Long-Term Effects of SARS-CoV-2 in the Brain: Clinical Consequences and Molecular Mechanisms
Abstract
1. Introduction
2. Common COVID-19-Related Neurological Symptoms
| Symptom | Age Range | Citation |
|---|---|---|
| Anosmia or hypogeusia | All ages | [41,57,58,61,62] |
| Encephalopathy | Older adults | [29,63] |
| Balance disturbances and limb force reductions | All ages | [58] |
| Cognitive impairment | 60 and older | [56,64] |
| Seizures | Older adults | [44,48] |
| Hemorrhagic events, including CVST | Females < 50 years old | [40,48,58] |
| Stroke (most common: cryptogenic stroke) | <55 years old | [59,60] |
| Delirium | Older adults/dementia | [65] |
| Severe headaches | 14–60% of all patients | [66] |
| General fatigue | All ages | [67] |
3. Cognitive Impairment and Alzheimer’s Disease
4. COVID-19 and Delirium
5. Increased Vulnerability in Patients with Traumatic Brain Injuries
6. Molecular Mechanisms for Brain and Cell Entry
6.1. Brain Aging and Aggregation of Misfolded Proteins
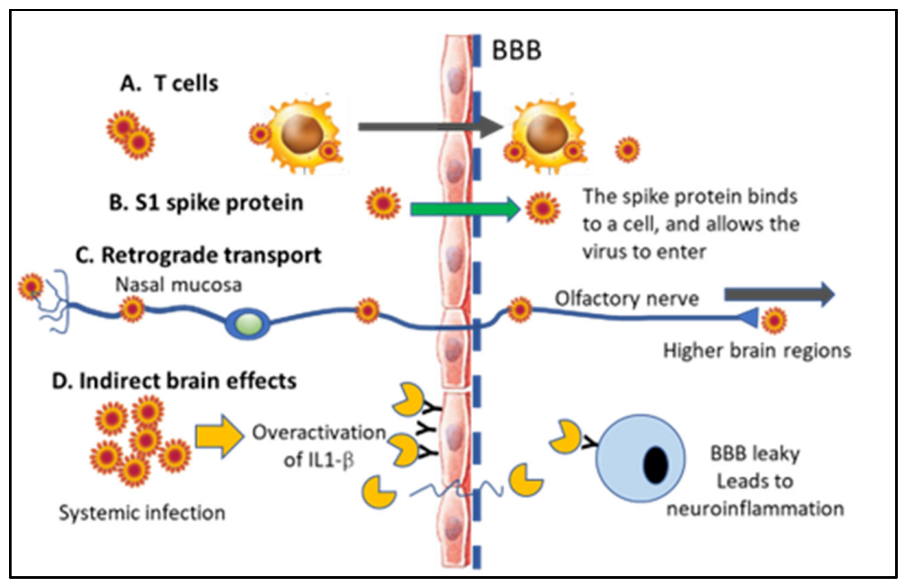
6.2. Direct vs. Indirect Effects in the CNS and Presence of the SARS-CoV-2 vs. in Neurons
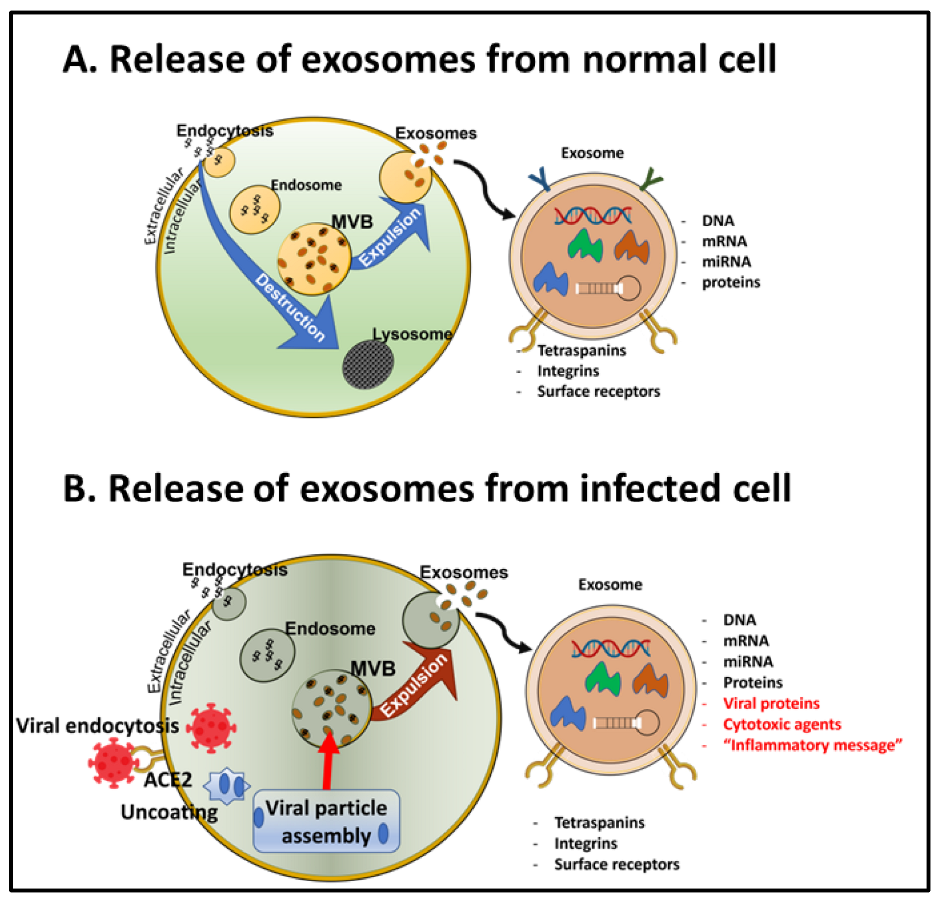
6.3. Transportation of the SARS-CoV-2 vs. in Exosomes
7. Mechanisms for Down Syndrome-Related Vulnerability
8. Conclusions and Next Steps
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef] [PubMed]
- De Lusignan, S.; Lopez Bernal, J.; Zambon, M.; Akinyemi, O.; Amirthalingam, G.; Andrews, N.; Borrow, R.; Byford, R.; Charlett, A.; Dabrera, G.; et al. Emergence of a Novel Coronavirus (COVID-19): Protocol for Extending Surveillance Used by the Royal College of General Practitioners Research and Surveillance Centre and Public Health England. JMIR Public Health Surveill. 2020, 6, e18606. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wei, F.; Hu, L.; Wen, L.; Chen, K. Epidemiology and Clinical Characteristics of COVID-19. Arch. Iran. Med. 2020, 23, 268–271. [Google Scholar] [CrossRef]
- Kooraki, S.; Hosseiny, M.; Myers, L.; Gholamrezanezhad, A. Coronavirus (COVID-19) Outbreak: What the Department of Radiology Should Know. J. Am. Coll. Radiol. 2020, 17, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Okba, N.M.A.; Muller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; de Bruin, E.; Chandler, F.D.; et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg. Infect. Dis. 2020, 26, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Palacios Cruz, M.; Santos, E.; Velazquez Cervantes, M.A.; Leon Juarez, M. COVID-19, a worldwide public health emergency. Rev. Clin. Esp. 2020, 221, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Wouk, J.; Rechenchoski, D.Z.; Rodrigues, B.C.D.; Ribelato, E.V.; Faccin-Galhardi, L.C. Viral infections and their relationship to neurological disorders. Arch. Virol. 2021, 221, 55–61. [Google Scholar] [CrossRef]
- De Chiara, G.; Marcocci, M.E.; Sgarbanti, R.; Civitelli, L.; Ripoli, C.; Piacentini, R.; Garaci, E.; Grassi, C.; Palamara, A.T. Infectious agents and neurodegeneration. Mol. Neurobiol. 2012, 46, 614–638. [Google Scholar] [CrossRef] [PubMed]
- Bagetta, G.; Nistico, G.; Bowery, N.G. Prevention by the NMDA receptor antagonist, MK801 of neuronal loss produced by tetanus toxin in the rat hippocampus. Br. J. Pharmacol. 1990, 101, 776–780. [Google Scholar] [CrossRef]
- Zhou, L.; Miranda-Saksena, M.; Saksena, N.K. Viruses and neurodegeneration. Virol. J. 2013, 10, 172. [Google Scholar] [CrossRef]
- Majde, J.A. Neuroinflammation resulting from covert brain invasion by common viruses—A potential role in local and global neurodegeneration. Med. Hypotheses 2010, 75, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Kirtipal, N.; Kumar, S.; Dubey, S.K.; Dwivedi, V.D.; Gireesh Babu, K.; Maly, P.; Bharadwaj, S. Understanding on the possible routes for SARS-CoV-2 invasion via ACE2 in the host linked with multiple organs damage. Infect. Genet. Evol. 2022, 99, 105254. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, K.; Yu, J.; Howard, D.; French, L.; Chen, Z.; Wen, C.; Xu, Z. The Spatial and Cell-Type Distribution of SARS-CoV-2 Receptor ACE2 in the Human and Mouse Brains. Front. Neurol. 2020, 11, 573095. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Shults, N.V.; Gychka, S.G.; Harris, B.T.; Suzuki, Y.J. Protein Expression of Angiotensin-Converting Enzyme 2 (ACE2) is Upregulated in Brains with Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 1687. [Google Scholar] [CrossRef]
- Hernandez, V.S.; Zetter, M.A.; Guerra, E.C.; Hernandez-Araiza, I.; Karuzin, N.; Hernandez-Perez, O.R.; Eiden, L.E.; Zhang, L. ACE2 expression in rat brain: Implications for COVID-19 associated neurological manifestations. Exp. Neurol. 2021, 345, 113837. [Google Scholar] [CrossRef]
- Qiao, J.; Li, W.; Bao, J.; Peng, Q.; Wen, D.; Wang, J.; Sun, B. The expression of SARS-CoV-2 receptor ACE2 and CD147, and protease TMPRSS2 in human and mouse brain cells and mouse brain tissues. Biochem. Biophys. Res. Commun. 2020, 533, 867–871. [Google Scholar] [CrossRef]
- Clough, E.; Inigo, J.; Chandra, D.; Chaves, L.; Reynolds, J.L.; Aalinkeel, R.; Schwartz, S.A.; Khmaladze, A.; Mahajan, S.D. Mitochondrial Dynamics in SARS-CoV-2 Spike Protein Treated Human Microglia: Implications for Neuro-COVID. J. Neuroimmune Pharmacol. 2021, 16, 770–784. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Jia, L.; Zhang, Y. Potential mechanism of SARS-CoV-2-associated central and peripheral nervous system impairment. Acta Neurol. Scand. 2022, 146, 225–236. [Google Scholar] [CrossRef]
- Lindskog, C.; Mear, L.; Virhammar, J.; Fallmar, D.; Kumlien, E.; Hesselager, G.; Casar-Borota, O.; Rostami, E. Protein Expression Profile of ACE2 in the Normal and COVID-19-Affected Human Brain. J. Proteome Res. 2022, 21, 2137–2145. [Google Scholar] [CrossRef]
- Jeong, G.U.; Lyu, J.; Kim, K.D.; Chung, Y.C.; Yoon, G.Y.; Lee, S.; Hwang, I.; Shin, W.H.; Ko, J.; Lee, J.Y.; et al. SARS-CoV-2 Infection of Microglia Elicits Proinflammatory Activation and Apoptotic Cell Death. Microbiol. Spectr. 2022, 10, e0109122. [Google Scholar] [CrossRef]
- Netland, J.; Meyerholz, D.K.; Moore, S.; Cassell, M.; Perlman, S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008, 82, 7264–7275. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Castaneda, A.; Lu, P.; Geraghty, A.C.; Song, E.; Lee, M.H.; Wood, J.; O’Dea, M.R.; Dutton, S.; Shamardani, K.; Nwangwu, K.; et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 2022, 185, 2452–2468.e16. [Google Scholar] [CrossRef] [PubMed]
- Bilinska, K.; Jakubowska, P.; Von Bartheld, C.S.; Butowt, R. Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem. Neurosci. 2020, 11, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Burks, S.M.; Rosas-Hernandez, H.; Alenjandro Ramirez-Lee, M.; Cuevas, E.; Talpos, J.C. Can SARS-CoV-2 infect the central nervous system via the olfactory bulb or the blood-brain barrier? Brain Behav. Immun. 2021, 95, 7–14. [Google Scholar] [CrossRef]
- Abbasi, A.Z.; Kiyani, D.A.; Hamid, S.M.; Saalim, M.; Fahim, A.; Jalal, N. Spiking dependence of SARS-CoV-2 pathogenicity on TMPRSS2. J. Med. Virol. 2021, 93, 4205–4218. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Wettstein, L.; Kirchhoff, F.; Munch, J. The Transmembrane Protease TMPRSS2 as a Therapeutic Target for COVID-19 Treatment. Int. J. Mol. Sci. 2022, 23, 1351. [Google Scholar] [CrossRef]
- Hinduja, A.; Moutairou, A.; Calvet, J.H. Sudomotor dysfunction in patients recovered from COVID-19. Neurophysiol. Clin. 2021, 51, 193–196. [Google Scholar] [CrossRef]
- Pizzanelli, C.; Milano, C.; Canovetti, S.; Tagliaferri, E.; Turco, F.; Verdenelli, S.; Nesti, L.; Franchi, M.; Bonanni, E.; Menichetti, F.; et al. Autoimmune limbic encephalitis related to SARS-CoV-2 infection: Case-report and review of the literature. Brain Behav. Immun. Health 2021, 12, 100210. [Google Scholar] [CrossRef]
- Emmerton, D.; Abdelhafiz, A.H. Care for Older People with Dementia During COVID-19 Pandemic. SN Compr. Clin. Med. 2021, 3, 437–443. [Google Scholar] [CrossRef]
- Yu, Y.; Travaglio, M.; Popovic, R.; Leal, N.S.; Martins, L.M. Alzheimer’s and Parkinson’s Diseases Predict Different COVID-19 Outcomes: A UK Biobank Study. Geriatrics 2021, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J.; Yong, M.H.; Teoh, S.L.; Soga, T.; Parhar, I.; Chew, J.; Lim, W.L. The Hippocampal Vulnerability to Herpes Simplex Virus Type I Infection: Relevance to Alzheimer’s Disease and Memory Impairment. Front. Cell. Neurosci. 2021, 15, 695738. [Google Scholar] [CrossRef]
- Readhead, B.; Haure-Mirande, J.V.; Funk, C.C.; Richards, M.A.; Shannon, P.; Haroutunian, V.; Sano, M.; Liang, W.S.; Beckmann, N.D.; Price, N.D.; et al. Multiscale Analysis of Independent Alzheimer’s Cohorts Finds Disruption of Molecular, Genetic, and Clinical Networks by Human Herpesvirus. Neuron 2018, 99, 64–82.e7. [Google Scholar] [CrossRef]
- Galeotti, C.; Bayry, J. Autoimmune and inflammatory diseases following COVID-19. Nat. Rev. Rheumatol. 2020, 16, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Al-Beltagi, M.; Saeed, N.K.; Bediwy, A.S. COVID-19 disease and autoimmune disorders: A mutual pathway. World J. Methodol. 2022, 12, 200–223. [Google Scholar] [CrossRef] [PubMed]
- Bhagavati, S. Autoimmune Disorders of the Nervous System: Pathophysiology, Clinical Features, and Therapy. Front. Neurol. 2021, 12, 664664. [Google Scholar] [CrossRef]
- Rodriguez, Y.; Novelli, L.; Rojas, M.; De Santis, M.; Acosta-Ampudia, Y.; Monsalve, D.M.; Ramirez-Santana, C.; Costanzo, A.; Ridgway, W.M.; Ansari, A.A.; et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J. Autoimmun. 2020, 114, 102506. [Google Scholar] [CrossRef]
- Tufan, A.; Avanoglu Guler, A.; Matucci-Cerinic, M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk. J. Med. Sci. 2020, 50, 620–632. [Google Scholar] [CrossRef]
- Al-Hazmi, A. Challenges presented by MERS corona virus, and SARS corona virus to global health. Saudi J. Biol. Sci. 2016, 23, 507–511. [Google Scholar] [CrossRef]
- Verstrepen, K.; Baisier, L.; De Cauwer, H. Neurological manifestations of COVID-19, SARS and MERS. Acta Neurol. Belg. 2020, 120, 1051–1060. [Google Scholar] [CrossRef]
- Anwar, M.M.; Badawi, A.M.; Eltablawy, N.A. Can the coronavirus infection penetrates the brain resulting in sudden anosmia followed by severe neurological disorders? eNeurologicalSci 2020, 21, 100290. [Google Scholar] [CrossRef] [PubMed]
- Desforges, M.; Le Coupanec, A.; Dubeau, P.; Bourgouin, A.; Lajoie, L.; Dube, M.; Talbot, P.J. Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System? Viruses 2019, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim Fouad, G. The neuropathological impact of COVID-19: A review. Bull. Natl. Res. Cent. 2021, 45, 19. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020, 87, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Cavallieri, F.; Sellner, J.; Zedde, M.; Moro, E. Neurologic complications of coronavirus and other respiratory viral infections. Handb. Clin. Neurol. 2022, 189, 331–358. [Google Scholar] [CrossRef]
- McEntire, C.R.S.; Song, K.W.; McInnis, R.P.; Rhee, J.Y.; Young, M.; Williams, E.; Wibecan, L.L.; Nolan, N.; Nagy, A.M.; Gluckstein, J.; et al. Neurologic Manifestations of the World Health Organization’s List of Pandemic and Epidemic Diseases. Front. Neurol. 2021, 12, 634827. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brunink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Harapan, B.N.; Yoo, H.J. Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19). J. Neurol. 2021, 268, 3059–3071. [Google Scholar] [CrossRef]
- Maiese, A.; Manetti, A.C.; Bosetti, C.; Del Duca, F.; La Russa, R.; Frati, P.; Di Paolo, M.; Turillazzi, E.; Fineschi, V. SARS-CoV-2 and the brain: A review of the current knowledge on neuropathology in COVID-19. Brain Pathol. 2021, 31, e13013. [Google Scholar] [CrossRef]
- Pajo, A.T.; Espiritu, A.I.; Apor, A.; Jamora, R.D.G. Neuropathologic findings of patients with COVID-19: A systematic review. Neurol. Sci. 2021, 42, 1255–1266. [Google Scholar] [CrossRef]
- Thakur, K.T.; Miller, E.H.; Glendinning, M.D.; Al-Dalahmah, O.; Banu, M.A.; Boehme, A.K.; Boubour, A.L.; Bruce, S.S.; Chong, A.M.; Claassen, J.; et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain 2021, 144, 2696–2708. [Google Scholar] [CrossRef] [PubMed]
- Arbour, N.; Day, R.; Newcombe, J.; Talbot, P.J. Neuroinvasion by human respiratory coronaviruses. J. Virol. 2000, 74, 8913–8921. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.S.C.; Zumla, A. Severe Acute Respiratory Syndrome: Historical, Epidemiologic, and Clinical Features. Infect. Dis. Clin. North. Am. 2019, 33, 869–889. [Google Scholar] [CrossRef]
- Pormohammad, A.; Ghorbani, S.; Khatami, A.; Farzi, R.; Baradaran, B.; Turner, D.L.; Turner, R.J.; Bahr, N.C.; Idrovo, J.P. Comparison of confirmed COVID-19 with SARS and MERS cases—Clinical characteristics, laboratory findings, radiographic signs and outcomes: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, e2112. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Coronavirus COVID-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ 2020, 368, m641. [Google Scholar] [CrossRef] [PubMed]
- Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P.; Ceccaldi, M.; et al. (18)F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2823–2833. [Google Scholar] [CrossRef]
- Grossberg, A.N.; Koza, L.A.; Ledreux, A.; Prusmack, C.; Krishnamurthy, H.K.; Jayaraman, V.; Granholm, A.C.; Linseman, D.A. A multiplex chemiluminescent immunoassay for serological profiling of COVID-19-positive symptomatic and asymptomatic patients. Nat. Commun. 2021, 12, 740. [Google Scholar] [CrossRef]
- Mirfazeli, F.S.; Sarabi-Jamab, A.; Jahanbakhshi, A.; Kordi, A.; Javadnia, P.; Shariat, S.V.; Aloosh, O.; Almasi-Dooghaee, M.; Faiz, S.H.R. Neuropsychiatric manifestations of COVID-19 can be clustered in three distinct symptom categories. Sci. Rep. 2020, 10, 20957. [Google Scholar] [CrossRef]
- Sweid, A.; Hammoud, B.; Bekelis, K.; Missios, S.; Tjoumakaris, S.I.; Gooch, M.R.; Herial, N.A.; Zarzour, H.; Romo, V.; DePrince, M.; et al. Cerebral ischemic and hemorrhagic complications of coronavirus disease 2019. Int. J. Stroke 2020, 15, 733–742. [Google Scholar] [CrossRef]
- Aghayari Sheikh Neshin, S.; Shahjouei, S.; Koza, E.; Friedenberg, I.; Khodadadi, F.; Sabra, M.; Kobeissy, F.; Ansari, S.; Tsivgoulis, G.; Li, J.; et al. Stroke in SARS-CoV-2 Infection: A Pictorial Overview of the Pathoetiology. Front. Cardiovasc. Med. 2021, 8, 649922. [Google Scholar] [CrossRef]
- Mutiawati, E.; Fahriani, M.; Mamada, S.S.; Fajar, J.K.; Frediansyah, A.; Maliga, H.A.; Ilmawan, M.; Emran, T.B.; Ophinni, Y.; Ichsan, I.; et al. Anosmia and dysgeusia in SARS-CoV-2 infection: Incidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms—A systematic review and meta-analysis. F1000Research 2021, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Pua Torrejon, R.C.; Ordono Saiz, M.V.; Gonzalez Alguacil, E.; Furones Garcia, M.; Cantarin Extremera, V.; Ruiz Falco, M.L.; Soto Insuga, V. Smell and Taste Dysfunction in Pediatric Patients With SARS-CoV-2 Infection. Pediatr. Neurol. 2022, 136, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Prado, E.; Simbana-Rivera, K.; Gomez-Barreno, L.; Rubio-Neira, M.; Guaman, L.P.; Kyriakidis, N.C.; Muslin, C.; Jaramillo, A.M.G.; Barba-Ostria, C.; Cevallos-Robalino, D.; et al. Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a comprehensive literature review. Diagn. Microbiol. Infect. Dis. 2020, 98, 115094. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, W.; Chen, F.; Cui, L. COVID-19 and cognitive impairment: Neuroinvasive and blood–brain barrier dysfunction. J. Neuroinflammation 2022, 19, 222. [Google Scholar] [CrossRef] [PubMed]
- Tyson, B.; Shahein, A.; Erdodi, L.; Tyson, L.; Tyson, R.; Ghomi, R.; Agarwal, P. Delirium as a Presenting Symptom of COVID-19. Cogn. Behav. Neurol. 2022, 35, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Caronna, E.; Ballve, A.; Llaurado, A.; Gallardo, V.J.; Ariton, D.M.; Lallana, S.; Lopez Maza, S.; Olive Gadea, M.; Quibus, L.; Restrepo, J.L.; et al. Headache: A striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia 2020, 40, 1410–1421. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Montero, M.T.V.; Rowe, K.; Kirton, R.; Kunik, F., Jr. Epidemiology, pathogenesis, clinical presentations, diagnosis and treatment of COVID-19: A review of current evidence. Expert. Rev. Clin. Pharmacol. 2021, 14, 601–621. [Google Scholar] [CrossRef]
- Rathore, V.; Galhotra, A.; Pal, R.; Sahu, K.K. COVID-19 Pandemic and Children: A Review. J. Pediatr. Pharmacol. Ther. 2020, 25, 574–585. [Google Scholar] [CrossRef]
- Rando, H.M.; Bennett, T.D.; Byrd, J.B.; Bramante, C.; Callahan, T.J.; Chute, C.G.; Davis, H.E.; Deer, R.; Gagnier, J.; Koraishy, F.M.; et al. Challenges in defining Long COVID: Striking differences across literature, Electronic Health Records, and patient-reported information. medRxiv 2021. [Google Scholar] [CrossRef]
- Tana, C.; Bentivegna, E.; Cho, S.J.; Harriott, A.M.; Garcia-Azorin, D.; Labastida-Ramirez, A.; Ornello, R.; Raffaelli, B.; Beltran, E.R.; Ruscheweyh, R.; et al. Long COVID headache. J. Headache Pain 2022, 23, 93. [Google Scholar] [CrossRef]
- Kakovan, M.; Ghorbani Shirkouhi, S.; Zarei, M.; Andalib, S. Stroke Associated with COVID-19 Vaccines. J. Stroke Cerebrovasc. Dis. 2022, 31, 106440. [Google Scholar] [CrossRef] [PubMed]
- Kantarcioglu, B.; Iqbal, O.; Lewis, J.; Carter, C.A.; Singh, M.; Lievano, F.; Ligocki, M.; Jeske, W.; Adiguzel, C.; Gerotziafas, G.T.; et al. An Update on the Status of Vaccine Development for SARS-CoV-2 Including Variants. Practical Considerations for COVID-19 Special Populations. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296211056648. [Google Scholar] [CrossRef] [PubMed]
- Sriwastava, S.; Sharma, K.; Khalid, S.H.; Bhansali, S.; Shrestha, A.K.; Elkhooly, M.; Srivastava, S.; Khan, E.; Jaiswal, S.; Wen, S. COVID-19 Vaccination and Neurological Manifestations: A Review of Case Reports and Case Series. Brain Sci. 2022, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, B.; Rayamajhi, G.; Lamichhane, P.; Shenoy, A.K. Adverse Events following Immunization with COVID-19 Vaccines: A Narrative Review. Biomed. Res. Int. 2022, 2022, 2911333. [Google Scholar] [CrossRef]
- Pyne, J.D.; Brickman, A.M. The Impact of the COVID-19 Pandemic on Dementia Risk: Potential Pathways to Cognitive Decline. Neurodegener. Dis. 2021, 21, 1–23. [Google Scholar] [CrossRef]
- Barrantes, F.J. Central Nervous System Targets and Routes for SARS-CoV-2: Current Views and New Hypotheses. ACS Chem. Neurosci. 2020, 11, 2793–2803. [Google Scholar] [CrossRef]
- Deng, Q.; Rasool, R.U.; Russell, R.M.; Natesan, R.; Asangani, I.A. Targeting androgen regulation of TMPRSS2 and ACE2 as a therapeutic strategy to combat COVID-19. iScience 2021, 24, 102254. [Google Scholar] [CrossRef]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef]
- Urmila, A.; Rashmi, P.; Nilam, G.; Subhash, B. Recent Advances in the Endogenous Brain Renin-Angiotensin System and Drugs Acting on It. J. Renin Angiotensin Aldosterone Syst. 2021, 2021, 9293553. [Google Scholar] [CrossRef]
- Wang, X.L.; Iwanami, J.; Min, L.J.; Tsukuda, K.; Nakaoka, H.; Bai, H.Y.; Shan, B.S.; Kan-No, H.; Kukida, M.; Chisaka, T.; et al. Deficiency of angiotensin-converting enzyme 2 causes deterioration of cognitive function. NPJ Aging Mech. Dis. 2016, 2, 16024. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Miura, Y.; Tanabe, C.; Maeda, T.; Terayama, Y.; Turner, A.J.; Zou, K.; Komano, H. Conversion of Abeta43 to Abeta40 by the successive action of angiotensin-converting enzyme 2 and angiotensin-converting enzyme. J. Neurosci. Res. 2014, 92, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Parit, R.; Jayavel, S. Association of ACE inhibitors and Angiotensin type II blockers with ACE2 overexpression in COVID-19 comorbidities: A pathway-based analytical study. Eur. J. Pharmacol. 2021, 896, 173899. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.E.; Esparza, T.J.; Lewis, H.A.; Kim, E.; Mac Donald, C.L.; Sullivan, P.M.; Brody, D.L. Human apolipoprotein E4 worsens acute axonal pathology but not amyloid-beta immunoreactivity after traumatic brain injury in 3xTG-AD mice. J. Neuropathol. Exp. Neurol. 2013, 72, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, Y.; Wang, Y.; Ke, Q.; Cui, L. The COVID-19 pandemic and Alzheimer’s disease: Mutual risks and mechanisms. Transl. Neurodegener. 2022, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Kurki, S.N.; Kantonen, J.; Kaivola, K.; Hokkanen, L.; Mayranpaa, M.I.; Puttonen, H.; FinnGen; Martola, J.; Poyhonen, M.; Kero, M.; et al. APOE epsilon4 associates with increased risk of severe COVID-19, cerebral microhaemorrhages and post-COVID mental fatigue: A Finnish biobank, autopsy and clinical study. Acta Neuropathol. Commun. 2021, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.; Marengoni, A.; Shenkin, S.; MacLullich, A. Delirium in COVID-19: Common, distressing and linked with poor outcomes... can we do better? Age Ageing 2021, 50, 1436–1438. [Google Scholar] [CrossRef]
- Voormolen, D.C.; Cnossen, M.C.; Polinder, S.; von Steinbuechel, N.; Vos, P.E.; Haagsma, J.A. Divergent Classification Methods of Post-Concussion Syndrome after Mild Traumatic Brain Injury: Prevalence Rates, Risk Factors, and Functional Outcome. J. Neurotrauma 2018, 35, 1233–1241. [Google Scholar] [CrossRef]
- Bullock, G.S.; Emery, C.A.; Nelson, V.R.; Prats-Uribe, A.; Gilliland, R.G.; Thigpen, C.A.; Shanley, E. Higher rates of concussion following COVID-19 infection in high school athletes. Br. J. Sport. Med. 2023, 57, 590–594. [Google Scholar] [CrossRef]
- Bodnar, C.N.; Watson, J.B.; Higgins, E.K.; Quan, N.; Bachstetter, A.D. Inflammatory Regulation of CNS Barriers After Traumatic Brain Injury: A Tale Directed by Interleukin-1. Front. Immunol. 2021, 12, 688254. [Google Scholar] [CrossRef]
- Welcome, M.O.; Mastorakis, N.E. Neuropathophysiology of coronavirus disease 2019: Neuroinflammation and blood brain barrier disruption are critical pathophysiological processes that contribute to the clinical symptoms of SARS-CoV-2 infection. Inflammopharmacology 2021, 29, 939–963. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Kochanek, P.M.; Valadka, A.B.; Clark, R.S.B.; Chou, S.H.; Au, A.K.; Horvat, C.; Jha, R.M.; Mannix, R.; Wisniewski, S.R.; et al. Blood Biomarkers for Detection of Brain Injury in COVID-19 Patients. J. Neurotrauma 2021, 38, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Zhang, J.; Dong, J.F.; Shi, F.D. Dissemination of brain inflammation in traumatic brain injury. Cell. Mol. Immunol. 2019, 16, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Fotuhi, M.; Mian, A.; Meysami, S.; Raji, C.A. Neurobiology of COVID-19. J. Alzheimers Dis. 2020, 76, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E.; Bielak-Zmijewska, A.; Dudkowska, M.; Krzystyniak, A.; Mosieniak, G.; Wesierska, M.; Wlodarczyk, J. Cellular Senescence in Brain Aging. Front. Aging Neurosci. 2021, 13, 646924. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef]
- Karnik, M.; Beeraka, N.M.; Uthaiah, C.A.; Nataraj, S.M.; Bettadapura, A.D.S.; Aliev, G.; Madhunapantula, S.V. A Review on SARS-CoV-2-Induced Neuroinflammation, Neurodevelopmental Complications, and Recent Updates on the Vaccine Development. Mol. Neurobiol. 2021, 58, 4535–4563. [Google Scholar] [CrossRef]
- Vargas, G.; Medeiros Geraldo, L.H.; Gedeao Salomao, N.; Viana Paes, M.; Regina Souza Lima, F.; Carvalho Alcantara Gomes, F. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and glial cells: Insights and perspectives. Brain Behav. Immun. Health 2020, 7, 100127. [Google Scholar] [CrossRef]
- Mavrikaki, M.; Lee, J.D.; Solomon, I.H.; Slack, F.J. Severe COVID-19 induces molecular signatures of aging in the human brain. medRxiv 2021. [Google Scholar] [CrossRef]
- Idrees, D.; Kumar, V. SARS-CoV-2 spike protein interactions with amyloidogenic proteins: Potential clues to neurodegeneration. Biochem. Biophys. Res. Commun. 2021, 554, 94–98. [Google Scholar] [CrossRef]
- Mysiris, D.S.; Vavougios, G.D.; Karamichali, E.; Papoutsopoulou, S.; Stavrou, V.T.; Papayianni, E.; Boutlas, S.; Mavridis, T.; Foka, P.; Zarogiannis, S.G.; et al. Post-COVID-19 Parkinsonism and Parkinson’s Disease Pathogenesis: The Exosomal Cargo Hypothesis. Int. J. Mol. Sci. 2022, 23, 9739. [Google Scholar] [CrossRef]
- Groh, N.; Buhler, A.; Huang, C.; Li, K.W.; van Nierop, P.; Smit, A.B.; Fandrich, M.; Baumann, F.; David, D.C. Age-Dependent Protein Aggregation Initiates Amyloid-beta Aggregation. Front. Aging Neurosci. 2017, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.H.; Prajapati, D.P.; Ritter, M.L.; DeConde, A.S. Persistent Smell Loss Following Undetectable SARS-CoV-2. Otolaryngol. Head Neck Surg. 2020, 63, 923–925. [Google Scholar] [CrossRef] [PubMed]
- Dell’Era, V.; Farri, F.; Garzaro, G.; Gatto, M.; Aluffi Valletti, P.; Garzaro, M. Smell and taste disorders during COVID-19 outbreak: A cross-sectional study on 355 patients. Head Neck 2020, 42, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Valdes, A.M.; Freidin, M.B.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Ganesh, S.; Varsavsky, T.; Cardoso, M.J.; El-Sayed Moustafa, J.S.; et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020, 26, 1037–1040. [Google Scholar] [CrossRef]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021, 218, e20202135. [Google Scholar] [CrossRef]
- Xu, J.; Lazartigues, E. Expression of ACE2 in Human Neurons Supports the Neuro-Invasive Potential of COVID-19 Virus. Cell. Mol. Neurobiol. 2020, 42, 305–309. [Google Scholar] [CrossRef]
- Schuler, B.A.; Habermann, A.C.; Plosa, E.J.; Taylor, C.J.; Jetter, C.; Negretti, N.M.; Kapp, M.E.; Benjamin, J.T.; Gulleman, P.; Nichols, D.S.; et al. Age-determined expression of priming protease TMPRSS2 and localization of SARS-CoV-2 in lung epithelium. J. Clin. Investig. 2021, 131, e140766. [Google Scholar] [CrossRef]
- Uversky, V.N.; Elrashdy, F.; Aljadawi, A.; Ali, S.M.; Khan, R.H.; Redwan, E.M. Severe acute respiratory syndrome coronavirus 2 infection reaches the human nervous system: How? J. Neurosci. Res. 2020, 99, 750–777. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Rana, V.; Parama, D.; Banik, K.; Girisa, S.; Sahu, H.; Thakur, K.K.; Dutta, U.; Garodia, P.; Gupta, S.C.; et al. COVID-19, cytokines, inflammation, and spices: How are they related? Life Sci. 2021, 284, 119201. [Google Scholar] [CrossRef]
- Agrawal, S.; Farfel, J.M.; Arfanakis, K.; Al-Harthi, L.; Shull, T.; Teppen, T.L.; Evia, A.M.; Patel, M.B.; Ely, E.W.; Leurgans, S.E.; et al. Brain autopsies of critically ill COVID-19 patients demonstrate heterogeneous profile of acute vascular injury, inflammation and age-linked chronic brain diseases. Acta Neuropathol. Commun. 2022, 10, 186. [Google Scholar] [CrossRef]
- Plini, E.R.G.; O’Hanlon, E.; Boyle, R.; Sibilia, F.; Rikhye, G.; Kenney, J.; Whelan, R.; Melnychuk, M.C.; Robertson, I.H.; Dockree, P.M. Examining the Role of the Noradrenergic Locus Coeruleus for Predicting Attention and Brain Maintenance in Healthy Old Age and Disease: An MRI Structural Study for the Alzheimer’s Disease Neuroimaging Initiative. Cells 2021, 10, 1829. [Google Scholar] [CrossRef] [PubMed]
- Bocci, M.; Oudenaarden, C.; Saenz-Sarda, X.; Simren, J.; Eden, A.; Sjolund, J.; Moller, C.; Gisslen, M.; Zetterberg, H.; Englund, E.; et al. Infection of Brain Pericytes Underlying Neuropathology of COVID-19 Patients. Int. J. Mol. Sci. 2021, 22, 11622. [Google Scholar] [CrossRef] [PubMed]
- Serrano, G.E.; Walker, J.E.; Arce, R.; Glass, M.J.; Vargas, D.; Sue, L.I.; Intorcia, A.J.; Nelson, C.M.; Oliver, J.; Papa, J.; et al. Mapping of SARS-CoV-2 Brain Invasion and Histopathology in COVID-19 Disease. medRxiv 2021. [Google Scholar] [CrossRef]
- Quan, D.; Luna Wong, L.; Shallal, A.; Madan, R.; Hamdan, A.; Ahdi, H.; Daneshvar, A.; Mahajan, M.; Nasereldin, M.; Van Harn, M.; et al. Impact of Race and Socioeconomic Status on Outcomes in Patients Hospitalized with COVID-19. J. Gen. Intern. Med. 2021, 36, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Maedler, K.; Dharmadhikari, G.; Schumann, D.M.; Storling, J. Interleukin-targeted therapy for metabolic syndrome and type 2 diabetes. In Diabetes-Perspectives in Drug Therapy; Handbook of Experimental Pharmacology; Schwanstecher, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 203, pp. 257–278. [Google Scholar] [CrossRef]
- Argaw, A.T.; Zhang, Y.; Snyder, B.J.; Zhao, M.L.; Kopp, N.; Lee, S.C.; Raine, C.S.; Brosnan, C.F.; John, G.R. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J. Immunol. 2006, 177, 5574–5584. [Google Scholar] [CrossRef] [PubMed]
- Friedman, W.J. Cytokines regulate expression of the type 1 interleukin-1 receptor in rat hippocampal neurons and glia. Exp. Neurol. 2001, 168, 23–31. [Google Scholar] [CrossRef]
- Lam, S.M.; Huang, X.; Shui, G. Neurological aspects of SARS-CoV-2 infection: Lipoproteins and exosomes as Trojan horses. Trends Endocrinol. Metab. 2022, 33, 554–568. [Google Scholar] [CrossRef]
- Pesce, E.; Manfrini, N.; Cordiglieri, C.; Santi, S.; Bandera, A.; Gobbini, A.; Gruarin, P.; Favalli, A.; Bombaci, M.; Cuomo, A.; et al. Exosomes Recovered From the Plasma of COVID-19 Patients Expose SARS-CoV-2 Spike-Derived Fragments and Contribute to the Adaptive Immune Response. Front. Immunol. 2021, 12, 785941. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef]
- DeLeo, A.M.; Ikezu, T. Extracellular Vesicle Biology in Alzheimer’s Disease and Related Tauopathy. J. Neuroimmune Pharmacol. 2018, 13, 292–308. [Google Scholar] [CrossRef]
- Hamlett, E.D.; Ledreux, A.; Potter, H.; Chial, H.J.; Patterson, D.; Espinosa, J.M.; Bettcher, B.M.; Granholm, A.C. Exosomal biomarkers in Down syndrome and Alzheimer’s disease. Free Radic. Biol. Med. 2018, 114, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Hamlett, E.D.; Goetzl, E.J.; Ledreux, A.; Vasilevko, V.; Boger, H.A.; LaRosa, A.; Clark, D.; Carroll, S.L.; Carmona-Iragui, M.; Fortea, J.; et al. Neuronal exosomes reveal Alzheimer’s disease biomarkers in Down syndrome. Alzheimers Dement. 2016, 13, 541–549. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Kim, J.H. Diverse Effects of Exosomes on COVID-19: A Perspective of Progress From Transmission to Therapeutic Developments. Front. Immunol. 2021, 12, 716407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, J.; Liu, J.; Zhang, G.; Lu, A. Advances in the discovery of exosome inhibitors in cancer. J. Enzym. Inhib. Med. Chem. 2020, 35, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L.; et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 2015, 11, 600–607.e601. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Elahi, F.M.; Mustapic, M.; Kapogiannis, D.; Pryhoda, M.; Gilmore, A.; Gorgens, K.A.; Davidson, B.; Granholm, A.C.; Ledreux, A. Altered levels of plasma neuron-derived exosomes and their cargo proteins characterize acute and chronic mild traumatic brain injury. FASEB J. 2019, 33, 5082–5088. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Mustapic, M.; Kapogiannis, D.; Eitan, E.; Lobach, I.V.; Goetzl, L.; Schwartz, J.B.; Miller, B.L. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 2016, 30, 3853–3859. [Google Scholar] [CrossRef]
- Guix, F.X.; Corbett, G.T.; Cha, D.J.; Mustapic, M.; Liu, W.; Mengel, D.; Chen, Z.; Aikawa, E.; Young-Pearse, T.; Kapogiannis, D.; et al. Detection of Aggregation-Competent Tau in Neuron-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2018, 19, 663. [Google Scholar] [CrossRef]
- Kenney, K.; Qu, B.X.; Lai, C.; Devoto, C.; Motamedi, V.; Walker, W.C.; Levin, H.S.; Nolen, T.; Wilde, E.A.; Diaz-Arrastia, R.; et al. Higher exosomal phosphorylated tau and total tau among veterans with combat-related repetitive chronic mild traumatic brain injury. Brain Inj. 2018, 32, 1276–1284. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Clift, A.K.; Coupland, C.A.C.; Keogh, R.H.; Hemingway, H.; Hippisley-Cox, J. COVID-19 Mortality Risk in Down Syndrome: Results From a Cohort Study Of 8 Million Adults. Ann. Intern. Med. 2020, 174, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, J.M. Down Syndrome and COVID-19: A Perfect Storm? Cell. Rep. Med. 2020, 1, 100019. [Google Scholar] [CrossRef]
- Huls, A.; Feany, P.T.; Zisman, S.I.; Costa, A.C.S.; Dierssen, M.; Balogh, R.; Bargagna, S.; Baumer, N.T.; Brandao, A.C.; Carfi, A.; et al. COVID-19 Vaccination of Individuals with Down Syndrome-Data from the Trisomy 21 Research Society Survey on Safety, Efficacy, and Factors Associated with the Decision to Be Vaccinated. Vaccines 2022, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.A.; Ademola, T.; Caslake, M.; Douglas, E.; Evans, J.; Greenlaw, N.; Haig, C.; Hassiotis, A.; Jahoda, A.; McConnachie, A.; et al. Towards onset prevention of cognition decline in adults with Down syndrome (The TOP-COG study): A pilot randomised controlled trial. Trials 2016, 17, 370. [Google Scholar] [CrossRef] [PubMed]
- Real de Asua, D.; Parra, P.; Costa, R.; Moldenhauer, F.; Suarez, C. A cross-sectional study of the phenotypes of obesity and insulin resistance in adults with down syndrome. Diabetes Metab. J. 2014, 38, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Wilcock, D.M.; Schmitt, F.A.; Head, E. Cerebrovascular contributions to aging and Alzheimer’s disease in Down syndrome. Biochim. Biophys. Acta 2016, 1862, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Illouz, T.; Biragyn, A.; Iulita, M.F.; Flores-Aguilar, L.; Dierssen, M.; De Toma, I.; Antonarakis, S.E.; Yu, E.; Herault, Y.; Potier, M.C.; et al. Immune Dysregulation and the Increased Risk of Complications and Mortality Following Respiratory Tract Infections in Adults With Down Syndrome. Front. Immunol. 2021, 12, 621440. [Google Scholar] [CrossRef]
- Colvin, K.L.; Yeager, M.E. What people with Down Syndrome can teach us about cardiopulmonary disease. Eur. Respir. Rev. 2017, 26, 160098. [Google Scholar] [CrossRef]
- Satge, D.; Seidel, M.G. The Pattern of Malignancies in Down Syndrome and Its Potential Context With the Immune System. Front. Immunol. 2018, 9, 3058. [Google Scholar] [CrossRef]
- Andolfo, I.; Russo, R.; Lasorsa, V.A.; Cantalupo, S.; Rosato, B.E.; Bonfiglio, F.; Frisso, G.; Abete, P.; Cassese, G.M.; Servillo, G.; et al. Common variants at 21q22.3 locus influence MX1 and TMPRSS2 gene expression and susceptibility to severe COVID-19. iScience 2021, 24, 102322. [Google Scholar] [CrossRef]
- Lockrow, J.P.; Fortress, A.M.; Granholm, A.C. Age-related neurodegeneration and memory loss in down syndrome. Curr. Gerontol. Geriatr. Res. 2012, 2012, 463909. [Google Scholar] [CrossRef]
- Bras, A.; Rodrigues, A.S.; Gomes, B.; Rueff, J. Down syndrome and microRNAs. Biomed. Rep. 2018, 8, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Pasca, S.; Jurj, A.; Petrushev, B.; Tomuleasa, C.; Matei, D. MicroRNA-155 Implication in M1 Polarization and the Impact in Inflammatory Diseases. Front. Immunol. 2020, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Derkow, K.; Rossling, R.; Schipke, C.; Kruger, C.; Bauer, J.; Fahling, M.; Stroux, A.; Schott, E.; Ruprecht, K.; Peters, O.; et al. Distinct expression of the neurotoxic microRNA family let-7 in the cerebrospinal fluid of patients with Alzheimer’s disease. PLoS ONE 2018, 13, e0200602. [Google Scholar] [CrossRef] [PubMed]
- Penarrubia, A.L.; Ruiz, M.; Porco, R.; Rao, S.N.; Juanola-Falgarona, M.; Manissero, D.; Lopez-Fontanals, M.; Pareja, J. Multiple assays in a real-time RT-PCR SARS-CoV-2 panel can mitigate the risk of loss of sensitivity by new genomic variants during the COVID-19 outbreak. Int. J. Infect. Dis. 2020, 97, 225–229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granholm, A.-C. Long-Term Effects of SARS-CoV-2 in the Brain: Clinical Consequences and Molecular Mechanisms. J. Clin. Med. 2023, 12, 3190. https://doi.org/10.3390/jcm12093190
Granholm A-C. Long-Term Effects of SARS-CoV-2 in the Brain: Clinical Consequences and Molecular Mechanisms. Journal of Clinical Medicine. 2023; 12(9):3190. https://doi.org/10.3390/jcm12093190
Chicago/Turabian StyleGranholm, Ann-Charlotte. 2023. "Long-Term Effects of SARS-CoV-2 in the Brain: Clinical Consequences and Molecular Mechanisms" Journal of Clinical Medicine 12, no. 9: 3190. https://doi.org/10.3390/jcm12093190
APA StyleGranholm, A.-C. (2023). Long-Term Effects of SARS-CoV-2 in the Brain: Clinical Consequences and Molecular Mechanisms. Journal of Clinical Medicine, 12(9), 3190. https://doi.org/10.3390/jcm12093190





