The Prognostic Characteristics and Recurrence Patterns of High Grade Endometrioid Endometrial Cancer: A Large Retrospective Analysis of a Tertiary Center
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; Xu, R.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019, A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer. Ann. Oncol. 2016, 26, 2–30. [Google Scholar] [CrossRef]
- Koskas, M.; Amant, F.; Mirza, M.R.; Creutzberg, C.L. Cancer of the corpus uteri: 2021 update. Int. J. Gynaecol. Obstet. Off. Organ. Int. Fed. Gynaecol. Obstet. 2021, 155, 45–60. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Soleymani majd, H.; Ferrari, F.; Gubbala, K.; Campanile, R.G.; Tozzi, R. Latest developments and techniques in gynaecological oncology surgery. Curr. Opin. Obstet. Gynecol. 2015, 27, 291–296. [Google Scholar] [CrossRef]
- Voss, M.A.; Ganesan, R.; Ludeman, L.; McCarthy, K.; Gornall, R.; Schaller, G.; Wei, W.; Sundar, S. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer-a clinical and pathological evaluation. Gynecol. Oncol. 2012, 124, 15–20. [Google Scholar] [CrossRef]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Felix, A.S.; Stone, R.A.; Bowser, R.; Chivukula, M.; Edwards, R.P.; Weissfeld, J.L.; Linkov, F. Comparison of survival outcomes between patients with malignant mixed mullerian tumors and high-grade endometrioid, clear cell, and papillary serous endometrial cancers. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2011, 21, 877–884. [Google Scholar] [CrossRef]
- Hamilton, C.A.; Cheung, M.K.; Osann, K.; Chen, L.; Teng, N.N.; A Longacre, T.; A Powell, M.; Hendrickson, M.R.; Kapp, D.S.; Chan, J.K. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br. J. Cancer 2006, 94, 642–646. [Google Scholar] [CrossRef]
- McGunigal, M.; Liu, J.; Kalir, T.; Chadha, M.; Gupta, V. Survival Differences Among Uterine Papillary Serous, Clear Cell and Grade 3 Endometrioid Adenocarcinoma Endometrial Cancers: A National Cancer Database Analysis. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2017, 27, 85–92. [Google Scholar] [CrossRef]
- Kato, M.K.; Yoshida, H.; Uehara, T.; Uno, M.; Ishikawa, M.; Miyasaka, N.; Kato, T. Unique prognostic features of grade 3 endometrioid endometrial carcinoma: Findings from 101 consecutive cases at a Japanese tertiary cancer center. Taiwan J. Obstet. Gynecol. 2021, 60, 238–244. [Google Scholar] [CrossRef]
- Wang, J.; Jia, N.; Li, Q.; Wang, C.; Tao, X.; Hua, K.; Feng, W. Analysis of recurrence and survival rates in grade 3 endometrioid endometrial carcinoma. Oncol. Lett. 2016, 12, 2860–2867. [Google Scholar] [CrossRef]
- Creutzberg, C.L.; Bosse, T. Molecular risk stratification to direct therapy in endometrial cancer: Ready for the clinic? Ann. Oncol. 2018, 29, 1081–1082. [Google Scholar] [CrossRef]
- Morotti, M.; Soleymani majd, H.; Casarin, J.; Alazzam, M.; Damato, S. Histomolecular features of high-grade endometrial cancers. Minerva Med. 2021, 112, 20–30. [Google Scholar] [CrossRef]
- Ferrari, F.; Forte, S.; Arrigoni, G.; Ardighieri, L.; Coppola, M.C.; Salinaro, F.; Barra, F.; Sartori, E.; Odicino, F. Impact of endometrial sampling technique and biopsy volume on the diagnostic accuracy of endometrial cancer. Transl. Cancer Res. 2020, 9, 7697–7705. [Google Scholar] [CrossRef]
- Zouridis, A.; Kehoe, S.T.; Soleymani Majd, H. Should laparoscopy be revisited in the management of stage II endometrial cancer in the post-LACC era? Minerva Obstet. Gynecol. 2023. [Google Scholar] [CrossRef]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; von Elm, E.; Langan, S.M. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med. 2015, 12, e1001885. [Google Scholar] [CrossRef]
- Di Donato, V.; D’oria, O.; Giannini, A.; Bogani, G.; Fischetti, M.; Santangelo, G.; Tomao, F.; Palaia, I.; Perniola, G.; Muzii, L.; et al. Age-Adjusted Charlson Comorbidity Index Predicts Survival in Endometrial Cancer Patients. Gynecol. Obstet. Investig. 2022, 87, 191–199. [Google Scholar] [CrossRef]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. Off. Organ. Int. Fed. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, T.-J.; Lee, Y.-Y.; Choi, C.H.; Lee, J.-W.; Bae, D.-S.; Kim, B.-G. A comparison of uterine papillary serous, clear cell carcinomas, and grade 3 endometrioid corpus cancers using 2009 FIGO staging system. J. Gynecol. Oncol. 2013, 24, 120–127. [Google Scholar] [CrossRef]
- Gayar, O.H.; Patel, S.; Schultz, D.; Mahan, M.; Rasool, N.; Elshaikh, M.A. The impact of tumor grade on survival end points and patterns of recurrence of 949 patients with early-stage endometrioid carcinoma: A single institution study. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2014, 24, 97–101. [Google Scholar] [CrossRef]
- Zhu, J.; Wen, H.; Bi, R.; Wu, X. Clinicopathological characteristics, treatment and outcomes in uterine carcinosarcoma and grade 3 endometrial cancer patients: A comparative study. J. Gynecol. Oncol. 2016, 27, e18. [Google Scholar] [CrossRef]
- Singh, N.; Hirschowitz, L.; Zaino, R.; Alvarado-Cabrero, I.; Duggan, M.A.; Ali-Fehmi, R.; Euscher, E.; Hecht, J.L.; Horn, L.-C.; Ioffe, O.; et al. Pathologic Prognostic Factors in Endometrial Carcinoma (Other Than Tumor Type and Grade). Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2019, 38 (Suppl. S1), S93–S113. [Google Scholar] [CrossRef]
- Rasool, N.; Fader, A.N.; Seamon, L.; Neubauer, N.L.; Abu Shahin, F.; Alexander, H.A.; Moore, K.; Moxley, K.; Secord, A.A.; Kunos, C.; et al. Stage I, grade 3 endometrioid adenocarcinoma of the endometrium: An analysis of clinical outcomes and patterns of recurrence. Gynecol. Oncol. 2010, 116, 10–14. [Google Scholar] [CrossRef]
- Larson, D.M.; Connor, G.P.; Broste, S.K.; Krawisz, B.R.; Johnson, K.K. Prognostic significance of gross myometrial invasion with endometrial cancer. Obstet. Gynecol. 1996, 88, 394–398. [Google Scholar] [CrossRef]
- Spagnol, G.; Noventa, M.; Bonaldo, G.; Marchetti, M.; Vitagliano, A.; Laganà, A.S.; Cavallin, F.; Scioscia, M.; Saccardi, C.; Tozzi, R. Three-dimensional transvaginal ultrasound vs magnetic resonance imaging for preoperative staging of deep myometrial and cervical invasion in patients with endometrial cancer: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2022, 60, 604–611. [Google Scholar] [CrossRef]
- Padilla-Iserte, P.; Lago, V.; Tauste, C.; Díaz-Feijoo, B.; Gil-Moreno, A.; Oliver, R.; Coronado, P.; Martín-Salamanca, M.B.; Pantoja-Garrido, M.; Marcos-Sanmartin, J.; et al. Impact of uterine manipulator on oncological outcome in endometrial cancer surgery. Am. J. Obstet. Gynecol. 2021, 224, 65.e1–65.e11. [Google Scholar] [CrossRef]
- Fung-Kee-Fung, M.; Dodge, J.; Elit, L.; Lukka, H.; Chambers, A.; Oliver, T. Follow-up after primary therapy for endometrial cancer: A systematic review. Gynecol. Oncol. 2006, 101, 520–529. [Google Scholar] [CrossRef]
- Newton, C.; Nordin, A.; Rolland, P.; Ind, T.; Larsen-Disney, P.; Martin-Hirsch, P.; Beaver, K.; Bolton, H.; Peevor, R.; Fernandes, A.; et al. British Gynaecological Cancer Society recommendations and guidance on patient-initiated follow-up (PIFU). Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 695–700. [Google Scholar] [CrossRef]
- Morrison, J.; Balega, J.; Buckley, L.; Clamp, A.; Crosbie, E.; Drew, Y.; Durrant, L.; Forrest, J.; Fotopoulou, C.; Gajjar, K.; et al. British Gynaecological Cancer Society (BGCS) uterine cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 270, 50–89. [Google Scholar] [CrossRef]
- Keys, H.M.; Roberts, J.A.; Brunetto, V.L.; Zaino, R.J.; Spirtos, N.M.; Bloss, J.D.; Pearlman, A.; Maiman, M.A.; Bell, J.G. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2004, 92, 744–751. [Google Scholar] [CrossRef]
- Sperling, C.; Sandager, M.; Jensen, H.; Knudsen, J.L. Current organisation of follow-up does not meet cancer patients’ needs. Dan. Med. J. 2014, 61, A4855. [Google Scholar]
- Nordin, A.J.; National Group of Gynaecology NSSG Leads. Mode of detection of recurrent gynecological malignancy: Does routine follow-up delay diagnosis and treatment? Int. J. Gynecol. Cancer Off J. Int. Gynecol. Cancer. Soc. 2006, 16, 1746–1748. [Google Scholar] [CrossRef]
- Department of Health NHS Hospital Outpatient Activity 2021–2022. 2022. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/hospital-outpatient-activity/2021-22 (accessed on 10 January 2023).
- Coleman, L.; Newton, C. Patient initiated follow up after gynaecological malignancy: National survey of current UK practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 248, 193–197. [Google Scholar] [CrossRef]
- Bellone, S.; Bignotti, E.; Lonardi, S.; Ferrari, F.; Centritto, F.; Masserdotti, A.; Pettinella, F.; Black, J.; Menderes, G.; Altwerger, G.; et al. Polymerase ε (POLE) ultra-mutation in uterine tumors correlates with T lymphocyte infiltration and increased resistance to platinum-based chemotherapy in vitro. Gynecol. Oncol. 2017, 144, 146–152. [Google Scholar] [CrossRef]
- Ardighieri, L.; Palicelli, A.; Ferrari, F.; Bugatti, M.; Drera, E.; Sartori, E.; Odicino, F. Endometrial Carcinomas with Intestinal-Type Metaplasia/Differentiation: Does Mismatch Repair System Defects Matter? Case Report and Systematic Review of the Literature. J. Clin. Med. 2020, 9, 2552. [Google Scholar] [CrossRef]
- Ravaggi, A.; Capoferri, D.; Ardighieri, L.; Ghini, I.; Ferrari, F.; Romani, C.; Bugatti, M.; Zanotti, L.; Vrede, S.; Tognon, G.; et al. Integrated Biomarker Analysis Reveals L1CAM as a Potential Stratification Marker for No Specific Molecular Profile High-Risk Endometrial Carcinoma. Cancers 2022, 14, 5429. [Google Scholar] [CrossRef]
- Shazly, S.A.; Coronado, P.J.; Yılmaz, E.; Melekoglu, R.; Sahin, H.; Giannella, L.; Ciavattini, A.; Carpini, G.D.; Di Giuseppe, J.; Yordanov, A.; et al. Endometrial Cancer Individualized Scoring System (ECISS): A machine learning-based prediction model of endometrial cancer prognosis. Int. J. Gynecol. Obstet. 2023, 14639. [Google Scholar] [CrossRef]
| N (%) | Recurrences | Cancer-Related Deaths | |||
|---|---|---|---|---|---|
| N | p-Value | N | p-Value | ||
| AGE | 0.377 | 0.139 | |||
| <65 | 30 (31.3) | 3 | 3 | ||
| ≥65 | 66 (68.8) | 12 | 15 | ||
| AACCS | 0.593 | 0.552 | |||
| 0–1 | 9 (9.4) | 1 | 1 | ||
| 2–3 | 59 (61.5) | 8 | 10 | ||
| >3 | 28 (29.2) | 6 | 7 | ||
| Surgical approach | 0.726 | 0.301 | |||
| Laparoscopy | 79 (82.3) | 12 | 13 | ||
| Laparotomy | 17 (17.7) | 3 | 5 | ||
| Pelvic lymph node dissection | 0.471 | 0.097 | |||
| No | 18 (18.8) | 4 | 6 | ||
| Yes | 78 (81.3) | 11 | 12 | ||
| Adjuvant treatment | 0.449 | 0.729 | |||
| No | 15 (16.9) | 1 | 3 | ||
| Yes | 74 (83.1) | 13 | 13 | ||
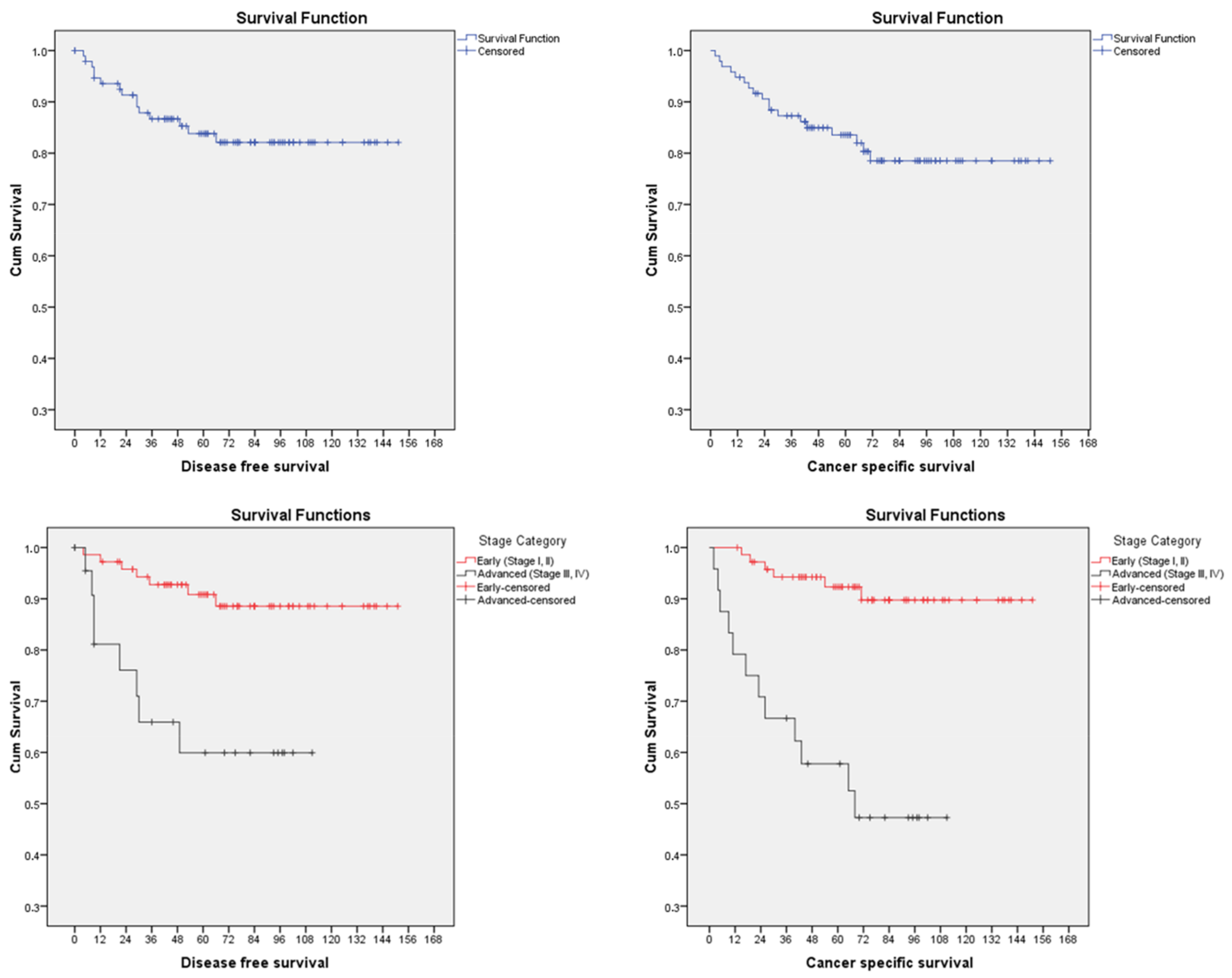
| FIGO Stage | 0.179 | 0.02 * | |||
| IA | 41 (42.7) | 3 | 3 | ||
| ΙΒ | 26 (27.1) | 3 | 2 | ||
| ΙΙ | 5 (5.2) | 1 | 1 | ||
| ΙΙΙA | 5 (5.2) | 2 | 2 | ||
| ΙΙΙΒ | 6 (6.3) | 1 | 3 | ||
| ΙΙΙC1 | 7 (7.3) | 3 | 4 | ||
| IIIC2 | 3 (3.1) | 1 | 1 | ||
| IVA | 0 (0) | 0 | 0 | ||
| IVB | 3 (3.1) | 1 | 2 | ||
| Depth of myometrial invasion | 0.135 | 0.028 * | |||
| <50% | 49 (51) | 5 | 5 | ||
| ≥50% | 47 (49) | 10 | 13 | ||
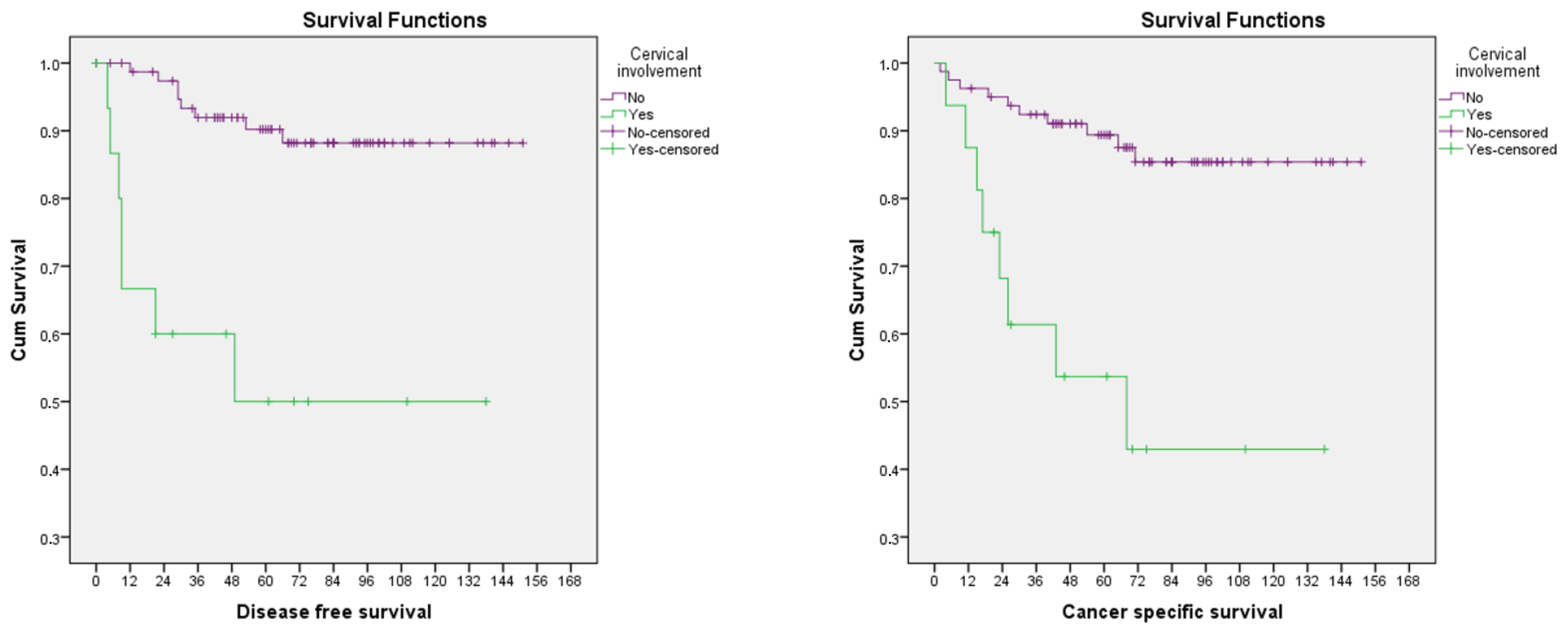
| Cervical stroma involvement | 0.003 * | 0.002 * | |||
| No | 80 (83.3) | 8 | 10 | ||
| Yes | 16 (16.7) | 7 | 9 | ||
| Adnexal involvement | 0.235 | 0.078 | |||
| No | 90 (93.8) | 13 | 15 | ||
| Yes | 6 (6.3) | 2 | 3 | ||
| Serosal breach | 0.370 | 0.010 * | |||
| No | 85 (88.5) | 12 | 11 | ||
| Yes | 11 (11.5) | 3 | 7 | ||
| Parametrial involvement | 0.653 | 0.019 * | |||
| No | 86 (89.6) | 13 | 13 | ||
| Yes | 10 (10.4) | 2 | 5 | ||
| Pelvic lymph node involvement | 0.043 * | 0.010 * | |||
| No | 67 (85.9) | 7 | 7 | ||
| Yes | 11 (14.1) | 4 | 5 | ||
| Distant metastases | 0.403 | 0.089 | |||
| No | 93 (96.9) | 14 | 16 | ||
| Yes | 3 (3.1) | 1 | 2 | ||
| LVSI | 0.736 | 0.716 | |||
| No | 41 (42.7) | 7 | 7 | ||
| Yes | 55 (57.3) | 8 | 11 | ||
| ESGO–ESTRO–ESP Risk stratification | 0.02 * | <0.001 * | |||
| Intermediate | 24 (25) | 3 | 3 | ||
| High-intermediate | 48 (50) | 4 | 3 | ||
| High | 24 (25) | 8 | 12 | ||
| Stage | Myometrial Invasion | Cervical Involvement | Adnexal Involvement | Serosal Breach | Parametrial Involvement | Pelvic LN Involvement | Paraaortic LN Involvement | Distant Metastasis | LVSI | EBRT | VBT | CT | Presenting Symptom at Recurrence | Site of Recurrence | Treatment for Recurrence | DFS (Months) | Survival after Recurrence (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IA | <50 | No | No | No | No | No | No | No | No | Yes | No | Weight loss and fatigue | Lung, kidney and brain | BSC | 66 | 5 | |
| IA | <50 | No | No | No | No | No | No | No | No | Yes | No | Vault, sigmoid colon, pelvic lymph nodes and lung | HT + EBRT | 35 | 19 | ||
| IA | <50 | No | No | No | No | No | No | No | No | Yes | No | Vaginal bleeding | Vault, pelvic bones, lung | BSC | 22 | 4 | |
| IB | ≥50 | No | No | No | No | No | No | Yes | Yes | Yes | No | Rectal bleeding | Lung | CT | 53 | ||
| IB | ≥50 | No | No | Νο | No | No | No | No | No | No | Hypoxia, vomiting and loss of weight | Vault | BSC | 29 | 1 | ||
| IB | ≥50 | No | No | No | No | No | No | Yes | No | No | Lung | EBRT | 12 | 7 | |||
| II | ≥50 | Yes | No | No | No | No | No | Yes | Yes | No | No | Shortness of breath | Vault, peritoneum, lung, liver | EBRT + CT | 4 | 11 | |
| IIIA | ≥50 | No | No | Yes | No | No | Yes | Yes | Asymptomatic (Unable to examine, hence CT) | Vault, pelvic lymph nodes, anterior abdominal wall | CT | 29 | 11 | ||||
| IIIA | <50 | Yes | Yes | No | No | No | No | Yes | No | Yes | Yes | Vault, liver, bones | HT | 21 | 2 | ||
| IIIB | ≥50 | Yes | No | Yes | Yes | No | No | No | Yes | No | No | Multifocal peritoneal deposits | BSC | 8 | 3 | ||
| IIIC1 | ≥50 | Yes | No | No | No | Yes | No | No | Yes | Yes | Yes | Right upper abdominal pain | Multifocal upper abdominal intra-abdominal nodules | HT + EBRT + CT | 49 | 19 | |
| IIIC1 | ≥50 | No | No | No | No | Yes | No | Yes | Right iliac fossa pain | Lung | CT | 30 | 35 | ||||
| IIIC1 | <50 | Yes | No | No | No | Yes | No | Yes | Yes | Yes | No | Hypoxia | Lung | CT | 9 | 8 | |
| IIIC2 | ≥50 | Yes | No | No | No | Yes | No | Yes | No | No | Yes | Asymptomatic (FU scan after radiotherapy) | 1st: Para-aortic lymph nodes, 2nd: Lung (11 months after 1st recurrence) | EBRT (for 1st recurrence) HT + CT (for 2nd recurrence) | 9 | 34 | |
| IVB | ≥50 | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No | Yes | Acute kidney injury | Vault, sigmoid colon, pelvic lymph nodes | CT | 5 | 21 |
| Recurrence | Cancer-Specific Death | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| AGE | 1.04 (0.98–1.11) | 0.192 | 1.05 (0.99–1.11) | 0.066 |
| MDT to Theatre interval | 1.01 (0.99–1.02) | 0.653 | 1.01 (0.99–1.02) | 0.471 |
| AACCS | ||||
| 0–1 | ||||
| 2–3 | 1.24 (0.16–9.89) | 0.842 | 1.53 (0.20–12.01) | 0.684 |
| >3 | 2.03 (0.24–16.81) | 0.513 | 2.36 (0.29–19.21) | 0.422 |
| Surgical approach | ||||
| Laparoscopy | ||||
| Laparotomy | 1.42 (0.40–5.02) | 0.591 | 2.18 (0.78–6.12) | 0.140 |
| Pelvic lymph node dissection | ||||
| No | ||||
| Yes | 0.46 (0.15–1.46) | 0.190 | 0.35 (0.13–0.94) | 0.038 * |
| Number of LN removed | 0.95 (0.87–1.03) | 0.215 | 0.95 (0.88–1.03) | 0.201 |
| Adjuvant treatment | ||||
| No | ||||
| Yes | 2.25 (0.30–17.25) | 0.434 | 0.72 (0.21–2.53) | 0.608 |
| FIGO Stage | ||||
| IA | ||||
| ΙΒ | 1.67 (0.34–8.28) | 0.530 | 1.12 (0.19–6.68) | 0.904 |
| ΙΙ | 4.25 (0.44–41.06) | 0.211 | 3.77 (0.39–36.41) | 0.251 |
| ΙΙΙA | 6.17 (1.03–36.99) | 0.046 * | 5.82 (0.97–34.84) | 0.054 |
| ΙΙΙΒ | 3.75 (0.39–36.12) | 0.253 | 10.59 (2.13–52.69) | 0.004 * |
| ΙΙΙC1 | 8.03 (1.61–40.06) | 0.011 * | 10.19 (2.27–45.72) | 0.002 * |
| IIIC2 | 6.19 (0.64–59.70) | 0.115 | 5.25 (0.55–50.62) | 0.151 |
| IVB | 11.09 (1.15–107.02) | 0.038 * | 15.20 (2.53–91.30) | 0.003 * |
| Stage category | ||||
| Early (I-II) | ||||
| Advanced (III-IV) | 4.72 (1.71–13.04) | 0.003 * | 7.53 (2.82–20.08) | <0.001 * |
| Depth of myometrial invasion | ||||
| <50% | ||||
| ≥50% | 2.53 (0.86–7.40) | 0.091 | 3.17 (1.13–8.90) | 0.029 * |
| Cervical stroma involvement | ||||
| No | ||||
| Yes | 7.11 (2.56–19.76) | <0.001 * | 5.59 (2.19–14.26) | <0.001 * |
| Adnexal involvement | ||||
| No | ||||
| Yes | 2.94 (0.66–13.06) | 0.155 | 3.37 (0.98–11.65) | 0.055 |
| Serosal breach | ||||
| No | ||||
| Yes | 3.11 (0.87–11.04) | 0.080 | 7.77 (3.00–20.12) | <0.001 * |
| Parametrial involvement | ||||
| No | ||||
| Yes | 2.21 (0.50–9.79) | 0.298 | 5.08 (1.80–14.30) | 0.002 * |
| Pelvic lymph node involvement | ||||
| No | ||||
| Yes | 4.58 (1.33–15.78) | 0.016 * | 5.27 (1.66–16.70) | 0.005 * |
| Distant metastases | ||||
| No | ||||
| Yes | 4.43 (0.58–33.70) | 0.150 | 5.93 (1.36–25.90) | 0.018 * |
| LVSI | ||||
| No | ||||
| Yes | 0.93 (0.34–2.57) | 0.890 | 1.25 (0.49–3.23) | 0.642 |
| ESGO–ESTRO–ESP Risk stratification | ||||
| Intermediate | ||||
| High–intermediate | 0.68 (0.15–3.04) | 0.614 | 0.51 (0.10–2.50) | 0.402 |
| High | 3.72 (0.99–14.05) | 0.053 | 5.05 (1.42–17.91) | 0.012 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouridis, A.; Zarrindej, K.; Rencher, J.; Pappa, C.; Kashif, A.; Smyth, S.L.; Sadeghi, N.; Sattar, A.; Damato, S.; Ferrari, F.; et al. The Prognostic Characteristics and Recurrence Patterns of High Grade Endometrioid Endometrial Cancer: A Large Retrospective Analysis of a Tertiary Center. J. Clin. Med. 2023, 12, 3141. https://doi.org/10.3390/jcm12093141
Zouridis A, Zarrindej K, Rencher J, Pappa C, Kashif A, Smyth SL, Sadeghi N, Sattar A, Damato S, Ferrari F, et al. The Prognostic Characteristics and Recurrence Patterns of High Grade Endometrioid Endometrial Cancer: A Large Retrospective Analysis of a Tertiary Center. Journal of Clinical Medicine. 2023; 12(9):3141. https://doi.org/10.3390/jcm12093141
Chicago/Turabian StyleZouridis, Andreas, Kianoush Zarrindej, Joshua Rencher, Christina Pappa, Ammara Kashif, Sarah Louise Smyth, Negin Sadeghi, Alisha Sattar, Stephen Damato, Federico Ferrari, and et al. 2023. "The Prognostic Characteristics and Recurrence Patterns of High Grade Endometrioid Endometrial Cancer: A Large Retrospective Analysis of a Tertiary Center" Journal of Clinical Medicine 12, no. 9: 3141. https://doi.org/10.3390/jcm12093141
APA StyleZouridis, A., Zarrindej, K., Rencher, J., Pappa, C., Kashif, A., Smyth, S. L., Sadeghi, N., Sattar, A., Damato, S., Ferrari, F., Laganà, A. S., Abdalla, M., Kehoe, S., Addley, S., & Soleymani majd, H. (2023). The Prognostic Characteristics and Recurrence Patterns of High Grade Endometrioid Endometrial Cancer: A Large Retrospective Analysis of a Tertiary Center. Journal of Clinical Medicine, 12(9), 3141. https://doi.org/10.3390/jcm12093141











