Impact of Various Atrial Fibrillation Treatment Strategies on Length of Stay in the Emergency Department and Early Complications—3 Years of a Single-Center Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection and Study Protocol
2.2. Safety Conditions for Sedation
2.3. Outcomes
2.4. Statistical Analysis
2.5. Ethical Considerations
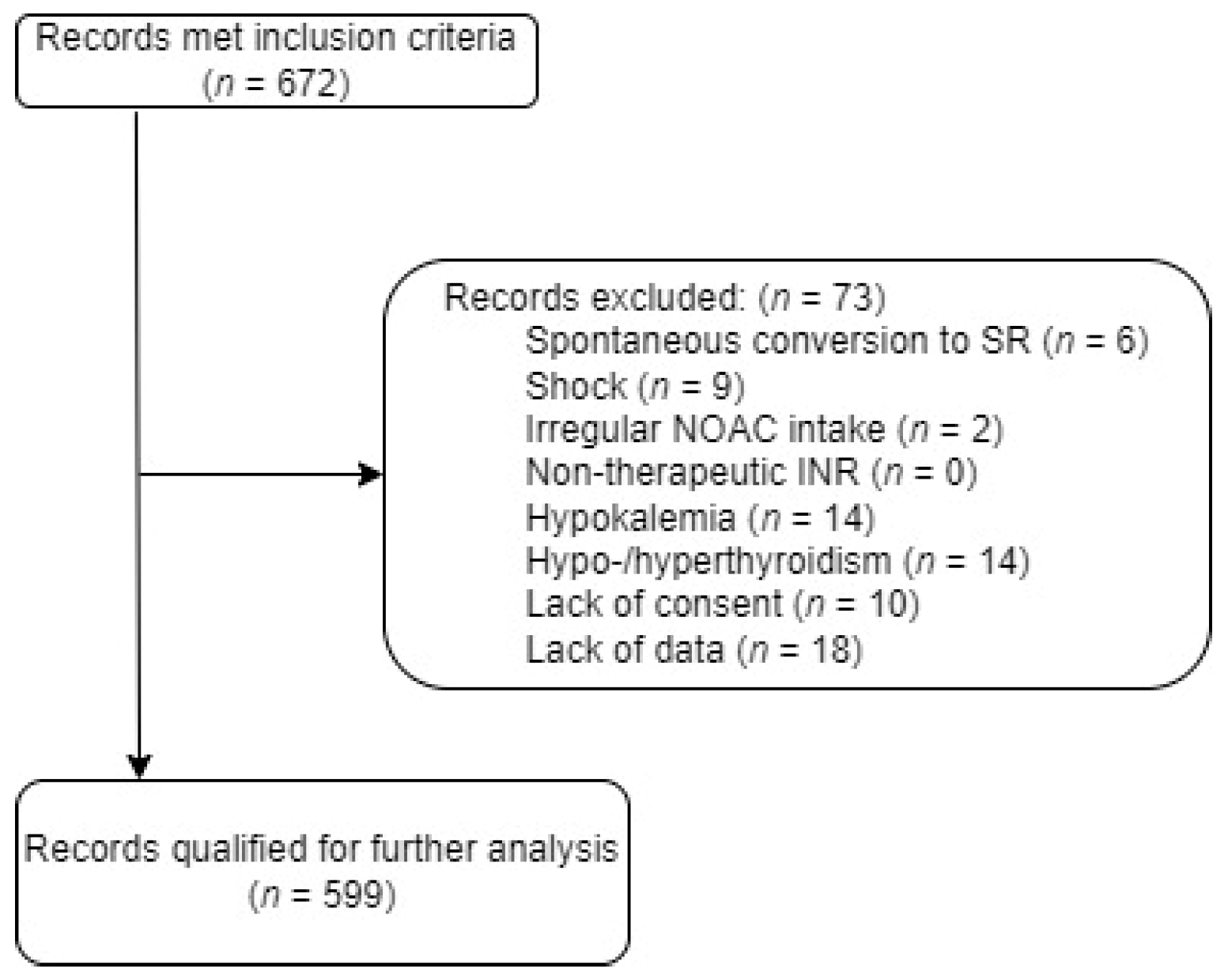
3. Results
3.1. Study Population
3.2. Effectiveness
3.3. Length of Stay
3.4. Complications
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucà, F.; La Meir, M.; Rao, C.M.; Parise, O.; Vasquez, L.; Carella, R.; Lorusso, R.; Daniela, B.; Maessen, J.; Gensini, G.F.; et al. Pharmacological Management of Atrial Fibrillation: One, None, One Hundred Thousand. Cardiol. Res. Pract. 2011, 2011, 874802. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Navarin, S.; Zampini, G.; Magrini, L.; Mann, C.; Muiesan, M.; Caterina, R.D.E.; Yilmaz, M.B.; Beton, O.; Monzani, V.; et al. Management of atrial fibrillation in the Emergency Department: Current approach and future expectations. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 3132–3147. [Google Scholar]
- Kłosiewicz, T.; Rozmarynowska, M.; Konieczka, P.; Mazur, M. Impact of Geriatric Admissions on Workload in the Emergency Department. Healthcare 2023, 11, 593. [Google Scholar] [CrossRef]
- Cintra, F.D.; de Oliveira Figueiredo, M.J. Atrial Fibrillation (Part 1): Pathophysiology, Risk Factors, and Therapeutic Basis. Arq. Bras. Cardiol. 2021, 116, 129–139. [Google Scholar] [CrossRef]
- Kalarus, Z.; Średniawa, B.; Mitręga, K.; Wierucki, Ł.; Sokal, A.; Lip, G.; Bandosz, P.; Stokwiszewski, J.; Boidol, J.; Zieleniewicz, P.; et al. Prevalence of atrial fibrillation in the 65 or over Polish population. Report of cross-sectional NOMED-AF study. Kardiol. Polska 2023, 81, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, L.; Annovi, A.; Sanchis-Gomar, F.; Saccenti, C.; Meschi, T.; Ticinesi, A.; Cervellin, G. Effectiveness and safety of electrical cardioversion for acute-onset atrial fibrillation in the emergency department: A real-world 10-year single center experience. Clin. Exp. Emerg. Med. 2019, 6, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yang, P.S.; Joung, B. Optimal Rhythm Control Strategy in Patients with Atrial Fibrillation. Korean Circ. J. 2022, 52, 496–512. [Google Scholar] [CrossRef]
- Sacchetti, A.; Williams, J.; Levi, S.; Akula, D. Impact of emergency department management of atrial fibrillation on hospital charges. West. J. Emerg. Med. 2013, 14, 55–57. [Google Scholar] [CrossRef]
- Green, S.M.; Roback, M.G.; Krauss, B.S.; Miner, J.R.; Schneider, S.; Kivela, P.D.; Nelson, L.S.; Chumpitazi, C.E.; Fisher, J.D.; Gesek, D.; et al. Unscheduled Procedural Sedation: A Multidisciplinary Consensus Practice Guideline. Ann. Emerg. Med. 2019, 73, e51–e65. [Google Scholar] [CrossRef]
- Green, S.M.; Leroy, P.L.; Roback, M.G.; Irwin, M.G.; Andolfatto, G.; Babl, F.E.; Barbi, E.; Costa, L.R.; Absalom, A.; Carlson, D.W.; et al. An international multidisciplinary consensus statement on fasting before procedural sedation in adults and children. Anaesthesia 2020, 75, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Purmah, Y.; Proietti, M.; Laroche, C.; Mazurek, M.; Tahmatzidis, D.; Boriani, G.; Novo, S.; Lip, G.Y.H.; The EORP-AF General Pilot Registry Investigators. Rate vs. rhythm control and adverse outcomes among European patients with atrial fibrillation. EP Eur. 2018, 20, 243–252. [Google Scholar] [CrossRef]
- Govindapillai, A.; Cox, J.L.; Thabane, L.; Doucette, S.; Xie, F.; MacKillop, J.H.; Ciaccia, A.; Choudhri, S.H.; Nemis-White, J.M.; Hamilton, L.M.; et al. Rhythm Control vs Rate Control in a Contemporary Ambulatory Atrial Fibrillation Cohort: Post Hoc Analysis of the IMPACT-AF Trial. CJC Open 2022, 4, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Rogenstein, C.; Kelly, A.; Mason, S.; Schneider, S.; Lang, E.; Clement, C.M.; Stiell, I.G. An international view of how recent-onset atrial fibrillation is treated in the emergency department. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2012, 19, 1255–1260. [Google Scholar] [CrossRef]
- Lee, G.A.; Farkowski, M.M.; Baker, E.; Sterliński, M.; van Gelder, I.C.; Dąbrowski, R.; Desteghe, L.; Szumowski, Ł.; Merino, J.L.; Collins, R.; et al. Multimorbid management in atrial fibrillation: The Polish perspective in the EHRA-PATHS study. Kardiol. Pol. 2023, 81, 580–586. [Google Scholar] [CrossRef]
- Pope, M.K.; Hall, T.S.; Schirripa, V.; Radic, P.; Virdone, S.; Pieper, K.S.; Le Heuzey, J.-Y.; Jansky, P.; A Fitzmaurice, D.; Cappato, R.; et al. Cardioversion in patients with newly diagnosed non-valvular atrial fibrillation: Observational study using prospectively collected registry data. BMJ 2021, 375, e066450. [Google Scholar] [CrossRef]
- Stiell, I.G.; A Sivilotti, M.L.; Taljaard, M.; Birnie, D.; Vadeboncoeur, A.; Hohl, C.M.; McRae, A.D.; Rowe, B.H.; Brison, R.J.; Thiruganasambandamoorthy, V.; et al. Electrical versus pharmacological cardioversion for emergency department patients with acute atrial fibrillation (RAFF2): A partial factorial randomised trial. Lancet Lond. Engl. 2020, 395, 339–349. [Google Scholar] [CrossRef]
- Grönberg, T.; Hartikainen, J.E.; Nuotio, I.; Biancari, F.; Vasankari, T.; Nikkinen, M.; Ylitalo, A.; Airaksinen, K.J. Can we predict the failure of electrical cardioversion of acute atrial fibrillation? The FinCV study. Pacing Clin. Electrophysiol. PACE 2015, 38, 368–375. [Google Scholar] [CrossRef]
- Um, K.J.; McIntyre, W.F.; Healey, J.S.; A Mendoza, P.; Koziarz, A.; Amit, G.; A Chu, V.; Whitlock, R.P.; Belley-Côté, E.P. Pre- and post-treatment with amiodarone for elective electrical cardioversion of atrial fibrillation: A systematic review and meta-analysis. EP Eur. 2019, 21, 856–863. [Google Scholar]
- Ramesh, T.; Lee, P.Y.K.; Mitta, M.; Allencherril, J. Intravenous magnesium in the management of rapid atrial fibrillation: A systematic review and meta-analysis. J. Cardiol. 2021, 78, 375–381. [Google Scholar] [CrossRef]
- Cacioppo, F.; Reisenbauer, D.; Herkner, H.; Oppenauer, J.; Schuetz, N.; Niederdoeckl, J.; Schnaubelt, S.; Gupta, S.; Lutnik, M.; Simon, A.; et al. Association of Intravenous Potassium and Magnesium Administration with Spontaneous Conversion of Atrial Fibrillation and Atrial Flutter in the Emergency Department. JAMA Netw. Open 2022, 5, e2237234. [Google Scholar] [CrossRef]
- Shepherd, J.; Jones, J.; Frampton, G.K.; Tanajewski, L.; Turner, D.; Price, A. Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: A systematic review and economic evaluation. Health Technol. Assess. Winch. Engl. 2008, 12, iii–iv, ix–95. [Google Scholar] [CrossRef]
- Hoffer, M.; Tran, Q.K.; Hodgson, R.; Atwater, M.; Pourmand, A. Utility of magnesium sulfate in the treatment of rapid atrial fibrillation in the emergency department: A systematic review and meta-analysis. Eur. J. Emerg. Med. 2022, 29, 253. [Google Scholar] [CrossRef]
- Eray, O.; Akça, S.; Pekdemir, M.; Eray, E.; Çete, Y.; Oktay, C. Magnesium efficacy in magnesium deficient and nondeficient patients with rapid ventricular response atrial fibrillation. Eur. J. Emerg. Med. 2000, 7, 287. [Google Scholar] [CrossRef]
- Pluymaekers, N.A.; Dudink, E.A.; Luermans, J.G.; Meeder, J.G.; Lenderink, T.; Widdershoven, J.; Bucx, J.J.; Rienstra, M.; Kamp, O.; Van Opstal, J.M.; et al. Early or Delayed Cardioversion in Recent-Onset Atrial Fibrillation. N. Engl. J. Med. 2019, 380, 1499–1508. [Google Scholar] [CrossRef]
- Doyle, B.; Reeves, M. “Wait and See” Approach to the Emergency Department Cardioversion of Acute Atrial Fibrillation. Emerg. Med. Int. 2011, 2011, 545023. [Google Scholar] [CrossRef]
- Boriani, G.; Imberti, J.F.; Valenti, A.C.; Malavasi, V.L.; Vitolo, M. Managing atrial fibrillation: The need for an individualized approach even in the emergency department. Intern. Emerg. Med. 2020, 15, 9–12. [Google Scholar] [CrossRef]
- Stiell, I.G.; Clement, C.M.; Perry, J.J.; Vaillancourt, C.; Symington, C.; Dickinson, G.; Birnie, D.; Green, M.S. Association of the Ottawa Aggressive Protocol with rapid discharge of emergency department patients with recent-onset atrial fibrillation or flutter. Can. J. Emerg. Med. 2010, 12, 181–191. [Google Scholar] [CrossRef]
- Bellone, A.; Etteri, M.; Vettorello, M.; Bonetti, C.; Clerici, D.; Gini, G.; Maino, C.; Mariani, M.; Natalizi, A.; Nessi, I.; et al. Cardioversion of acute atrial fibrillation in the emergency department: A prospective randomised trial. Emerg. Med. J. EMJ 2012, 29, 188–191. [Google Scholar] [CrossRef]
- Dryver, E.; Larsson, D.; Pahlm, U. Swedish emergency physicians can safely sedate patients with propofol prior to cardioversion. Lakartidningen 2019, 116, FDIZ. [Google Scholar]
- Fried, A.M.; Strout, T.D.; Perron, A.D. Electrical cardioversion for atrial fibrillation in the emergency department: A large single-center experience. Am. J. Emerg. Med. 2021, 42, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Scheuermeyer, F.X.; Andolfatto, G.; Christenson, J.; Villa-Roel, C.; Rowe, B. A Multicenter Randomized Trial to Evaluate a Chemical-first or Electrical-first Cardioversion Strategy for Patients with Uncomplicated Acute Atrial Fibrillation. Acad. Emerg. Med. 2019, 26, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Scheuermeyer, F.X.; Grafstein, E.; Stenstrom, R.; Innes, G.; Heslop, C.; MacPhee, J.; Pourvali, R.; Heilbron, B.; McGrath, L.; Christenson, J. Thirty-day and 1-year outcomes of emergency department patients with atrial fibrillation and no acute underlying medical cause. Ann. Emerg. Med. 2012, 60, 755–765.e2. [Google Scholar] [CrossRef] [PubMed]
- Faessler, L.; Kutz, A.; Haubitz, S.; Mueller, B.; Perrig-Chiello, P.; Schuetz, P. Psychological distress in medical patients 30 days following an emergency department admission: Results from a prospective, observational study. BMC Emerg. Med. 2016, 16, 33. [Google Scholar] [CrossRef]
- Aldulaymi, R.; Al Meslamani, A.Z. Systematic review of the safety and efficacy of antazoline in the treatment of atrial fibrillation. J. Pharm. Pharmacogn. Res. 2022, 10, 147–157. [Google Scholar] [CrossRef]
- Wybraniec, M.; Maciąg, A.; Miśkowiec, D.; Ceynowa-Sielawko, B.; Balsam, P.; Wójcik, M.; Wróbel, W.; Farkowski, M.; Ćwiek-Rębowska, E.; Szołkiewicz, M.; et al. Efficacy and safety of antazoline for cardioversion of atrial fibrillation: Propensity score matching analysis of a multicenter registry (CANT II Study). Pol. Arch. Med. Wewnętrznej 2022, 132, 16234. [Google Scholar] [CrossRef]
| Variable | Total | MED | EC | COMB | p-Value |
|---|---|---|---|---|---|
| Median age, years [IQR] | 71 (62–79) | 72 (63–81) § | 68 (60–75) | 67 (57–73) § | * < 0.05 § < 0.05 |
| Median LOS, min [IQR] | 206 (127–308) | 173 (112–275) † | 180 (131–295) § | 295 (214–392) §,† | * < 0.05 § < 0.05 † < 0.05 |
| Male, n (%) | 291 (47.47) | 162 (42.40) §,† | 45 (56.96) § | 84 (55.26) † | * 0.0082 § 0.0081 † 0.0093 |
| Past medical history, n (%) | |||||
| Hypertension | 209 (34.09) | 148 (38.74) § | 23 (29.11) | 38 (25.00) § | * 0.0026 § 0.0201 |
| Coronary artery disease | 67 (10.51) | 45 (11.78) | 6 (7.59) | 16 (10.52) | * 0.5428 |
| Heart failure | 24 (3.91) | 15 (3.92) | 2 (2.53) | 7 (4.60) | * 0.7290 |
| Diabetes mellitus | 46 (7.50) | 37 (9.68) §,† | 2 (2.53) † | 7 (4.60) § | * 0.0173 § 0.0222 † 0.0185 |
| Chronic obstructive pulmonary disease | 22 (3.59) | 14 (3.66) | 4 (5.06) | 4 (2.36) | * 0.7476 |
| History of hypo-/hyperthyroidism | 39 (6.36) | 22 (5.75) | 6 (7.59) | 11 (7.23) | * 0.7621 |
| Drug | n (% of MED Group) | Effectiveness (%) |
|---|---|---|
| Amiodarone | 133 (34.82) | 40 |
| Propafenone | 14 (3.66) | 83 |
| Antazoline | 155 (40.58) | 88 |
| Amiodarone, antazoline | 64 (16.75) | 53 |
| Propafenone, antazoline | 16 (4.19) | 79 |
| Variable | Total | EC | MED | COMB | p-Value |
|---|---|---|---|---|---|
| LOS (min) 1 | 223 [144–328] | 180 [131–295] † | 173 [112–275] § | 295 [214–392] §,† | * < 0.05 § < 0.05 † < 0.05 |
| Effectiveness | 74.29% | 92.40% † | 64.95% §,† | 87.91% § | * < 0.05 § < 0.05 † < 0.05 |
| Complication rate | 2.67% | 2.53% | 0.81% § | 7.38% § | * < 0.05 § < 0.05 † < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłosiewicz, T.; Cholerzyńska, H.; Zasada, W.A.; Shadi, A.; Olszewski, J.; Konieczka, P.; Podlewski, R.; Puślecki, M. Impact of Various Atrial Fibrillation Treatment Strategies on Length of Stay in the Emergency Department and Early Complications—3 Years of a Single-Center Experience. J. Clin. Med. 2024, 13, 190. https://doi.org/10.3390/jcm13010190
Kłosiewicz T, Cholerzyńska H, Zasada WA, Shadi A, Olszewski J, Konieczka P, Podlewski R, Puślecki M. Impact of Various Atrial Fibrillation Treatment Strategies on Length of Stay in the Emergency Department and Early Complications—3 Years of a Single-Center Experience. Journal of Clinical Medicine. 2024; 13(1):190. https://doi.org/10.3390/jcm13010190
Chicago/Turabian StyleKłosiewicz, Tomasz, Hanna Cholerzyńska, Wiktoria Antonina Zasada, Amira Shadi, Jakub Olszewski, Patryk Konieczka, Roland Podlewski, and Mateusz Puślecki. 2024. "Impact of Various Atrial Fibrillation Treatment Strategies on Length of Stay in the Emergency Department and Early Complications—3 Years of a Single-Center Experience" Journal of Clinical Medicine 13, no. 1: 190. https://doi.org/10.3390/jcm13010190
APA StyleKłosiewicz, T., Cholerzyńska, H., Zasada, W. A., Shadi, A., Olszewski, J., Konieczka, P., Podlewski, R., & Puślecki, M. (2024). Impact of Various Atrial Fibrillation Treatment Strategies on Length of Stay in the Emergency Department and Early Complications—3 Years of a Single-Center Experience. Journal of Clinical Medicine, 13(1), 190. https://doi.org/10.3390/jcm13010190







