Ranibizumab Modifies the Expression of Metalloproteinases and Their Tissue Inhibitors in Peripheral Blood Mononuclear Cells in Patients with Exudative Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Test Group
2.3. Intravitreal Injections
2.4. Preparation of Material and Molecular Analysis
2.5. Ribonucleic Acid Isolation and Qualitative and Quantitative Evaluation of the Extracts Obtained
2.6. Evaluation of Gene Expression Profile Using Oligonucleotide Microarray Technique
2.7. Reverse Transcription-Quantitative Polymerase Chain Reaction
2.8. Statistical Analysis
3. Results
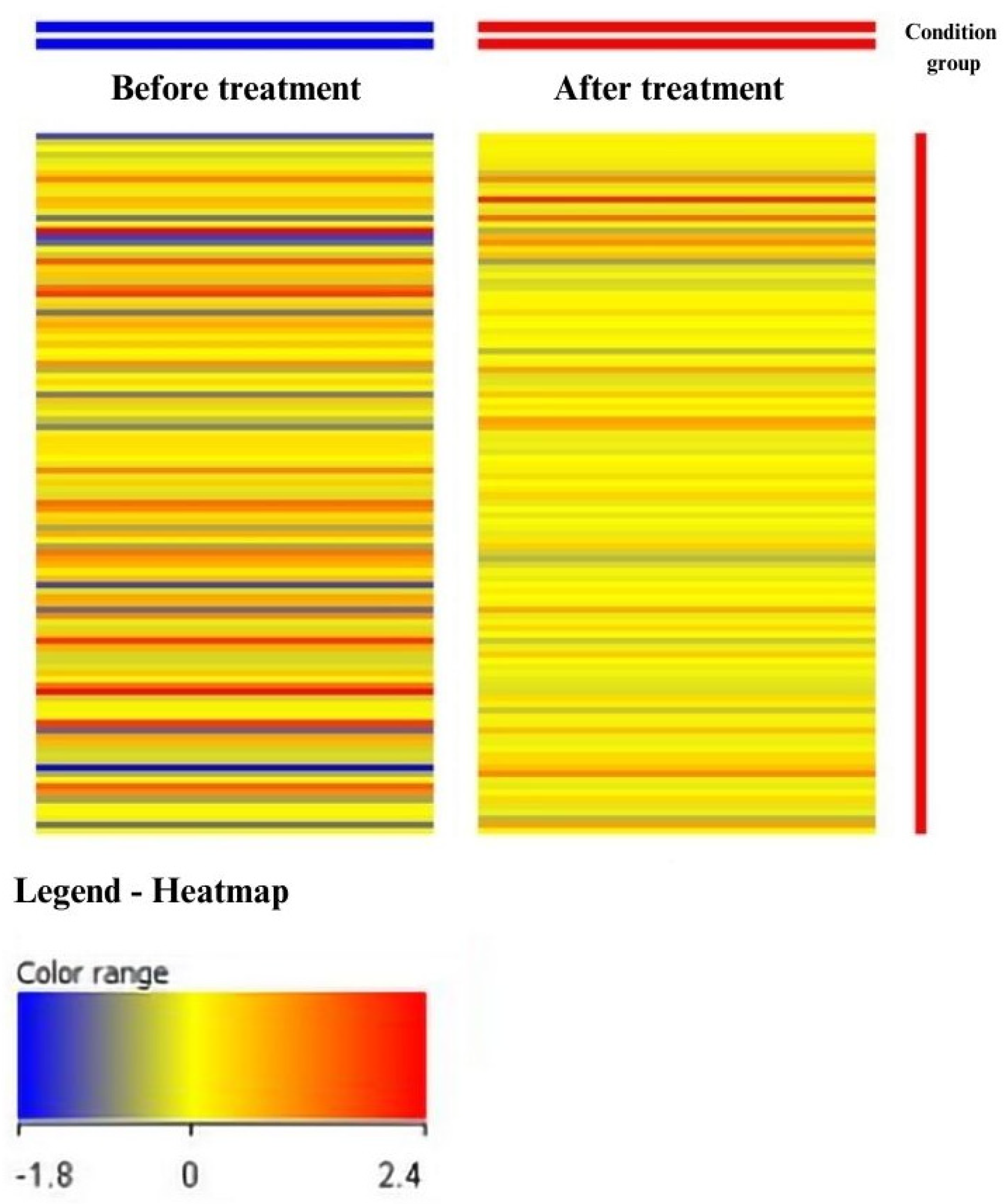
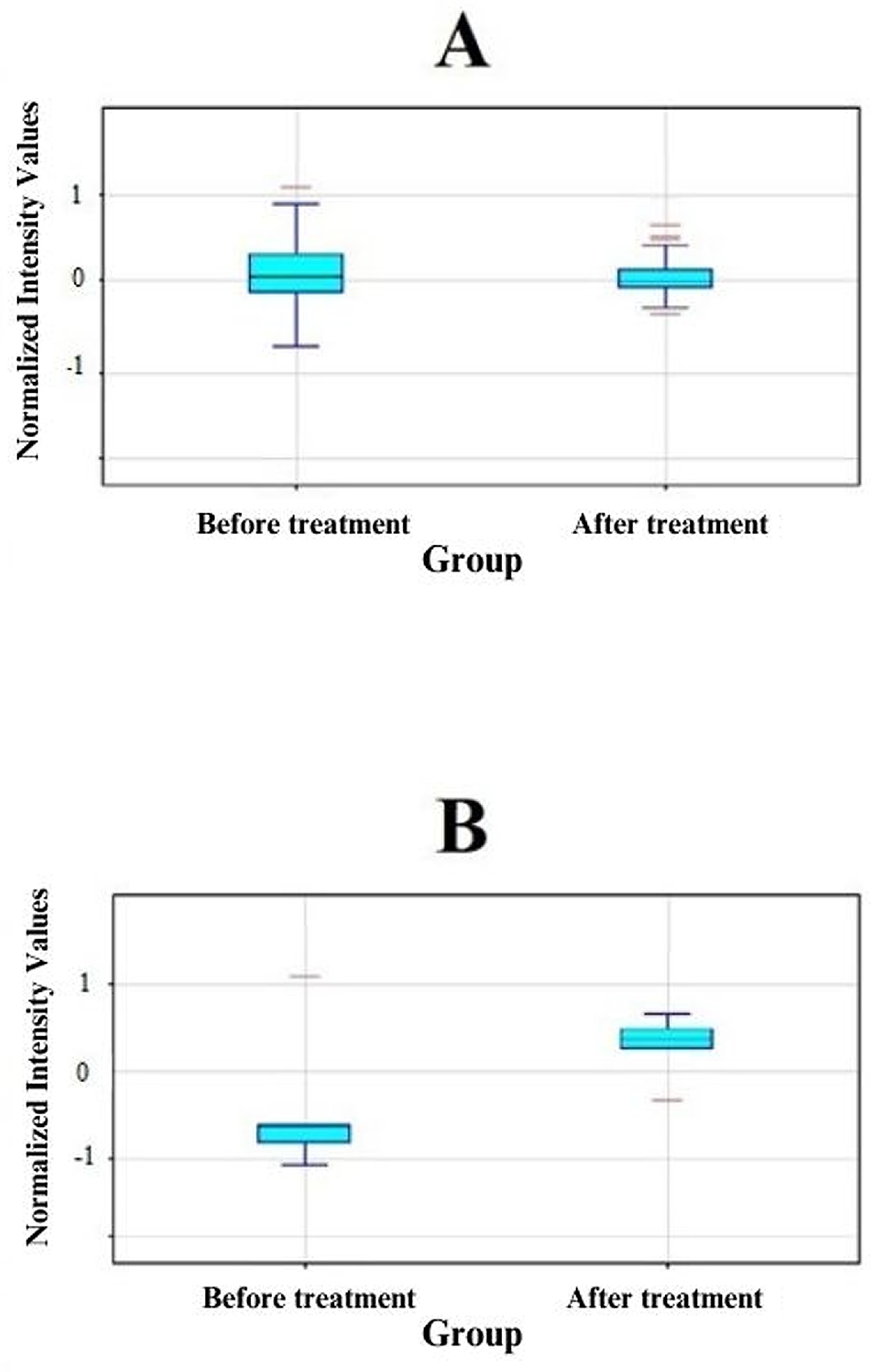
3.1. Analysis of Results Obtained from Oligonucleotide Microarrays
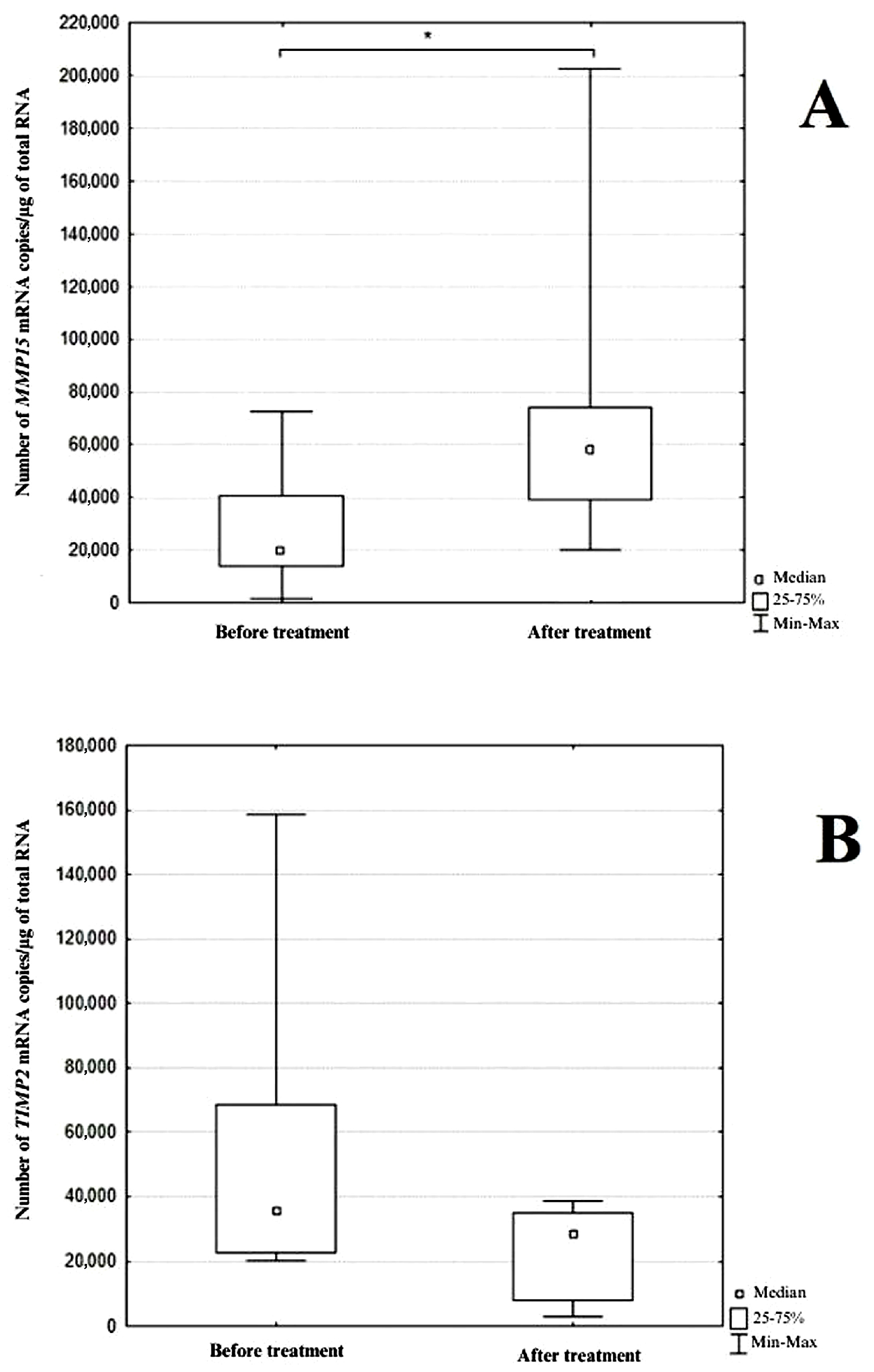
3.2. Analysis of Results Obtained by Real-Time RT-qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Liang, Y.; Xie, J.; Li, D.; Hu, Q.; Li, X.; Zheng, W.; He, R. Conbercept for patients with age-related macular degeneration: A systematic review. BMC Ophthalmol. 2018, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of age-related macular degeneration in Europe: The past and the future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet. Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Teper, S.J.; Nowińska, A.; Figurska, M.; Rękas, M.; Wylęgała, E. The need for treatment of neovascular age-related macular degeneration: A study based on the Polish national registry. Ophthalmol. Ther. 2022, 11, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, A.; Pinkas, J.; Goryński, P.; Jankowski, M. A national registry-based epidemiological study to evaluate 395 646 patients hospitalized due to eye diseases in Poland in 2019. Med. Sci. Monit. 2023, 29, e939351. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Jiang, S.; Gericke, A. Age-related macular degeneration: Role of oxidative stress and blood vessels. Int. J. Mol. Sci. 2021, 22, 1296. [Google Scholar] [CrossRef]
- Nita, M.; Strzałka-Mrozik, B.; Grzybowski, A.; Mazurek, U.; Romaniuk, W. Age-related macular degeneration and changes in the extracellular matrix. Med. Sci. Monit. 2014, 20, 1003–1016. [Google Scholar]
- Cabral, T.; Lima, L.H.; Mello, L.G.M.; Polido, J.; Correa, É.P.; Oshima, A.; Duong, J.; Serracarbassa, P.; Regatieri, C.V.; Mahajan, V.B.; et al. Bevacizumab injection in patients with neovascular age-related macular degeneration increases angiogenic biomarkers. Ophthalmol. Retina 2018, 2, 31–37. [Google Scholar] [CrossRef]
- Amin, M.; Pushpakumar, S.; Muradashvili, N.; Kundu, S.; Tyagi, S.C.; Sen, U. Regulation and involvement of matrix metalloproteinases in vascular diseases. Front. Biosci. 2016, 21, 89–118. [Google Scholar]
- Caban, M.; Owczarek, K.; Lewandowska, U. The role of metalloproteinases and their tissue inhibitors on ocular diseases: Focusing on potential mechanisms. Int. J. Mol. Sci. 2022, 23, 4256. [Google Scholar] [CrossRef]
- Hussain, A.A.; Lee, Y.; Zhang, J.J.; Marshall, J. Disturbed matrix metalloproteinase activity of Bruch’s membrane in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4459–4466. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Chan, M.F.; Werb, Z. Metalloproteinases: A functional pathway for myeloid cells. Microbiol. Spectr. 2016, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Cieplak, P.; Strongin, A.Y. Matrix metalloproteinases—From the cleavage data to the prediction tools and beyond. Biochim. Biophys. Acta. Mol. Cell Res. 2017, 1864, 1952–1963. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, S.; Gamba, P.; Poli, G.; Leonarduzzi, G. Metalloproteinases and metalloproteinase inhibitors in age-related diseases. Curr. Pharm. Des. 2014, 20, 2993–3018. [Google Scholar] [CrossRef] [PubMed]
- Kamei, M.; Hollyfield, J.G. TIMP-3 in Bruch’s membrane: Changes during aging and in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2367–2375. [Google Scholar] [PubMed]
- Deng, Y.; Qiao, L.; Du, M.; Qu, C.; Wan, L.; Li, J.; Huang, L. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 2021, 9, 62–79. [Google Scholar] [CrossRef]
- Michalska-Małecka, K.; Kabiesz, A.; Kimsa, M.W.; Strzałka-Mrozik, B.; Formińska-Kapuścik, M.; Nita, M.; Mazurek, U. Effects of intravitreal ranibizumab on the untreated eye and systemic gene expression profile in age-related macular degeneration. Clin. Interv. Aging 2016, 11, 357–365. [Google Scholar] [CrossRef]
- Strzalka-Mrozik, B.; Stanik-Walentek, A.; Kapral, M.; Kowalczyk, M.; Adamska, J.; Gola, J.; Mazurek, U. Differential expression of transforming growth factor-beta isoforms in bullous keratopathy corneas. Mol. Vis. 2010, 16, 161–166. [Google Scholar]
- Zhao, B.; Wang, M.; Xu, J.; Li, M.; Yu, Y. Identification of pathogenic genes and upstream regulators in age-related macular degeneration. BMC Ophthalmol. 2017, 17, 102. [Google Scholar] [CrossRef]
- Holash, J.; Davis, S.; Papadopoulos, N.; Croll, S.D.; Ho, L.; Russell, M.; Boland, P.; Leidich, R.; Hylton, D.; Burova, E.; et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc. Natl. Acad. Sci. USA 2002, 99, 11393–11398. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yu, D.; Yang, C.; Xia, Q.; Li, W.; Liu, B.; Li, H. The pharmacology study of a new recombinant human VEGF receptor-fc fusion protein on experimental choroidal neovascularization. Pharm. Res. 2009, 26, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, G.; Wang, Y.; Xu, X.; Liu, X.; Tang, S.; Zhang, F.; Zhang, J.; Tang, L.; Wu, Q.; et al. Safety and efficacy of conbercept in neovascular age-related macular degeneration: Results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology 2014, 121, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Khanani, A.M.; Patel, S.S.; Ferrone, P.J.; Osborne, A.; Sahni, J.; Grzeschik, S.; Basu, K.; Ehrlich, J.S.; Haskova, Z.; Dugel, P.U. Efficacy of every four monthly and quarterly dosing of faricimab vs ranibizumab in neovascular age-related macular degeneration: The STAIRWAY phase 2 randomized clinical trial. JAMA Ophthalmol. 2020, 138, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Kunimoto, D.; Ohji, M.; Maturi, R.K.; Sekiryu, T.; Wang, Y.; Pan, G.; Li, X.Y.; Schneider, S.; BAMBOO and CYPRESS Study Groups. Evaluation of abicipar pegol (an Anti-VEGF DARPin therapeutic) in patients with neovascular age-related macular degeneration: Studies in Japan and the United States. Ophthalmic. Surg. Lasers Imaging Retina 2019, 50, e10–e22. [Google Scholar] [CrossRef]
- Bellezza, I. Oxidative stress in age-related macular degeneration: nrf2 as therapeutic target. Front. Pharmacol. 2018, 9, 1280. [Google Scholar] [CrossRef]
- Cheung, C.M.; Wong, T.Y. Is age-related macular degeneration a manifestation of systemic disease? New prospects for early intervention and treatment. J. Intern. Med. 2014, 276, 140–153. [Google Scholar] [CrossRef]
- Acharya, N.R.; Sittivarakul, W.; Qian, Y.; Hong, K.C.; Lee, S.M. Bilateral effect of unilateral ranibizumab in patients with uveitis-related macular edema. Retina 2011, 31, 1871–1876. [Google Scholar] [CrossRef]
- Wu, Z.; Sadda, S.R. Effects on the contralateral eye after intravitreal bevacizumab and ranibizumab injections: A case report. Ann. Acad. Med. Singap. 2008, 37, 591–593. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Nawaz, M.I.; Allegaert, E.; Siddiquei, M.M.; Ahmad, A.; Gikandi, P.; De Hertogh, G.; Opdenakker, G. Differential expression and localization of ADAMTS proteinases in proliferative diabetic retinopathy. Molecules 2022, 27, 5977. [Google Scholar] [CrossRef] [PubMed]
- Ortak, H.; Demir, S.; Ateş, Ö.; Benli, İ.; Söğüt, E.; Sahin, M. The role of MMP2 (-1306C>T) and TIMP2 (-418 G>C) promoter variants in age-related macular degeneration. Ophthalmic. Genet. 2013, 34, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Wride, M.A.; Geatrell, J.; Guggenheim, J.A. Proteases in eye development and disease. Birth Defects Res. C Embryo. Today 2006, 78, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate -phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Shughoury, A.; Sevgi, D.D.; Ciulla, T.A. Molecular genetic mechanisms in age-related macular degeneration. Genes 2022, 13, 1233. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, L.M.; Duncan, G.; Wang, L.; Pennington, C.J.; Edwards, D.R.; Wormstone, I.M. MMP and TIMP expression in quiescent, dividing, and differentiating human lens cells. Investig. Ophthalmol. Vis Sci. 2007, 48, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- West-Mays, J.A.; Pino, G. Matrix metalloproteinases as mediators of primary and secondary cataracts. Expert Rev. Ophthalmol. 2007, 2, 931–938. [Google Scholar] [CrossRef]
- Kim, M.H.; Lim, S.H. Matrix metalloproteinases and glaucoma. Biomolecules 2022, 12, 1368. [Google Scholar] [CrossRef]
- Naj, A.C.; Scott, W.K.; Courtenay, M.D.; Cade, W.H.; Schwartz, S.G.; Kovach, J.L.; Agarwal, A.; Wang, G.; Haines, J.L.; Pericak-Vance, M.A. Genetic factors in nonsmokers with age-related macular degeneration revealed through genome-wide gene-environment interaction analysis. Ann. Hum. Genet. 2013, 77, 215–231. [Google Scholar] [CrossRef]
- Miller, J.W.; Bagheri, S.; Vavvas, D.G. Advances in age-related macular degeneration understanding and therapy. US Ophthalmic Rev. 2017, 10, 119–130. [Google Scholar] [CrossRef]
- Nashine, S.; Cohen, P.; Chwa, M.; Lu, S.; Nesburn, A.B.; Kuppermann, B.D.; Kenney, M.C. Humanin G (HNG) protects age-related macular degeneration (AMD) transmitochondrial ARPE-19 cybrids from mitochondrial and cellular damage. Cell Death Dis. 2017, 8, e2951. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Yashar, B.M.; Hiriyanna, S.; Swaroop, A. Microarray analysis of gene expression in the aging human retina. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2554–2560. [Google Scholar]
- Strzalka-Mrozik, B.; Madej, M.; Kurowska, N.; Kruszniewska-Rajs, C.; Kimsa-Dudek, M.; Adamska, J.; Gola, J.M. Changes in the expression profile of pyroptosis-related genes in senescent retinal pigment epithelial cells after lutein treatment. Curr. Issues Mol. Biol. 2023, 45, 1500–1518. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Park, C.; Barner, J.C. Ranibizumab for age-related macular degeneration: A meta-analysis of dose effects and comparison with no anti-VEGF treatment and bevacizumab. J. Clin. Pharm. Ther. 2014, 39, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, D.; London, N.J.; Khurana, R.N.; Dugel, P.U.; Gune, S.; Hill, L.; Tuomi, L. Ranibizumab treatment for pigment epithelial detachment secondary to neovascular age-related macular degeneration: Post hoc analysis of the HARBOR study. Ophthalmology 2016, 123, 2213–2224. [Google Scholar] [CrossRef] [PubMed]
- Moore, N.A.; Bracha, P.; Hussain, R.M.; Morral, N.; Ciulla, T.A. Gene therapy for age-related macular degeneration. Expert. Opin. Biol. Ther. 2017, 17, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Ahir, A.; Guo, L.; Hussain, A.A.; Marshall, J. Expression of metalloproteinases from human retinal pigment epithelial cells and their effects on the hydraulic conductivity of Bruch’s membrane. Investig. Ophthalmol. Vis. Sci. 2002, 43, 458–465. [Google Scholar]
- Naim, A.; Pan, Q.; Baig, M.S. Matrix metalloproteinases (MMPs) in liver diseases. J. Clin. Exp. Hepatol. 2017, 7, 367–372. [Google Scholar] [CrossRef]
- Pescosolido, N.; Giannotti, R.; Buomprisco, G. Metalloproteinases and eye diseases. Biomed. Pharmacother. 2013, 3, 97–105. [Google Scholar] [CrossRef]
- Macgregor, A.M.; Eberhart, C.G.; Fraig, M.; Lu, J.; Halushka, M.K. Tissue inhibitor of matrix metalloproteinase-3 levels in the extracellular matrix of lung, kidney, and eye increase with age. J. Histochem. Cytochem. 2009, 57, 207–213. [Google Scholar] [CrossRef]
- Ecker, S.M.; Pfahler, S.M.; Hines, J.C.; Lovelace, A.S.; Glaser, B.M. Sequential in-office vitreous aspirates demonstrate vitreous matrix metalloproteinase 9 levels correlate with the amount of subretinal fluid in eyes with wet age-related macular degeneration. Mol. Vis. 2012, 18, 1658–1667. [Google Scholar] [PubMed]
- Bevitt, D.J.; Mohamed, J.; Catterall, J.B.; Li, Z.; Arris, C.E.; Hiscott, P.; Sheridan, C.; Langton, K.P.; Barker, M.D.; Clarke, M.P.; et al. Expression of ADAMTS metalloproteinases in the retinal pigment epithelium derived cell line ARPE-19: Transcriptional regulation by TNFalpha. Biochim. Biophys. Acta. 2003, 1626, 83–91. [Google Scholar] [CrossRef] [PubMed]
| Neovascular AMD Patients (n) | Total 29 |
|---|---|
| Gender (F/M) (n) | 15/14 |
| Age (years; mean ± SD) | 76.8 ± 6.1 |
| Female | 78.2 ± 6.5 |
| Male | 79.5 ± 6.2 |
| AMD in family | |
| Yes | 0 |
| No | 29 |
| Tabacco smoking | |
| Ever | 17 |
| Never | 12 |
| Gene | Oligonucleotide Sequence | Amplimer Length (bp) | Tm (°C) |
|---|---|---|---|
| MMP15 | Forward: 5′GAAACAACCTCTTCCTGGTGGCAGTGCA3′ Reverse: 5′CGTGCCTCGGGCAGCTTGAAGTTGTC3′ | 135 | 84.6 |
| TIMP2 | Forward: 5′CCCCAAGCAGGAGTTTCTCGACATCG3′ Reverse: 5′TGGACCAGTCGAAACCCTTGGAGGCT3′ | 100 | 80.4 |
| ACTB | Forward: 5′TCACCCACACTGTGCCCATCTACGA3′ Reverse: 5′CAGCGGAACCGCTCATTGCCAATGG3′ | 295 | 86.2 |
| FC | Number of ID mRNAs | |||||
|---|---|---|---|---|---|---|
| p all | p < 0.05 | p < 0.02 | p < 0.01 | p < 0.005 | p < 0.001 | |
| FC ≥ 1.0 | 110 | 16 | 10 | 6 | 4 | 1 |
| FC ≥ 2.0 | 9 | 6 | 3 | 2 | 1 | 0 |
| Probe ID | Gene Symbol | Gene Name | FC before vs. after | Change Direction |
|---|---|---|---|---|
| 202828_s_at | MMP14 | Matrix Metallopeptidase 14 | 2.20 | ↑ |
| 203167_at | TIMP2 | TIMP Metallopeptidase Inhibitor 2 | −2.68 | ↓ |
| 203365_s_at | MMP15 | Matrix Metallopeptidase 15 | 2.41 | ↑ |
| 203876_s_at | MMP11 | Matrix Metallopeptidase 11 | 2.19 | ↑ |
| 220705_s_at | ADAMTS7 | ADAM Metallopeptidase With Thrombospondin Type 1 Motif 7 | 2.55 | ↑ |
| 221953_s_at | MMP24A | Matrix Metallopeptidase 24A | 2.04 | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzalka-Mrozik, B.; Paprzycka, O.; Gruszka, O.; Madej, M.; Kruszniewska-Rajs, C.; Gola, J.M.; Turek, A. Ranibizumab Modifies the Expression of Metalloproteinases and Their Tissue Inhibitors in Peripheral Blood Mononuclear Cells in Patients with Exudative Age-Related Macular Degeneration. J. Clin. Med. 2024, 13, 295. https://doi.org/10.3390/jcm13010295
Strzalka-Mrozik B, Paprzycka O, Gruszka O, Madej M, Kruszniewska-Rajs C, Gola JM, Turek A. Ranibizumab Modifies the Expression of Metalloproteinases and Their Tissue Inhibitors in Peripheral Blood Mononuclear Cells in Patients with Exudative Age-Related Macular Degeneration. Journal of Clinical Medicine. 2024; 13(1):295. https://doi.org/10.3390/jcm13010295
Chicago/Turabian StyleStrzalka-Mrozik, Barbara, Olga Paprzycka, Oliwia Gruszka, Marcel Madej, Celina Kruszniewska-Rajs, Joanna Magdalena Gola, and Artur Turek. 2024. "Ranibizumab Modifies the Expression of Metalloproteinases and Their Tissue Inhibitors in Peripheral Blood Mononuclear Cells in Patients with Exudative Age-Related Macular Degeneration" Journal of Clinical Medicine 13, no. 1: 295. https://doi.org/10.3390/jcm13010295
APA StyleStrzalka-Mrozik, B., Paprzycka, O., Gruszka, O., Madej, M., Kruszniewska-Rajs, C., Gola, J. M., & Turek, A. (2024). Ranibizumab Modifies the Expression of Metalloproteinases and Their Tissue Inhibitors in Peripheral Blood Mononuclear Cells in Patients with Exudative Age-Related Macular Degeneration. Journal of Clinical Medicine, 13(1), 295. https://doi.org/10.3390/jcm13010295







