela, A.; Bretcha-Boix, P.; Ruíz Ramírez, J.C.; Paz, A.; Munoz, P.; Ortega, M.A.; Álvarez-Mon, M.; Farré-Alegre, J. Follow-up for More than 10 Years of Patients with Peritoneal Metastases Treated with Cytoreductive Surgery + Hyperthermic Intraperitoneal Chemotherapy in a Specialized Unit. J. Clin. Med. 2024, 13, 297. https://doi.org/10.3390/jcm13010297
Fernández-Candela A, Bretcha-Boix P, Ruíz Ramírez JC, Paz A, Munoz P, Ortega MA, Álvarez-Mon M, Farré-Alegre J. Follow-up for More than 10 Years of Patients with Peritoneal Metastases Treated with Cytoreductive Surgery + Hyperthermic Intraperitoneal Chemotherapy in a Specialized Unit. Journal of Clinical Medicine. 2024; 13(1):297. https://doi.org/10.3390/jcm13010297
Chicago/Turabian StyleFernández-Candela, Alba, Pedro Bretcha-Boix, Juan Carlos Ruíz Ramírez, Alejandro Paz, Paula Munoz, Miguel A. Ortega, Melchor Álvarez-Mon, and José Farré-Alegre. 2024. "Follow-up for More than 10 Years of Patients with Peritoneal Metastases Treated with Cytoreductive Surgery + Hyperthermic Intraperitoneal Chemotherapy in a Specialized Unit" Journal of Clinical Medicine 13, no. 1: 297. https://doi.org/10.3390/jcm13010297
APA StyleFernández-Candela, A., Bretcha-Boix, P., Ruíz Ramírez, J. C., Paz, A., Munoz, P., Ortega, M. A., Álvarez-Mon, M., & Farré-Alegre, J. (2024). Follow-up for More than 10 Years of Patients with Peritoneal Metastases Treated with Cytoreductive Surgery + Hyperthermic Intraperitoneal Chemotherapy in a Specialized Unit. Journal of Clinical Medicine, 13(1), 297. https://doi.org/10.3390/jcm13010297









