Effects of Different Kinds of Physical Activity on Vascular Function
Abstract
:1. Introduction
2. Effects of Different Types of Exercise on Arterial Distensibility
3. Effect of Short-Term versus Long-Term Physical Activity on Arterial Distensibility
4. Effect of Regular Exercise on Vascular Function in Hypertensive and Normotensive Patients
5. Role of Physical Activity in the Elderly
6. Conclusions
Funding
Conflicts of Interest
References
- Fihn, S.D.; Blankenship, J.C.; Alexander, K.P.; Bittl, J.A.; Byrne, J.G.; Fletcher, B.J.; Fonarow, G.C.; Lange, R.A.; Levine, G.N.; Maddox, T.M.; et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2014, 130, 1749–1767. [Google Scholar] [PubMed]
- Visseren, F.L.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. J. Prev. Cardiol. 2022, 29, 5–115. [Google Scholar] [CrossRef] [PubMed]
- Joseph, G.; Marott, J.L.; Torp-Pedersen, C.; Biering-Sørensen, T.; Nielsen, G.; Christensen, A.-E.; Johansen, M.B.; Schnohr, P.; Sogaard, P.; Mogelvang, R. Dose-response association between level of physical activity and mortality in normal, elevated, and high blood pressure. Hypertension 2019, 74, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.F.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [PubMed]
- Pekas, E.J.; Shin, J.; Son, W.M.; Headid III, R.J.; Park, S.Y. Habitual Combined Exercise Protects against age-associated decline in vascular function and lipid profiles in elderly postmenopausal women. Int. J. Environ. Res. Public Health 2020, 17, 3893. [Google Scholar] [CrossRef] [PubMed]
- Palatini, P.; Graniero, G.R.; Mormino, P.; Nicolosi, L.; Mos, L.; Visentin, P.; Pessina, A.C. Relation between physical training and ambulatory blood pressure in stage I hypertensive subjects. Results of the HARVEST Trial. Hypertension and Ambulatory Recording Venetia Study. Circulation 1994, 90, 2870–2876. [Google Scholar] [CrossRef]
- De Ciuceis, C.; Rizzoni, D.; Palatini, P. Microcirculation and physical exercise In hypertension. Hypertension 2023, 80, 730–739. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Buys, R.; Smart, N.A. Endurance exercise beneficially affects ambulatory blood pressure: A systematic review and meta-analysis. J. Hypertens. 2013, 31, 639–648. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Smart, N.A. Exercise training for blood pressure: A systematic review and meta-analysis. J. Am. Heart Assoc. 2013, 2, e004473. [Google Scholar] [CrossRef] [PubMed]
- Saladini, F.; Benetti, E.; Mos, L.; Mazzer, A.; Casiglia, E.; Palatini, P. Regular physical activity is associated with improved small artery distensibility in young to middle-age stage 1 hypertensives. Vasc. Med. 2014, 19, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Vriz, O.; Mos, L.; Palatini, P. Leisure-time physical activity has a more favourable impact on carotid artery stiffness than vigorous physical activity in hypertensive human beings. J. Clin. Med. 2022, 11, 5303. [Google Scholar] [CrossRef] [PubMed]
- Palatini, P.; Canali, C.; Graniero, G.R.; Rossi, G.; de Toni, R.; Santonastaso, M.; dal Follo, M.; Zanata, G.; Ferrarese, E.; Mormino, P.; et al. Relationship of plasma renin activity with caffeine intake and physical training in mild hypertensive men. HARVEST Study Group. Eur. J. Epidemiol. 1996, 12, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Lantelme, P.; Mestre, C.; Lievre, M.; Gressard, A.; Milon, H. Heart rate: An important confounder of pulse wave velocity assessment. Hypertension 2002, 39, 1083–1087. [Google Scholar] [CrossRef]
- Cunha, R.S.; Pannier, B.; Benetos, A.; Siché, J.-P.; London, G.M.; Mallion, J.M.; Safar, M.E. Association between high heart rate and high arterial rigidity in normotensive and hypertensive subjects. J. Hypertens. 1997, 15, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-L.; Gatzka, C.D.; Du, X.-J.; Cameron, J.D.; Kingwell, B.A. Effects of heart rate on arterial compliance in men. Clin. Exp. Pharmacol. Physiol. 1999, 26, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, I.B.; McEniery, C.M. ARTERIAL Stiffness, endothelial function and novel pharmacological approaches. Clin. Exp. Pharmacol. Physiol. 2004, 31, 795–799. [Google Scholar] [CrossRef]
- Pedralli, M.L.; Marschner, R.A.; Kollet, D.P.; Neto, S.G.; Eibel, B.; Tanaka, H.; Lehnen, A.M. Different exercise training modalities produce similar endothelial function improvements in individuals with prehypertension or hypertension: A randomized clinical trial Exercise, endothelium and blood pressure. Sci. Rep. 2020, 10, 7628. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H.; Tan, X.; Hu, M.; Shen, B. Exercise restores impaired endothelium-derived hyperpolarizing factor–mediated vasodilation in aged rat aortic arteries via the TRPV4-KCa2.3 signaling complex. Clin. Interv. Aging 2019, 14, 1579–1587. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Zhang, C.; Xu, G.; Tang, Z.; Zhang, Z.; Liu, Y.; Wang, Z. Effects of aerobic exercise on the expressions and activities of nitric oxide synthases in the blood vessel endothelium in prediabetes mellitus. Exp. Ther. Med. 2019, 17, 4205–4212. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.P.; Nascimento, A.R.; Lopes, G.O.; Medeiros-Lima, D.J.M.; Coelho, M.P.; Nascimento, P.M.C.; Kopiler, D.A.; Matsuura, C.; Mediano, M.F.F.; Tibirica, E. The impact of exercise frequency upon microvascular endothelium function and oxidative stress among patients with coronary artery disease. Clin. Physiol. Funct. Imaging 2018, 38, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, V.A.; Fagard, R.H.; Coeckelberghs, E.; Vanhees, L. Impact of resistance training on blood pressure and other cardiovascular risk factors: A metaanalysis of randomized, controlled trials. Hypertension 2011, 58, 950–958. [Google Scholar] [CrossRef] [PubMed]
- López-Valenciano, A.; Ruiz-Pérez, I.; Ayala, F.; Sánchez-Meca, J.; Vera-Garcia, F.J. Updated systematic review and meta-analysis on the role of isometric resistance training for resting blood pressure management in adults. J. Hypertens. 2019, 37, 1320–1333. [Google Scholar] [CrossRef]
- Smart, N.A.; Way, D.; Carlson, D.; Millar, P.; McGowan, C.; Swaine, I.; Baross, A.; Howden, R.; Ritti-Dias, R.; Wiles, J.; et al. Effects of isometric resistance training on resting blood pressure: Individual participant data meta-analysis. J. Hypertens. 2019, 37, 1927–1938. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Lara, J.; Siervo, M.; Celis-Morales, C.; Mathers, J.C. Effects of exercise modalities on arterial stiffness and wave reflection: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e110034. [Google Scholar] [CrossRef]
- Miyachi, M. Effects of resistance training on arterial stiffness: A meta-analysis. Br. J. Sports Med. 2013, 47, 393–396. [Google Scholar] [CrossRef]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Effect of low-intensity resistance training on arterial function. Eur. J. Appl. Physiol. 2011, 111, 743–748. [Google Scholar] [CrossRef]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Upper but not lower limb resistance training increases arterial stiffness in humans. Eur. J. Appl. Physiol. 2009, 107, 127–134. [Google Scholar] [CrossRef]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Effects of muscle contraction timing during resistance training on vascular function. J. Hum. Hypertens. 2009, 23, 470–478. [Google Scholar] [CrossRef]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Combined aerobic and resistance training and vascular function: Effect of aerobic exercise before and after resistance training. J. Appl. Physiol. 2007, 103, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Marzolini, S.; Oh, P.I.; Brooks, D. Effect of combined aerobic and resistance training versus aerobic training alone in individuals with coronary artery disease: A meta-analysis. Eur. J. Prev. Cardiol. 2012, 19, 81–94. [Google Scholar] [CrossRef]
- Snowling, N.J.; Hopkins, W.G. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: A meta-analysis. Diabetes Care 2006, 29, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Vriz, O.; Mos, L.; Frigo, G.; Sanigi, C.; Zanata, G.; Pegoraro, F.; Palatini, P. Effects of physical exercise on clinic and 24-hour ambulatory blood pressure in young subjects with mild hypertension. J. Sports Med. Phys. Fitness 2002, 42, 83–88. [Google Scholar] [PubMed]
- Cardoso, C.G.; Gomides, R.S.; Queiroz, A.C.C.; Pinto, L.G.; Lobo, F.d.S.; Tinucci, T.; Mion, D.; Forjaz, C.L.d.M. Acute and chronic Effects of aerobic and resistance exercise on ambulatory blood pressure. Clinics 2010, 65, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Palatini, P.; Visentin, P.; Dorigatti, F.; Guarnieri, C.; Santonastaso, M.; Cozzio, S.; Pegoraro, F.; Bortolazzi, A.; Vriz, O.; Mos, L. Regular physical activity prevents development of left ventricular hypertrophy in hypertension. Eur. Heart J. 2009, 30, 225–232. [Google Scholar] [CrossRef]
- Sasaki, N.; Matsuura, H.; Kajiyama, G.; Oshima, T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: Role of endothelium-derived nitric oxide. Circulation 1999, 100, 1194–1202. [Google Scholar]
- Stewart, K.J. Exercise training and the cardiovascular consequences of type 2 diabetes and hypertension: Plausible mechanisms for improving cardiovascular health. JAMA 2002, 288, 1622–1631. [Google Scholar] [CrossRef]
- Palatini, P.; Puato, M.; Rattazzi, M.; Pauletto, P. Effect of regular physical activity on carotid intima-media thickness. Results from a 6-year prospective study in the early stage of hypertension. Blood Press. 2011, 20, 37–44. [Google Scholar] [CrossRef]
- Meyer, A.A.; Kundt, G.; Lenschow, U.; Schuff-Werner, P.; Kienast, W. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. J. Am. Coll. Cardiol. 2006, 48, 1865–1870. [Google Scholar] [CrossRef]
- Whelton, S.P.; Chin, A.; Xin, X.; He, J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized controlled trials. Ann. Intern. Med. 2002, 136, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.A.; Tran, Z.V. Aerobic exercise and resting blood pressure: A meta-analytic review of randomized, controlled trials. Prev. Cardiol. 2001, 4, 73–80. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, S.W.; Wilcken, D.E.; Macdonald, G.J. The effect of weight reduction on left ventricular mass. A randomized controlled trial in young, overweight hypertensive patients. N. Engl. J. Med. 1986, 314, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Himeno, E.; Nishino, K.; Nakashima, Y.; Kuroiwa, A.; Ikeda, M. Weight reduction regresses left ventricular mass regardless of blood pressure level in obese subjects. Am. Hearth J. 1996, 131, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, V.A.; Fagard, R.H. Effects of endurance training on blood pressure, blood pressure–Regulating mechanisms, and cardiovascular risk factors. Hypertension 2005, 46, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.; Spronck, B.; Kiat, H.; Barin, E.; Reesink, K.D.; Delhaas, T.; Avolio, A.P.; Butlin, M. Heart rate dependency of large artery stiffness. Hypertension 2016, 68, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, H.; Hashimoto, H.; Tanaka, H.; Matsumoto, C.; Odaira, M.; Yamada, J.; Yoshida, M.; Shiina, K.; Nagata, M.; Yamashina, A. Synergistic relationship between changes in the pulse wave velocity and changes in the heart rate in middle-aged Japanese adults: A prospective study. J. Hypertens. 2010, 28, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Benetos, A.; Adamopoulos, C.; Bureau, J.M.; Temmar, M.; Labat, C.; Bean, K.; Thomas, F.; Pannier, B.; Asmar, R.; Zureik, M.; et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation 2002, 105, 1202–1207. [Google Scholar] [CrossRef]
- Palatini, P.; Saladini, F.; Mos, L.; Fania, C.; Mazzer, A.; Casiglia, E. Low night-time heart rate is longitudinally associated with lower augmentation index and central systolic blood pressure in hypertension. Eur. J. Appl. Physiol. 2018, 118, 543–550. [Google Scholar] [CrossRef]
- Palatini, P.; Thijs, L.; Staessen, J.A.; Fagard, R.H.; Bulpitt, C.J.; Clement, D.L.; de Leeuw, P.W.; Jaaskivi, M.; Leonetti, G.; Nachev, C.; et al. Predictive value of clinic and ambulatory heart rate for mortality in elderly subjects with systolic hypertension. Arch. Intern. Med. 2002, 162, 2313–2321. [Google Scholar] [CrossRef]
- Jouven, X.; Empana, J.-P.; Schwartz, P.J.; Desnos, M.; Courbon, D.; Ducimetière, P. Heart-rate profile during exercise as a predictor of sudden death. N. Engl. J. Med. 2005, 352, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Kolloch, R.; Legler, U.F.; Champion, A.; Cooper-DeHoff, R.M.; Handberg, E.; Zhou, Q.; Pepine, C.J. Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: Findings from the INternational VErapamil-SR/trandolapril STudy (INVEST). Eur. Heart J. 2008, 29, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Dekleva, M.; Lazic, J.S.; Arandjelovic, A.; Mazic, S. Beneficial and harmful effects of exercise in hypertensive patients: The role of oxidative stress. Hypertens. Res. 2017, 40, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Völz, S.; Svedlund, S.; Andersson, B.; Li-Ming, G.; Rundqvist, B. Coronary flow reserve in patients with resistant hypertension. Clin. Res. Cardiol. 2017, 106, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; De Ciuceis, C.; Porteri, E.; Paiardi, S.; Boari, G.E.; Mortini, P.; Cornali, C.; Cenzato, M.; Rodella, L.F.; Borsani, E.; et al. Altered structure of small cerebral arteries in patients with essential hypertension. J. Hypertens. 2009, 27, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Paiardi, S.; Rodella, L.F.; De Ciuceis, C.; Porteri, E.; Boari, G.E.; Rezzani, R.; Rizzardi, N.; Platto, C.; Tiberio, G.A.; Giulini, S.M.; et al. Immunohistochemical evaluation of microvascular rarefaction in hypertensive humans and in spontaneously hypertensive rats. Clin. Hemorheol. Microcirc. 2009, 42, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.L. Aortic Stiffness in Aging and Hypertension: Prevention and Treatment with Habitual Aerobic Exercise. Curr. Hypertens. Rep. 2017, 19, 90–99. [Google Scholar] [CrossRef]
- Kraft, K.A.; Arena, R.; Arrowood, J.A.; Fei, D.-Y. High aerobic capacity does not attenuate aortic stiffness in hypertensive subjects. Am. Heart J. 2007, 154, 976–982. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Xanthopoulos, A.; Lampropoulos, K.; Briasoulis, A.; Sarafidis, P.; Skoularigis, J.; Boudoulas, H. Aortic Stiffness: A Major Risk Factor for Multimorbidity in the Elderly. J. Clin. Med. 2023, 12, 2321. [Google Scholar] [CrossRef]
- Alvarez-Bueno, C.; Cunha, P.G.; Martinez-Vizcaino, V.; Pozuelo-Carrascosa, D.P.; Visier-Alfonso, M.E.; Jimenez-Lopez, E.; Cavero-Redondo, I. Arterial Stiffness and Cognition Among Adults: A Systematic Review and Meta-Analysis of Observational and Longitudinal Studies. J. Am. Heart Assoc. 2020, 9, e014621. [Google Scholar] [CrossRef]
- Zhang, Y.; Miyai, N.; Abe, K.; Utsumi, M.; Uematsu, Y.; Terada, K.; Nakatani, T.; Takeshita, T.; Arita, M. Muscle mass reduction, low muscle strength, and their combination are associated with arterial stiffness in community-dwelling elderly population: The Wakayama Study. J. Hum. Hypertens. 2021, 35, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Vizzi, L.; Padua, E.; D’Amico, A.G.; Tancredi, V.; D’Arcangelo, G.; Cariati, I.; Scimeca, M.; Maugeri, G.; D’Agata, V.; Montorsi, M. Beneficial Effects of Physical Activity on Subjects with Neurodegenerative Disease. J. Funct. Morphol. Kinesiol. 2020, 5, 94. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Kwak, Y.-S.; Pekas, E.J. Impacts of aquatic walking on arterial stiffness, exercise tolerance, and physical function in patients with peripheral artery disease: A randomized clinical trial. J. Appl. Physiol. 2019, 127, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.W. Effect of physical exercise and medication on enhancing cognitive function in older adults with vascular risk. Geriatr. Gerontol. Int. 2020, 20, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.K.T.; Menêses, A.L.; Parmenter, B.J.; Ritti-Dias, R.M.; Farah, B.Q. Effects of resistance training on endothelial function: A systematic review and meta-analysis. Atherosclerosis 2021, 333, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qi, L.; Xu, L.; Sun, X.; Liu, W.; Zhou, S.; van de Vosse, F.; Greenwald, S.E. Effects of exercise modalities on central hemodynamics, arterial stiffness and cardiac function in cardiovascular disease: Systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2018, 13, e0200829. [Google Scholar] [CrossRef]
- Campos, H.O.; Rodrigues, Q.T.; Drummond, L.R.; Lima, P.M.A.; Monteiro, M.d.C.; Wanner, S.P.; Coimbra, C.C. Exercise-based cardiac rehabilitation after myocardial revascularization: A systematic review and meta-analysis. Rev. Cardiovasc. Med. 2022, 23, 74. [Google Scholar] [CrossRef]
- Mora-Rodriguez, R.; Ramirez-Jimenez, M.; Fernandez-Elias, V.E.; de Prada, M.V.G.; Morales-Palomo, F.; Pallares, J.G.; Nelson, R.K.; Ortega, J.F. Effects of aerobic interval training on arterial stiffness and microvascular function in patients with metabolic syndrome. J. Clin. Hypertens. 2018, 20, 11–18. [Google Scholar] [CrossRef]
- Koskinen, J.; Magnussen, C.G.; Taittonen, L.; Räsänen, L.; Mikkilä, V.; Laitinen, T.; Rönnemaa, T.; Kähönen, M.; Viikari, J.S.; Raitakari, O.T.; et al. Arterial structure and function after recovery from the metabolic syndrome: The cardiovascular risk in Young Finns Study. Circulation 2010, 121, 392–400. [Google Scholar] [CrossRef]
- Fryer, S.; Paterson, C.; Turner, L.; Moinuddin, A.; Faulkner, J.; Stoner, L.; Daykin, A.; Stone, K. Localized activity attenuates the combined impact of a high fat meal and prolonged sitting on arterial stiffness: A randomized, controlled cross-over trial. Front. Physiol. 2023, 14, 1107456. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, M.; Stoner, L. Blood glucose responses are associated with prolonged sitting-induced changes in arterial stiffness: A randomized crossover trial. Blood Press. Monit. 2022, 27, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, M.; Stoner, L. Macrovascular and microvascular responses to prolonged sitting with and without bodyweight exercise interruptions: A randomized cross-over trial. Vasc Med. 2022, 27, 127–135. [Google Scholar] [CrossRef] [PubMed]
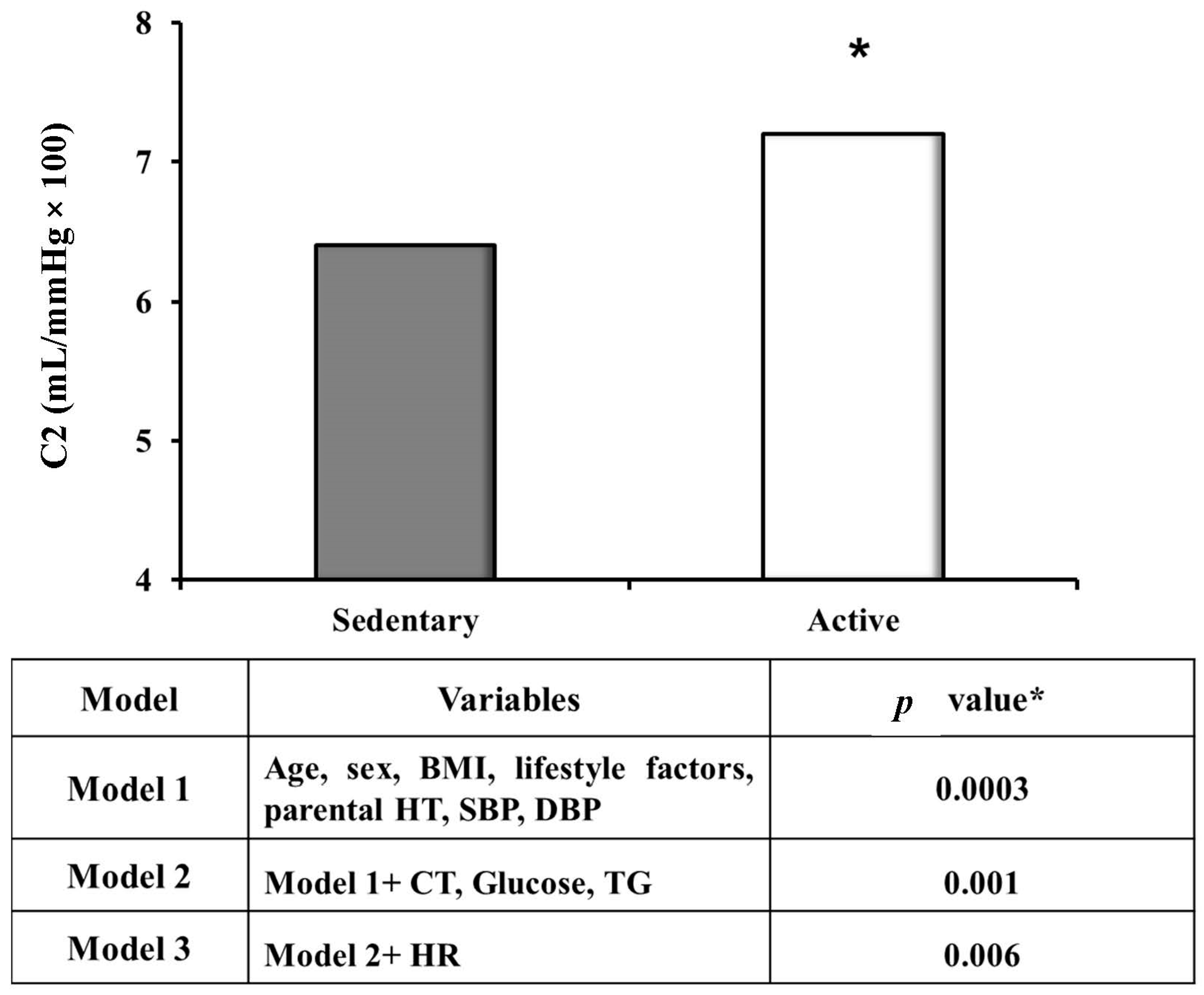
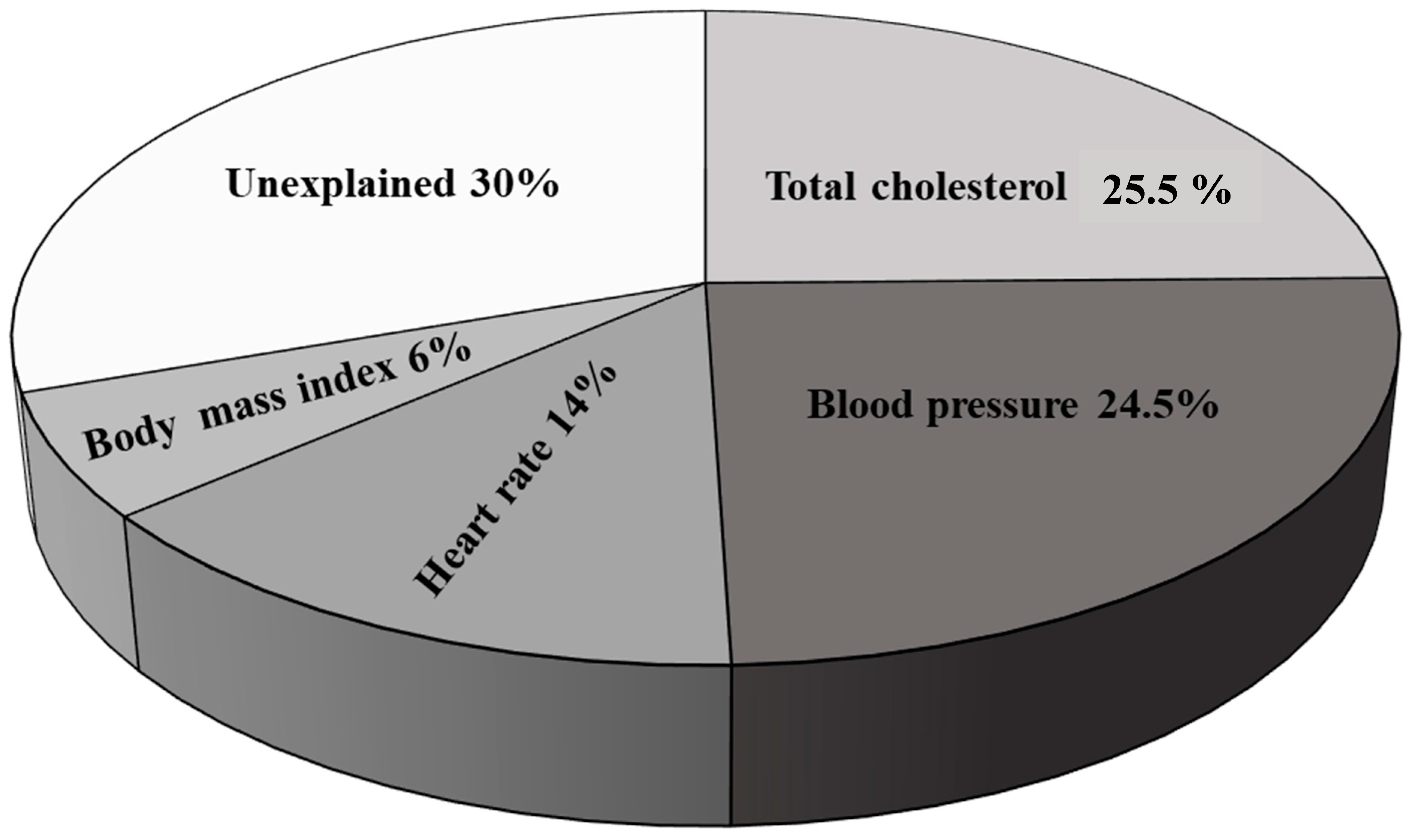
| Variables | Sedentary Subjects (n = 110) | Active Subjects (n = 42) | p * |
|---|---|---|---|
| C1, mL/mmHg × 10 | 16.4 ± 4.5 | 17.0 ± 4.8 | n.s. |
| C2, mL/mmHg × 100 | 6.2 ± 2.8 | 7.9 ± 2.5 | 0.009 |
| AIx, % | 25.8 ± 22.6 | 14.5 ± 0.24 | n.s. |
| Carotid-radial PWV, m/s | 8.8 ± 2.1 | 9.1 ± 2.2 | n.s. |
| Peripheral resistance, dyne × s × cm−5 | 1466.0 ± 236 | 1357.0 ± 228 | n.s. |
| Central SBP, mmHg | 120.4 ± 15.0 | 118.2 ± 10.9 | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saladini, F. Effects of Different Kinds of Physical Activity on Vascular Function. J. Clin. Med. 2024, 13, 152. https://doi.org/10.3390/jcm13010152
Saladini F. Effects of Different Kinds of Physical Activity on Vascular Function. Journal of Clinical Medicine. 2024; 13(1):152. https://doi.org/10.3390/jcm13010152
Chicago/Turabian StyleSaladini, Francesca. 2024. "Effects of Different Kinds of Physical Activity on Vascular Function" Journal of Clinical Medicine 13, no. 1: 152. https://doi.org/10.3390/jcm13010152
APA StyleSaladini, F. (2024). Effects of Different Kinds of Physical Activity on Vascular Function. Journal of Clinical Medicine, 13(1), 152. https://doi.org/10.3390/jcm13010152






