Abstract
Background: Vertebral osteomyelitis (VO) often necessitates surgical intervention due to bone loss-induced spinal instability. Anterior column reconstruction, utilizing expandable vertebral body replacement (VBR) implants, is a recognized approach to restore stability and prevent neurological compromise. Despite various techniques, clinical evidence regarding the safety and efficacy of these implants in VO remains limited. Methods: A retrospective cohort analysis, spanning 2000 to 2020, was conducted on 24 destructive VO cases at a Level 1 orthopedic trauma center. Diagnosis relied on clinical, radiological, and microbiological criteria. Patient demographics, clinical presentation, surgical interventions, and radiological outcomes were assessed. Results: The study included 24 patients (62.5% male; mean age 65.6 ± 35.0 years), with 58% having healthcare-associated infections (HAVO). The mean radiological follow-up was 137.2 ± 161.7 weeks. Surgical intervention significantly improved the bi-segmental kyphotic endplate angle (BKA) postoperatively (mean −1.4° ± 13.6°). However, a noticeable loss of correction was observed over time. The study reported a mortality rate of 1/24. Conclusions: Anterior column reconstruction using expandable VBR effectively improved local spinal alignment in destructive VO. However, the study underscores the necessity for prolonged follow-up and continuous research to refine surgical techniques and postoperative care. Addressing long-term complications and refining surgical approaches will be pivotal as the field progresses.
1. Introduction
Vertebral osteomyelitis (VO) is the most common manifestation of osteomyelitis in the adult population with an increasing incidence rate [1,2]. A feared complication is spinal instability due to progressive bone loss of the infected vertebral bodies (VB) [3]. However, VO requires complete surgical debridement only in chosen, severe cases [4]. In most other cases, conservative treatment, or a limited surgical regime is sufficient. In cases in which surgery is indicated, it has been suggested to significantly reduce pain, enhance neurologic function, and result in a high percentage of patients going back to their previous functional/work status [5]. In these cases, segmental stabilization with dorsal instrumentation and the option of interbody fusion, combined with systemic antibiotic therapy, usually is the therapy of choice [6,7]. Indications for surgical treatment of pyogenic spondylodiscitis are sepsis, an epidural abscess, neurological deficits/complications, and instabilities/deformities in the affected motion segment, which are also included in current classification systems [8]. Preservation of vertebral body integrity, development of spinal deformities, refractory back pain, inadequate patient compliance, and failure of conservative therapy are considered relative surgical indications. Segmental kyphosis >15°, vertebral body loss >50%, and/or translation >5 mm are considered instability criteria [9,10]. Combined posterior–anterior surgery is considered in cases of large anterior defects. It has been demonstrated that a better reconstruction of the sagittal profile could be achieved with posterior–anterior stabilization in comparison to posterior only constructs [11]. The indication for the posterior–anterior stabilization [12] mainly depends on the clinical course, radiological parameters for segmental instability, and on the experience of the treating surgeons [13,14]. An autologous iliac bone crest can be used to restore the ventral load-bearing column. This has the disadvantage of donor site morbidity and the risk of subsequent graft failure due to collapse of the bone chip. Alternatively, cages filled with autologous bone or, in the case of larger defects, (expandable) vertebral body replacement implants can be used. The titanium cage is considered the gold standard, although polyetheretherketone (PEEK) cages show comparable results in the medium term [15]. Expandable vertebral body replacement (VBR) implants have been shown to be an effective alternative for bone blocks and cages [16,17,18,19]. Data on restoring the bi-segmental kyphotic endplate angle (BKA) in VO patients are scarce, despite the fact that it has been demonstrated that they are effective in supplying primary stability. It is noteworthy that reports have been made of cage subsidence and loss of ventral support over time [20,21]. The ensuing kyphotic malalignment may also cause subsequent neurological signs and diseases, impairing spinal function [22,23].
This study aimed to examine the safety and radiological outcome of posterior–anterior treatment with anterior column reconstruction of destructive vertebral osteomyelitis at the thoracolumbar spine with an expandable VBR (ObeliscTM, Ulrich Medical, Ulm, Germany).
2. Materials and Methods
This retrospective study conducted at a Level 1 orthopedic trauma center in Germany focused on patients with spondylodiscitis who underwent vertebral body replacement (VBR) between 1 January 2000 and 31 December 2020. The evaluation considered three time points: pre-operation, post-operation, and the final follow-up, ensuring a minimum follow-up period of 6 weeks.
2.1. Patient Selection and Characterization
Patients eligible for this study were those aged 18 years or older diagnosed with vertebral osteomyelitis (VO) per ICD-10 codes: M46.2 (osteomyelitis of vertebra), M46.3 (infection of the intervertebral disc, pyogenic), M46.4 (discitis, unspecified), and M46.5 (other infective spondylopathies). The cases were meticulously screened, and the diagnosis was confirmed by compatible clinical features, radiological evidence in CT and/or MRI, and microbiologic demonstration of bacterial pathogens.
The study differentiated between healthcare-associated vertebral osteomyelitis (HAVO) and community-acquired vertebral osteomyelitis (CAVO) [24,25]. HAVO was identified if symptoms developed a month after hospitalization without prior evidence of VO, or if there was a hospital admission or outpatient diagnostic or therapeutic manipulation six months before symptom onset. If none of these criteria were met, VO cases were classified as CAVO.
The inclusion criterion was patients with vertebral osteomyelitis in the thoracolumbar spine who were treated with a VBR implant and had at least two radiological follow-ups after surgery, with the latter one after a minimum follow-up period of 6 weeks. Exclusion criteria were patients under 18, those with non-operative treatment or surgical treatment other than an expandable VBR, and those with incomplete radiological follow-up. Given the retrospective nature of the data-set, VO patients with heterogeneous infection courses were indicated for ventral column reconstruction. In general, we consider vertebral body replacement for defects encompassing 50% or more of the vertebral body, progressive osteolysis, persistent symptoms despite dorsal instrumentation and antibiotic therapy, and cases with severe local sagittal deformity. The indication primarily depended on the individual patient’s situation.
2.2. Data Collection and Ethics
Data were retrospectively collected, focusing on patient demographics, injury mechanism, neurological status, treatment details, and microbiological details on the causative pathogens and treatments. Septic patients admitted to the Intensive Care Unit were classified according to the Sequential Organ Failure Assessment score. The study adhered to the Declaration of Helsinki and was approved by the University of Regensburg’s ethics committee (Number: 12-218_2-101 09/2021).
2.3. Radiological Assessment
Radiological assessment involved pre- and postoperative CT scans and X-rays for surgical planning and implant verification, with subsequent X-rays conducted at least 6 weeks post-surgery. The evaluation utilized the bi-segmental kyphotic endplate angle (BKA), visualized in Figure 1, to measure medio-lateral X-rays. In the assessment, BKA values below zero denote kyphosis, while values above zero denote lordosis. In a subset analysis, we separately evaluated the BKA at the thoracic spine (T1-T10), thoracolumbar transition (T11-L2), and the lumbar spine (L3-L5). Fusion at the final follow-up was assessed using the Bridwell [26] classification system. Briefly, the evaluation of fusion rates was conducted via lateral X-ray examination, where the absence of radiolucency, lack of bone sclerosis, and presence of bridging trabecular bone within the fusion area were assessed. Additionally, the observation of screw loosening or implant displacement indicated the presumption of insufficient fusion.
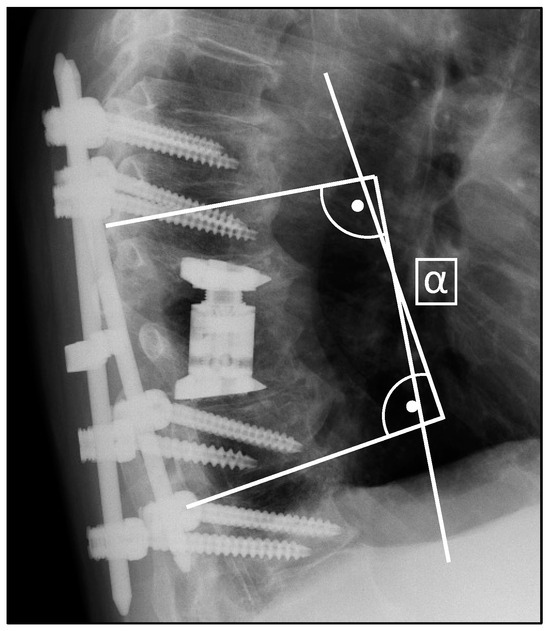
Figure 1.
Measurement of the bi-segmental kyphotic endplate angle (BKA) in medio-lateral X-rays.
2.4. Statistical Analysis
Statistical analysis was conducted using SPSS software, version 28. Tests, including Mann–Whitney U, Kruskal–Wallis, and independent t-tests, were performed as appropriate. The associations between implant specifications and the loss of correction were assessed using Pearson correlation analysis. p-values < 0.05 were deemed statistically significant. Continuous variables are presented as mean ± standard deviation, and categorical data as frequencies.
3. Results
The study included 24 patients (Figure 2), with a male predominance (62.5%) and a mean age of 65.6 ± 35.0 years. The average BMI was 29.5 ± 6.3. Symptoms had been present for 71.0 ± 46.3 days on average before hospitalization. Healthcare-associated infections (HAVO) were identified in 58% of cases (Table 1), with 54.2% potentially being iatrogenic. The mean hospital stay was 33.2 ± 22.3 days, and 75.0% (18/24) of patients required ICU (Intensive Care Unit) admission, with an average ICU (Intensive Care Unit) stay of 3.3 ± 3.4 days. A third of the patients (33.3%) developed sepsis during their hospitalization, according to the reviewed diagnoses in the patient charts. Initial CRP levels upon admission were high at 149.3 ± 11.2 mg/L, decreasing to a mean of 83.6 ± 9.7 mg/L by the end of the hospitalization. Leukocyte counts increased slightly from an initial mean of 9.4 ± 3.5 × 109/L to a final mean of 10.0 ± 7.5 × 109/L.
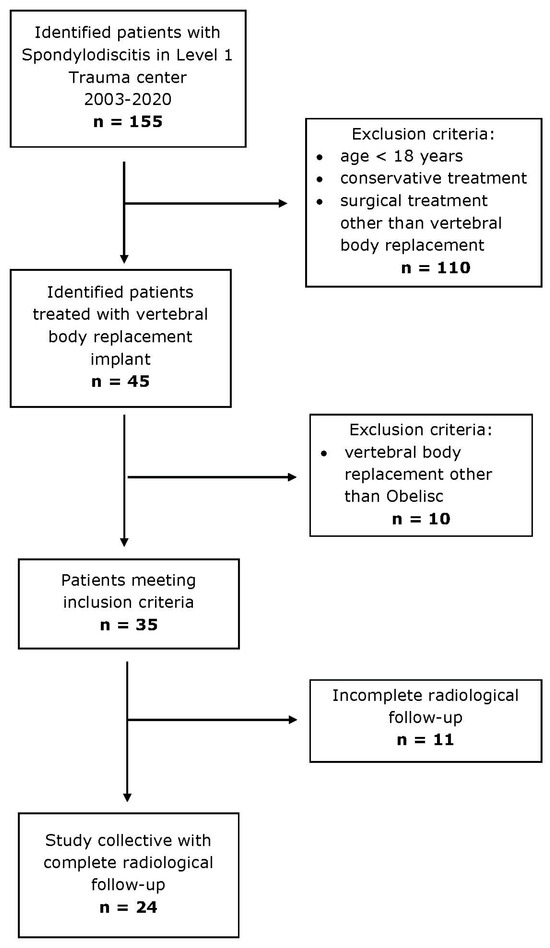
Figure 2.
Inclusion and exclusion criteria of study cohort.

Table 1.
Baseline statistics of study cohort.
Six (25.0%) patients had a paravertebral abscess, eight (33.3%) had a psoas abscess, and three (12.5%) had an epidural abscess. Neurological complications were also present: eight (33.3%) patients had paresis, two (8.3%) had hyposensitivity, and two (8.3%) had paresthesia. Six (25.0%) patients experienced a spinal cord injury. Additionally, 19 patients (79.2%) reported back pain.
An acute fulminant septic course was observed in eight cases (33.4%). With Staphylococcus aureus being the most isolated pathogen (n = 10, 41.7%), Streptococcus species (n = 1, 4.2%) and Enterococcus (n = 1, 4.2%) were detected in patients’ microbiological samples. In 12 cases, no pathogen could be isolated. The most frequent comorbidity was diabetes mellitus (n = 10, 41.7%), followed by congestive heart failure (n = 7, 29.2%). Perioperative abscess formation occurred in 15 cases (62.5%), with percutaneous drainage performed in 11 cases. All patients received empirical broad-spectrum antibiotic therapy, which was subsequently adjusted according to antibiotic susceptibility testing in cases where pathogens were identified. Nine patients were treated with antibiotic monotherapy, while the remaining patients received combination antibiotic regimens, including the addition of rifampicin in five cases, among other variations. The mean duration of antibiotic therapy was 62.4 ± 28 days.
Among the cohort, 22 (91.6%) cases underwent dorsal instrumentation. The reconstruction of the anterior column and implantation of the VBR was conducted using a thoracoscopic approach in n = 11 cases (45.8%) and a lumbotomy in n = 4 (16.7%) cases, whereas in n = 9 (37.5%) cases an isolated dorsal approach was used. In 20 cases (83.3%), implants with 0° angulation base- and endplates were used, while in two cases (8.3%) 5° and 10° angulations were utilized, respectively. A total of seven different VBR sizes were utilized, ranging from 17–23 mm to 40–62 mm.
A total of 17 patients underwent a one-staged procedure, and in seven cases a two-staged procedure was performed. The mean surgical duration for the reconstruction of the anterior column was calculated at 155.5 ± 65 min. Complications were observed in six patients (25.0%), encompassing a dislocation of the VBR implant in one case (4.2%), material irritation in three cases (12.5%), postoperative hematoma in one case (4.2%), and screw dislocation in one case (4.2%). Among these cases, five (20.8%) required subsequent revision surgeries to address the identified complications. In the study population, material irritation manifested as localized discomfort and pain at the site of the implanted dorsal instrumentation material, while VBR dislocation, observed radiographically during follow-up, did not exhibit any clinical symptoms. However, it necessitated implant removal due to a high risk of potential further damage. The time to failure, defined as the need for revision surgery, was recorded at an average of 280.5 ± 386.8 days (as shown in Table 2). The in-hospital mortality rate was observed to be 4.2% (n = 1). Recurrent infection related to the VBR was not recorded during the follow-up period.

Table 2.
Complications and revisions after surgical implantation of VBR.
Radiological Outcome
The mean radiological follow-up was after 137.2 ± 161.7 weeks with a minimum follow-up of 6 weeks. Examining the entire cohort initially, we found a mean preoperative BKA of −7.3° (±17.9°). Post-surgery, a significant correction in the BKA was observed, demonstrated by a postoperative mean of −1.4° (±13.6°; p = 0.023). At the follow-up, we observed a decrease of the BKA to a mean of −8.3° (±14.4°), indicating a significant loss of surgical correction over time by 8.7 ± 7.7° (p < 0.000, Figure 3).
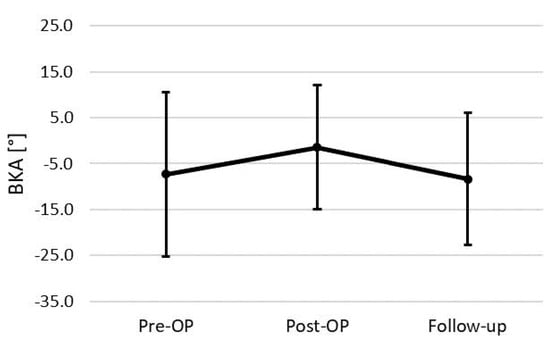
Figure 3.
BKA changes of the total cohort (n = 24).
For the thoracic spine subset of patients, the preoperative mean BKA was −19.3 ± 12.7°, showing a more pronounced kyphotic deformity than the overall cohort. Post-surgery, a correction was noted with a postoperative mean BKA of −10.8 ± 8.6° (p = 0.123). By the time of follow-up, the mean BKA had decreased to −17.8 ± 11.4°, reflecting a significant loss of correction by 8.3 ± 7.1° (p = 0.021; Figure 4A).
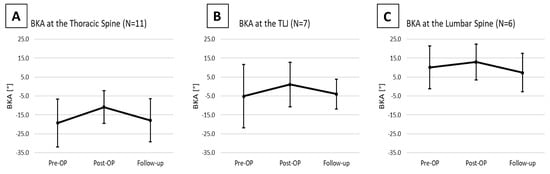
Figure 4.
Changes in the BKA in the Thoracic Spine (A), the Thoracolumbar junction (B), and the Lumbar Spine (C).
At the thoracolumbar junction the preoperative mean BKA was −5.1 ± 16.7°. Postoperative measurements indicated a corrected mean of 1.1 ± 11.7°, yet the change did not reach statistical significance (p = 0.245). By the time of follow-up, the mean BKA was −4.0 ± 7.8°, implying loss of correction by 11.1 ± 10.1° (p = 0.067, Figure 4B).
At the lumbar spine, the preoperative mean BKA was 10.1° ± 11.2°. Postoperatively, there was an observed correction to a postoperative mean of 12.9 ± 9.4°, showing a correction by 4.1 ± 3.4° (p = 0.210). At follow-up, the mean BKA decreased to 7.4 ± 10.2°, demonstrating a loss of correction by 6.8 ± 6.1° (p = 0.109; Figure 4C).
We did not detect a correlation between the VBR size and the amount of loss of correction (r = 0.178; p = 0.400). There was a medium positive correlation between the used VBR base-and endplate angulation and the loss of correction at the final follow-up (r = 0.513; p < 0.05). A subsidence rate of 5/24 was documented in patients but did not exhibit a significant correlation with changes in the BKA.
In 18 of 24 patients, we were able to assess the bony consolidation progress using the Bridwell classification [26]. Among those 18 patients, we observed varying progress in fusion at the follow-up period. Specifically, 4.2% of the patients were classified as Bridwell stage 1, 58.4% as Bridwell stage 2, 8.4% as Bridwell stage 3 and 4.2% as stage 4 (as shown in Table 2).
4. Discussion
The present study provides a comprehensive analysis of the surgical treatment and vertebral body replacement in patients with VO, focusing on the thoracolumbar spine. Our findings suggest that surgical intervention, specifically posterior–anterior treatment with anterior column reconstruction using an expandable VBR (ObeliscTM, Ulrich Medical, Ulm, Germany), can significantly improve the bi-segmental kyphotic endplate angle (BKA) postoperatively. However, a significant loss of this surgical correction was observed over time, indicating the potential for long-term complications, cage subsidence and the need for further research and improvement in surgical techniques and postoperative care.
Our findings align with previous studies that have highlighted the effectiveness of surgical intervention in spondylodiscitis cases. For instance, a study by Kehrer et al. reported that surgical intervention could significantly reduce pain, enhance neurological function, and enable a high percentage of patients to return to their previous functional or work status [27]. Similarly, our study found that surgical intervention could significantly improve the BKA postoperatively, suggesting an improvement in spinal alignment and potentially reducing pain and enhancing neurological function. However, this study did not include a detailed clinical follow-up. It has been recently demonstrated that spondylodiscitis patients show acceptable but impaired patient-reported outcome measurements, regardless the therapy strategy [15]. A recent study by Neuhoff et al. demonstrated a sagittal correction of 10° (range 0–54°) in a cohort of 100 patients with spinal infections treated via vertebral body replacements. This aligns with our study’s results, which indicated an approximate average 6° correction in the BKA [28].
Our study also revealed a significant loss of surgical correction over time, as evidenced by the decrease in the BKA at follow-up. This finding is consistent with previous reports of cage subsidence and loss of ventral support over time in thoracolumbar vertebral body replacement [19,29]. The ensuing kyphotic malalignment may also cause subsequent neurological impairment, limiting spinal function [22,23]. This suggests that while surgical intervention can provide immediate relief and improvement, long-term complications may arise.
In the cohort studied by Neuhoff et al., 31 patients were followed up for more than one year. They found that the main causes for revision surgery were wound healing disorders (12%) and implant failure (11%), with specific complications including posterior pedicle screw loosening (8%) and anterior cage subsidence (3%) [28]. Aseptic mechanical complications were more common in longer pedicle-screw constructs, occurring significantly less in shorter constructs (0–4 levels). In comparison, 6/24 patients in our study experienced complications, including VBR implant dislocation (n = 1), material irritation (n = 3), postoperative hematoma (n = 1), and screw dislocation (n = 1), leading to revision surgeries in five cases. The average time until the unplanned revision surgery was 280.5 days. A subsidence rate of 5/24 was documented in patients but did not exhibit a significant correlation with changes in the BKA. A subsidence rate of 5 out of 24 was noted, but this did not show a significant association with alterations in the BKA during the overall follow-up period. The study’s findings underscore the complexity of managing VO, particularly within the thoracolumbar region, and depending on the complexity of the surgical intervention, due to the intricate interplay of anatomical, biomechanical, and infectious factors.
The choice of surgical intervention for anterior column reconstruction using an expandable VBR represents a strategic approach to address the multifaceted challenges posed by VO. However, it is important to acknowledge that surgical management in this context demands meticulous patient selection, thorough preoperative planning, and a nuanced assessment of risk factors to ensure optimal outcomes [2].
Furthermore, the noted decrease in the BKA over time highlights the ongoing biomechanical challenges associated with vertebral body replacement. Darwich et al. showed overall low complication rates and a good functional outcome within their cohort treated with VBR, but also that significant height gain was associated with higher complication rates [30]. While immediate improvements in spinal alignment are evident, the long-term biomechanical implications require continuous scrutiny. The interplay between surgical correction, fusion, and load distribution underscores the importance of biomechanical studies that provide insights into the longevity and sustainability of surgical outcomes.
Additionally, a reported mortality rate of 4.2% and septic complications in one third of the cohort indicate the potential severity of VO and its associated challenges. This underlines the importance of early diagnosis, timely intervention, and comprehensive management to mitigate adverse outcomes [2,31].
In analyzing our results, the study revealed a statistically significant improvement in the bi-segmental kyphotic endplate angle (BKA) postoperatively across the entire cohort, emphasizing the effectiveness of posterior–anterior treatment with anterior column reconstruction using an expandable VBR. However, the observed long-term loss of surgical correction underscores the need for continuous scrutiny and further research to address potential complications such as cage subsidence, contributing to the ongoing biomechanical challenges associated with vertebral body replacement [32,33].
Limitations
An important limitation of our study is the unavailability of longstanding X-rays for all the cases to assess global spinal alignment. In our study, follow-up X-rays were conducted with the patient standing upright. However, we did not apply X-rays of the whole spine because global deformity correction was not the main surgical goal for the mostly severely ill patients, but rather local reconstruction and stabilization. It is worth noting that comparison between the pre- and postoperative kyphotic angle might partially be influenced by the difference of patient positioning [34]. While the preoperative CT was conducted with the patient in supine position, the postoperative follow-up X-rays were taken with the patient standing upright. Additionally, beyond these considerations, 11 patients were excluded from the analysis because of incomplete radiological follow-up. The reported follow-up time of 137 ± 161.7 weeks exhibits a notably uneven distribution. This asymmetry in follow-up duration is a relevant consideration that may have influenced the interpretation of the radiographic results. Another limitation is that our retrospective design restricts our study’s capability to thoroughly analyze the multifaceted factors, including heterogeneous infection courses in VO patients, that influenced the decision for patients to undergo either one- or two-staged procedures. Generally, the two-staged procedure is selected for patients with multimorbidity, as it allows for a more cautious surgical approach, minimizing operative time and blood loss. Furthermore, it is worth emphasizing that a more comprehensive understanding of the reasons behind the loss of correction is desirable, for example through a comparison with another patient group. This could be achieved through the implementation of a prospectively designed study, which might also include an evaluation of the height of the VBR in correlation to postoperative outcomes. Such an approach could provide valuable insights into optimizing patient care and surgical strategies in the future. Additionally, the evaluation of the role of osteoporosis, with VBR combined allo- or autografts as well as local antibiotics, should be taken into consideration.
In light of the retrospective design and limited sample size acknowledged in our study, it is imperative to recognize that our findings offer a valuable mid-term analysis of surgical interventions for vertebral osteomyelitis in the thoracolumbar spine. While the study’s limitations necessitate careful consideration, the observed biomechanical challenges and long-term complications underscore the importance of ongoing research and refinement in surgical techniques and postoperative care to optimize patient outcomes in this complex clinical scenario. Also, due to the retrospective design of our study, a notable limitation is the absence of an assessment of functional outcomes or other patient-associated symptoms, which could have provided a more comprehensive understanding of the clinical impact of the procedures. The potential influence of patient positioning on the comparison between pre- and postoperative kyphotic angles emphasizes the importance of standardized imaging protocols in future studies, with a recommendation for consistent positioning, such as standing lateral radiographs, to ensure more accurate and reliable measurements.
While the study has certain limitations inherent to its retrospective design and small sample size, its comprehensive analysis, clinical relevance, and focus on mid-term considerations contribute valuable insights into the outcomes of surgical interventions for VO. The findings provide a foundation for further research and optimization of treatment strategies to improve patient outcomes in this challenging clinical scenario.
5. Conclusions
In conclusion, the study’s radiological outcomes highlight the promising potential of surgical interventions in the management of spondylodiscitis, specifically within the thoracolumbar spine. The immediate benefits in spinal alignment improvement need to be balanced with a keen awareness of potential long-term complications and the imperative for ongoing research to refine surgical techniques and postoperative care. As the field advances, collaborative efforts that integrate biomechanical, clinical, and microbiological insights will be essential in optimizing treatment strategies and enhancing patient outcomes in this complex clinical scenario.
Author Contributions
L.K.: Conceptualization, resources, supervision, investigation, data curation, writing. M.E.: Formal analysis, writing—original draft preparation, writing—review and editing. L.H.: Supervision, writing—original draft preparation, writing—review and editing. M.R. (Moritz Riedl): Investigation, data curation, writing—original draft preparation, writing. M.S.: Formal analysis, writing—original draft preparation, writing—review and editing. M.R. (Markus Rupp): Writing—original draft preparation, writing—review and editing. V.A.: supervision, writing—original draft preparation, writing—review and editing. M.K.: Conceptualization, methodology, supervision, statistical analysis. S.L.: Conceptualization, resources, supervision, investigation, data curation, writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study adhered to the Declaration of Helsinki and was approved by the University of Regensburg’s ethics committee (Number: 12-218_2-101 09/2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Conan, Y.; Laurent, E.; Belin, Y.; Lacasse, M.; Amelot, A.; Mulleman, D.; Rosset, P.; Bernard, L.; Grammatico-Guillon, L. Large increase of vertebral osteomyelitis in France: A 2010–2019 cross-sectional study. Epidemiol. Infect. 2021, 149, e227. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Walter, N.; Schindler, M.; Baertl, S.; Szymski, D.; Loibl, M.; Alt, V.; Rupp, M. The Epidemiology of Spondylodiscitis in Germany: A Descriptive Report of Incidence Rates, Pathogens, In-Hospital Mortality, and Hospital Stays between 2010 and 2020. J. Clin. Med. 2023, 12, 3373. [Google Scholar] [CrossRef] [PubMed]
- Schömig, F.; Li, Z.; Perka, L.; Vu-Han, T.-L.; Diekhoff, T.; Fisher, C.G.; Pumberger, M. Georg schmorl prize of the German spine society (DWG) 2021: Spinal Instability Spondylodiscitis Score (SISS)—A novel classification system for spinal instability in spontaneous spondylodiscitis. Eur. Spine J. 2022, 31, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Pola, E.; Pambianco, V.; Autore, G.; Cipolloni, V.; Fantoni, M. Minimally invasive surgery for the treatment of thoraco lumbar pyogenic spondylodiscitis: Indications and outcomes. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.G.; Buchholz, A.L.; Sure, D.R.; Buell, T.J.; Nguyen, J.H.; Chen, C.-J.; Diamond, J.M.; Washburn, P.A.; Harrop, J.; Shaffrey, C.I.; et al. Presentation and Outcomes After Medical and Surgical Treatment Versus Medical Treatment Alone of Spontaneous Infectious Spondylodiscitis: A Systematic Literature Review and Meta-Analysis. Glob. Spine J. 2018, 8, 49S–58S. [Google Scholar] [CrossRef] [PubMed]
- Nasto, L.A.; Colangelo, D.; Mazzotta, V.; Di Meco, E.; Neri, V.; Nasto, R.A.; Fantoni, M.; Pola, E. Is posterior percutaneous screw-rod instrumentation a safe and effective alternative approach to TLSO rigid bracing for single-level pyogenic spondylodiscitis? Results of a retrospective cohort analysis. Spine J. 2014, 14, 1139–1146. [Google Scholar] [CrossRef]
- Rutges, J.P.H.J.; Kempen, D.H.; van Dijk, M.; Oner, F.C. Outcome of conservative and surgical treatment of pyogenic spondylodiscitis: A systematic literature review. Eur. Spine J. 2015, 25, 983–999. [Google Scholar] [CrossRef]
- Almansour, H.; Pepke, W.; Akbar, M. Pyogenic spondylodiscitis. Orthopäde 2019, 49, 482–493. [Google Scholar] [CrossRef]
- Dietze, D.D.; Fessler, R.G.; Jacob, R.P. Primary reconstruction for spinal infections. J. Neurosurg. 1997, 86, 981–989. [Google Scholar] [CrossRef]
- Fleege, C.; Wichelhaus, T.; Rauschmann, M. Systemische und lokale Antibiotikatherapie bei konservativ und operativ behandelten Spondylodiszitiden. Orthopäde 2012, 41, 727–735. [Google Scholar] [CrossRef]
- von der Hoeh, N.H.; Voelker, A.; Hofmann, A.; Zajonz, D.; Spiegl, U.A.; Jarvers, J.-S.; Heyde, C.-E. Pyogenic Spondylodiscitis of the Thoracic Spine: Outcome of 1-Stage Posterior Versus 2-Stage Posterior and Anterior Spinal Reconstruction in Adults. World Neurosurg. 2018, 120, e297–e303. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Loibl, M.; Neumann, C.; Alt, V. Einsatz der videoassistierten Thorakoskopie bei der dorsoventralen Stabilisierung einer osteodestruktiven pyogenen Spondylodiszitis der Brustwirbelsäule. Unfallchirurg 2021, 124, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Rupp, M.; Hanses, F.; Neumann, C.; Loibl, M.; Alt, V. Infektionen der Wirbelsäule. Unfallchirurg 2021, 124, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Blecher, R.; Frieler, S.; Qutteineh, B.; Pierre, C.A.; Yilmaz, E.; Ishak, B.; Von Glinski, A.; Oskouian, R.J.; Kramer, M.; Drexler, M.; et al. Who Needs Surgical Stabilization for Pyogenic Spondylodiscitis? Retrospective Analysis of Non-Surgically Treated Patients. Glob. Spine J. 2021, 13, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Walter, N.; Froemming, A.; Baertl, S.; Szymski, D.; Alt, V.; Rupp, M. Long-term patient-related quality of life outcomes and ICD-10 symptom rating (ISR) of patients with pyogenic vertebral osteomyelitis: What is the psychological impact of this life-threatening disease? Eur. Spine J. 2023, 32, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Schnake, K.J.; Stavridis, S.I.; Kandziora, F. Five-year clinical and radiological results of combined anteroposterior stabilization of thoracolumbar fractures. J. Neurosurg. Spine 2014, 20, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Knop, C.; Blauth, M.; Bühren, V.; Arand, M.; Egbers, H.J.; Hax, P.M.; Wentzensen, A. Surgical treatment of injuries of the thoracolumbar tran-sition—3: Follow-up examination. Results of a prospective multi-center study by the “Spinal” Study Group of the German Society of Trauma Surgery. Unfallchirurg 2001, 104, 583–600. [Google Scholar] [CrossRef]
- Kreinest, M.; Schmahl, D.; Grützner, P.A.; Matschke, S. Radiological Results and Clinical Patient Outcome After Implantation of a Hydraulic Expandable Vertebral Body Replacement following Traumatic Vertebral Fractures in the Thoracic and Lumbar Spine: A 3-Year Follow-Up. Spine 2017, 42, E482–E489. [Google Scholar] [CrossRef]
- Lang, S.; Neumann, C.; Schwaiger, C.; Voss, A.; Alt, V.; Loibl, M.; Kerschbaum, M. Radiological and mid- to long-term patient-reported outcome after stabilization of traumatic thoraco-lumbar spinal fractures using an expandable vertebral body replacement implant. BMC Musculoskelet. Disord. 2021, 22, 744. [Google Scholar] [CrossRef]
- Schmieder, K.; Wolzik-Grossmann, M.; Pechlivanis, I.; Engelhardt, M.; Scholz, M.; Harders, A. Subsidence of the Wing titanium cage after anterior cervical interbody fusion: 2-year follow-up study. J. Neurosurg. Spine 2006, 4, 447–453. [Google Scholar] [CrossRef]
- Briem, D.; Lehmann, W.; Ruecker, A.; Windolf, J.; Rueger, J.; Linhart, W. Factors influencing the quality of life after burst fractures of the thoracolumbar transition. Arch. Orthop. Trauma Surg. 2004, 124, 461–468. [Google Scholar] [CrossRef] [PubMed]
- McLain, R.F. Functional Outcomes After Surgery for Spinal Fractures: Return to Work and Activity. Spine 2004, 29, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Gertzbein, S.D. Neurologic Deterioration in Patients with Thoracic and Lumbar Fractures After Admission to the Hospital. Spine 1994, 19, 1723–1725. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Frömming, A.; Walter, N.; Freigang, V.; Neumann, C.; Loibl, M.; Ehrenschwender, M.; Alt, V.; Rupp, M. Is There a Difference in Clinical Features, Microbiological Epidemiology and Effective Empiric Antimicrobial Therapy Comparing Healthcare-Associated and Community-Acquired Vertebral Osteomyelitis? Antibiotics 2021, 10, 1410. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-H.; Kim, D.Y.; Lee, Y.-M.; Lee, M.S.; Kang, K.-C.; Lee, J.-H.; Park, S.Y.; Moon, C.; Chong, Y.P.; Kim, S.-H.; et al. Selection of an appropriate empiric antibiotic regimen in hematogenous vertebral osteomyelitis. PLoS ONE 2019, 14, e0211888. [Google Scholar] [CrossRef] [PubMed]
- Bridwell, K.H.; Lenke, L.G.; McEnery, K.W.; Baldus, C.; Blanke, K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine 1995, 20, 1410–1418. [Google Scholar] [CrossRef]
- Kehrer, M.; Pedersen, C.; Jensen, T.G.; Lassen, A.T. Increasing incidence of pyogenic spondylodiscitis: A 14-year population-based study. J. Infect. 2014, 68, 313–320. [Google Scholar] [CrossRef]
- Neuhoff, J.; Berkulian, O.; Kramer, A.; Thavarajasingam, S.; Wengert, A.; Schleicher, P.; Pingel, A.; Kandziora, F. Single- and Multilevel Corpectomy and Vertebral body replacement for treatment of spinal infections. A retrospective single-center study of 100 cases. Brain Spine 2024, 4, 102721. [Google Scholar] [CrossRef]
- Thaker, R.A.; Gautam, V.K. Study of Vertebral Body Replacement with Reconstruction Spinal Cages in Dorsolumbar Traumatic and Koch’s Spine. Asian Spine J. 2014, 8, 786–792. [Google Scholar] [CrossRef][Green Version]
- Darwich, A.; Vogel, J.; Dally, F.-J.; Hetjens, S.; Gravius, S.; Faymonville, C.; Bludau, F. Cervical vertebral body replacement using a modern in situ expandable and angulable corpectomy cage system: Early clinical and radiological outcome. Br. J. Neurosurg. 2022, 37, 1101–1111. [Google Scholar] [CrossRef]
- Pluemer, J.; Freyvert, Y.; Pratt, N.; E Robinson, J.; Cooke, J.A.; Tataryn, Z.L.; Godolias, P.; Daher, Z.A.; Oskouian, R.J.; Chapman, J.R. An Assessment of the Safety of Surgery and Hardware Placement in de-novo Spinal Infections. A Systematic Review and Meta-Analysis of the Literature. Glob. Spine J. 2022, 13, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Liebsch, C.; Kocak, T.; Aleinikov, V.; Kerimbayev, T.; Akshulakov, S.; Jansen, J.U.; Vogt, M.; Wilke, H.-J. Thoracic Spinal Stability and Motion Behavior Are Affected by the Length of Posterior Instrumentation After Vertebral Body Replacement, but Not by the Surgical Approach Type: An in vitro Study with Entire Rib Cage Specimens. Front. Bioeng. Biotechnol. 2020, 8, 572. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.; Abd-El-Barr, M.M.; Doppenberg, E.; Suki, D.; Gokaslan, Z.; Mendel, E.; Rao, G.; Rhines, L.D. Initial experience with the use of an expandable titanium cage as a vertebral body replacement in patients with tumors of the spinal column: A report of 95 patients. Eur. Spine J. 2011, 21, 84–92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, E.S.; Ko, C.W.; Suh, S.W.; Kumar, S.; Kang, I.K.; Yang, J.H. The effect of age on sagittal plane profile of the lumbar spine according to standing, supine, and various sitting positions. J. Orthop. Surg. Res. 2014, 9, 11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).