Abstract
Vonoprazan (VPZ) inhibits gastric acid secretion more potently than proton pump inhibitors. Recently, attention has been focused on the dual therapy with VPZ and amoxicillin (AMOX) for the eradication of H. pylori. The dual VPZ/AMOX therapy attains the sufficient eradication rate with lowering the risk of adverse events in comparison with the triple therapy and quadruple therapy. Therefore, the dual VPZ/AMOX therapy is considered a useful eradication regimen for H. pylori infection.
1. Introduction
One of the first-line eradication regimens for H. pylori has long been a triple therapy with proton pump inhibitor (PPI), amoxicillin (AMOX), and clarithromycin (CAM). However, with the recent increase in CAM-resistant strains of H. pylori, the eradication rates of PPI/AMOX/CAM therapy have been declining [1], which is a global problem. The reported incidences of CAM-resistance strains of H. pylori are approximately 25–32% [2,3,4]. In addition, since the effects of PPIs are influenced by the genetic polymorphism of CYP2C19, which is the main metabolic enzyme of PPIs, it has been pointed out that the eradication rates in the CYP2C19 extensive metabolizers are lower in comparison with other metabolizers [5,6]. In order to recover the eradication rates of the first-line eradication therapy, bismuth or non-bismuth quadruple therapies are being used, which are recommended especially in areas with high CAM-resistance rates [7]. Although the eradication rates of quadruple therapy are high, multiple drugs must be taken and the frequency of side effects is high.
There have been some reports of dual therapy with PPIs and AMOX as listed in Table 1 [5,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. Originally, eradication therapy for H. pylori started with classical triple therapy followed by the dual therapy. However, the eradication rates of the dual therapy have varied as shown in Table 1. After the triple therapy for 1 week was developed [14], the dual therapy with PPI and AMOX was no longer the main therapy for H. pylori infection.

Table 1.
Lists of reports of dual therapy with PPI and AMOX.
Vonoprazan (VPZ) has been clinically available since 2015. VPZ inhibits gastric acid secretion more potently than PPIs [26]. The eradication rate of the triple therapy with VPZ 20 mg bid, CAM 200 mg bid, and AMOX 750 mg bid was reported 92.6%, which was significantly higher than that of the triple therapy with lansoprazole 30 mg and the same doses of CAM and AMOX [27]. Interestingly, in this report, the eradication rate by the triple therapy with VPZ, CAM, and AMOX in patients infected with CAM-resistant strains of H. pylori was 82.0%, suggesting that the dual therapy with VPZ and AMOX for 1 week can attain the eradication rate higher than 80.0%. Then, the attention has recently been focused on the dual therapy with VPZ and AMOX.
2. Gastric Acid Secretion Required for the Dual Therapy with Amoxicillin
AMOX exerts its antibacterial action by binding to penicillin-binding protein (PBP) and inhibiting the synthesis of bacterial cell wall. According to a report examining the relationship between PBP expression in H. pylori and pH, H. pylori does not proliferate and PBP expression is low at around pH 3.0 [28]. However, at pH 7.4, H. pylori proliferates vigorously and the expression of PBP also increases, indicating that the number of targets of AMOX increases, and it is thought that AMOX becomes more effective. In addition, inhibition of gastric acid secretion leads to stabilization of antibacterial drugs in the stomach and increases their concentration in gastric juice [29], which greatly contributes to the success of eradication.
There is a report examining the relationship among the success or failure of eradication by triple therapy, CAM-resistance of H. pylori and intragastric pH [30]. When the mean 24-h intragastric pH is less than 4.5 and when the percent time for intragastric pH < 4.0 is longer than 40%, eradication will fail even if H. pylori strain is sensitive to CAM (Figure 1 blue area). In contrast, when the mean 24-h intragastric pH exceeds 5, CAM-susceptible bacteria can be successfully eradicated (Figure 1 yellow area). Interestingly, if the mean 24-h intragastric pH in the stomach is higher than 6.5 and the percent time for intragastric pH < 4.0 is 5% or less (pink part in Figure 1), in other words, if the pH 4 holding time ratio (pH 4 HTR) is 95% or more and if the mean 24 h intragastric pH is no less than 6.5, CAM-resistant strains can be eradicated. Therefore, it is suggested that eradication of H. pylori can be attained by a single antibiotic, such as AMOX, when the intragastric pH is strictly controlled to be neutral.
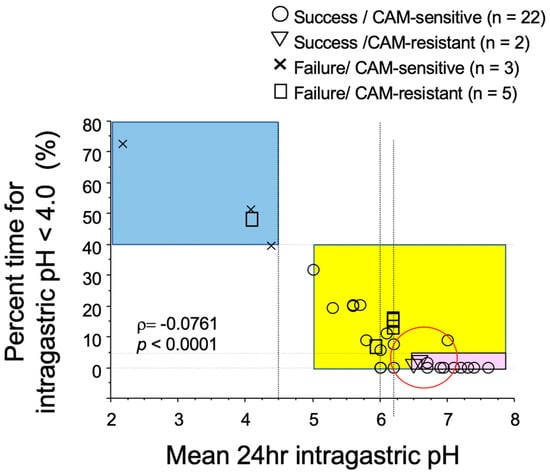
Figure 1.
Relationship of success or failure of eradication of H. pylori by the triple therapy with PPI, amoxicillin, and clarithromycin (CAM) with CAM-resistance, 24 h intragastric pH, and percent time for intragastric pH < 4.0. When the mean intragastric pH is less than 4.5 and the percent time for intragastric pH < 4.0 was longer than 40% (blue area), eradication of H. pylori fails in patients infected with not only CAM-resistant strains (□) but also CAM-sensitive strains (×) of H. pylori. When 24 h intragastric pH is higher than 5.0 (yellow area), eradication of H. pylori succeeds for CAM-sensitive strains (○) of H. pylori. When the mean intragastric pH is no less than 6.5 and the percent time for intragastric pH < 4.0 is less than 5% (pink area), eradication of H. pylori succeeds in patients infected with CAM-resistant strains (∇) as well as CAM-sensitive strains (○) of H. pylori. Modified [30].
The relationship between dosing schemes of PPI and eradication rates of the dual therapies with PPI and AMOX listed in Table 1 is plotted in Figure 2. As the dosing frequency of PPI increases, the eradication rates increase. Because the acid inhibitory effect of PPI is enhanced by the divided dosing [31], the eradication rates with dual therapy with PPI and AMOX increase with the grade of acid inhibition.
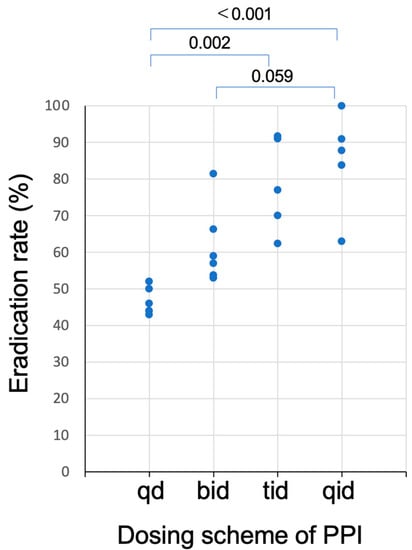
Figure 2.
Plots of the eradication rates of studies of dual therapy with PPI and amoxicillin as a function of dosing schemes of PPI listed in Table 1. Eradication rates of regimen with three (tid) or four (qid) times daily dosing of PPI was significantly higher than those with once (qd) or twice (bid) daily dosing.
Kagami et al. [32] compared the acid inhibitory effects of VPZ and EPZ and found that 95% of pH 4 HTR could be achieved by VPZ 20 mg once daily and that 100.0% of pH 4 HTR and 6.8 of the mean 24 h intragastric pH could be attained by VPZ 20 mg twice daily, but not EPZ (Figure 3). Moreover, the acid inhibitory action of VPZ was not influenced by CYP2C19 polymorphism, meaning that the gastric acid inhibition required for eradication of the H. pylori by the dual therapy with AMOX can be achieved by VPZ 20 mg twice a day irrespective of CYP2C19 polymorphism.
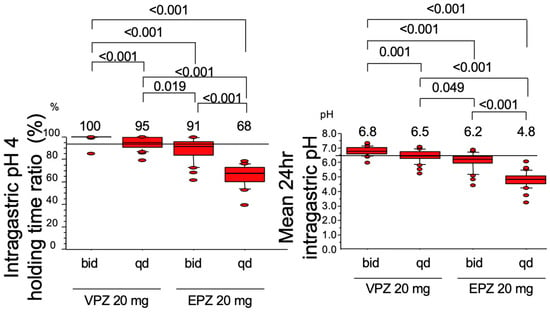
Figure 3.
Intragastric pH 4 holding time ratio (Left panel) and mean 24 h intragastric pH (Right panel) attained by the twice (bid) or once (qd) daily dosing of 20 mg of vonoprazan (VPZ) or esomeprazole (EPZ) on day 7. VPZ 20 mg twice daily can attain both the intragastric pH 4 holding time ratio 100.0% and the mean 24 h intragastric pH 6.8.
3. Antibacterial Effect and Optimal Dosing Scheme of Amoxicillin
The relationship between the eradication rates and dosing scheme of AMOX is shown in Figure 4. When AMOX was dosed twice daily, the eradication rates were all less than 60%. To attain eradication rates higher than 90.0%, at least 3 times daily dosing seems necessary for AMOX in the dual therapy. Because the antibacterial effect of AMOX is time-dependent [33] and because it has no post antibiotic effect [34], its antibacterial activity depends on the percent time above MIC (%T > MIC) [35]. Since the plasma half-life of AMOX is as short as around 1 h, it will decrease below MIC in a few hours after dosing, and therefore, it is necessary to be dosed 3–4 times a day for AMOX to be effective. This is the reason why results of the twice-daily dosing regimens of AMOX are insufficient. In contrast, in reports that achieved high eradication rates, AMOX was administered 3–4 times daily when PPI was also administered at high doses 3–4 times a day.
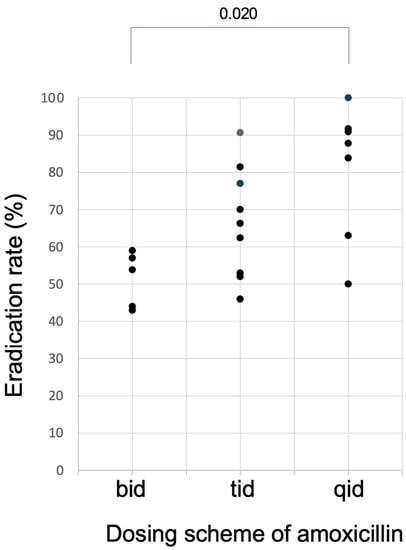
Figure 4.
Plots of the eradication rates of studies of dual therapy with PPI and amoxicillin listed in Table 1 as a function of dosing schemes of amoxicillin. Eradication rates higher than 90.0% can be attained when amoxicillin was dosed at least three time daily (tid).
There was no significant correlation between the total daily dose of AMOX and eradication rates in the regimens listed in Table 1 (p = 0.414)
Accordingly, to attain the sufficient eradication rates by the dual therapy with AMOX and an acid inhibitor, it is considered necessary to administer AMOX in 3 or more divided doses under the potent acid inhibition that can be attained by the higher doses of PPI dosed 3 or 4 times daily or VPZ 20 mg twice daily. Because the acid inhibitory effect of PPI is influenced by CYP2C19 polymorphism, but not for VPZ, VPZ is ideal and should be used for the dual therapy.
It is obvious that the time above the MIC obtained with 3 doses of AMOX 500 mg is longer than the time above the MIC obtained with 2 doses of AMOX 750 mg (Figure 5). Furthermore, since the MIC is lowered by strongly suppressing gastric acid, it is speculated that the time above the MIC will be even longer when an ultra-potent acid inhibitor, such as VPZ, is used.
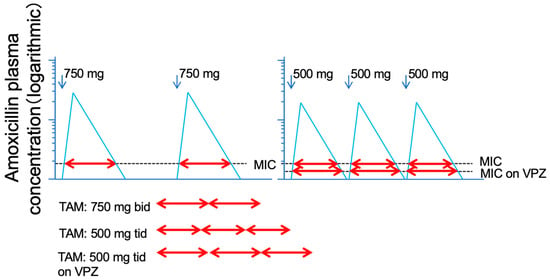
Figure 5.
Logarithmic model of Time above MIC (T > MIC) of plasma level of amoxicillin 750 mg dosed twice and 500 mg three times daily. The total of T > MIC of amoxicillin 500 mg three times daily is longer than that of 750 mg twice daily. By using the vonoprazan, MIC is expected to be lowered, resulting in the further elongation of T > MIC of amoxicillin.
4. Eradication Rates of Dual Therapy with Vonoprazan and Amoxicillin
The eradication rate reported in the first study of the dual therapy with VPZ and AMOX was 92.9%, which was identical to that of the triple therapy with VPZ, AMOX, and CAM [36]. In this report, there were no statistically significant differences in adverse events between dual therapy and triple therapy, but tended to be less with dual therapy. Therefore, it was considered that the dual VPZ/AMOX therapy could be a regimen for the first-line eradication therapy.
Since this report, there have been several studies on the dual therapy with VPZ and AMOX as listed in Table 2 [37,38,39,40,41,42,43,44,45]. Although the sufficient eradication rate could be attained by the first study [36], the eradication rates of the dual VPZ/AMOX therapies varies by different dosing schemes of AMOX and treatment periods as in the cases of PPIs.

Table 2.
Lists of reports of dual therapy with VPZ and AMOX.
As the duration of the treatment period, Lin et al. [42] reported that the dual VPZ/AMOX therapies for 7 days could not achieve an acceptable eradication rates (58.3% and 60.7%). Hu et al. [45] compared the 7 day and 10 day regimen with different AMOX dosing schemes and found that none of the 7 or 10 day regimens could attain the sufficient eradication rates. However, they reported that a 14 day regimen could attained a sufficient eradication rate (89.1%) [37]. However, studies by Suzuki et al. [38] Gotoda et al. [39] and Sue et al. [40], which were all from Japan, demonstrated that 7 days seemed a sufficient treatment period for the dual VPZ/AMMOX therapy. The optimal treatment periods for the dual VPZ/AMOX therapy seem to differ among different regions and ethnic groups.
Qian et al. [41] compared two types of ten-day dual VPZ/AMOX therapies with bismuth-containing quadruple therapy. The dual therapy with VPZ 20 mg bid and AMOX 750 mg qid attained the 93.4% of eradication rate, which was as high as that of the quadruple therapy (90.9%). Interestingly, the incidence of side effects of the dual therapy was significantly lower than that of the quadruple therapy. Zuberi [44] also reported that dual VPZ/AMOX therapy provides an acceptable and higher eradication rate (93.5%) with fewer adverse events in comparison with the triple therapy with omeprazole, CAM, and AMOX. Gao [43] reported that the dual VPZ/AMOX therapies were useful as the rescue therapy. However, the eradication rate of the report by Chey [46] was 78.5%, although the regimen was almost the same as that of study of Gao [43]. Accordingly, the optimal dosing schemes of the dual VPZ/AMOX therapy remains to be determined. The best regimens of the dual VPZ/AMOX therapy should be developed for each region and ethnicity.
5. Merits of the Dual Therapy with Vonoprazan and Amoxicillin
One of merits of the dual VPZ/AMOX therapy is to reduce the incidence of adverse events. As noted above, the incidence of adverse events observed in the dual therapies was lower in comparison with the triple therapy and quadruple therapy [36,41].
Pharmacologically, the greatest advantage of the dual therapy is that fewer drugs are used, reducing the risk of drug–drug interactions. CAM, which is often used in triple therapy, is a potent inhibitor of CYP3A4 and p-glycoprotein [47]. CAM has been reported to increase plasma levels of drugs metabolized and transported by these enzymes [29]. However, CAM is not involved in the dual VPZ/AMOX therapy. Therefore, the risk of drug–drug interactions related to CAM can be avoided. Of course, AMOX and VPZ are not without risk of interaction. VPZ has also been reported to affect the antiplatelet effects of Clopidogrel and Prasugrel [48], and has also been reported to affect CYPs [49]. Therefore, it cannot be said that the dual VPZ/AMOX is completely safe. However, it is considered safer than triple therapy from the viewpoint of drug–drug interactions. CAM also has an arrhythmia risk [50]. Therefore, dual therapy with VPZ and AMOX is particularly useful in patients with a risk of arrythmia, such as QT prolongation.
6. Dual Therapy with Vonoprazan and Amoxicillin with Reference to Clarithromycin Susceptibility
Suzuki et al. investigated influence of the CAM resistance in the comparative study of the dual therapy with VPZ 20 mg bid + AMOX 750 mg bid for 1 week and the triple therapy with VPZ 20 mg bid + CAM 20 mg bid + AMOX 750 mg bid for 1 week. In their study in patients infected with CAM-sensitive strains of H. pylori, the eradication rates of the dual and triple therapies were 85.5% and 95.1%, respectively. However, in patients infected with CAM-resistant strains of H. pylori, the respective eradication rates were 92.3% and 76.2% (p < 0.05). In other words, CAM has a negative effect on patients infected with CAM-resistant strains of H. pylori. There are many possible reasons, but one possible explanation is the pharmacodynamic antagonism between CAM and APMC.
In other bacteria, the combination of AMOX and CAM has been reported to be antagonistic [51,52]. The target of AMOX is PBP as noted above, which is the enzyme involved in biosynthesis of bacterial cell wall. When H. pylori grows, the expression of PBP is enhanced, which means that the expression of target of AMOX is increased, resulting in the bactericidal effect of AMOX being enhanced. In contrast, when the growth of H. pylori is inhibited, the expression of PBP is decreased. In this situation, the bactericidal effect of AMOX is decreased because the expression of target of AMOX is decreased. CAM is known as the inhibitor of rRNA, indicating that CAM inhibits the protein synthesis including PBP. Then, the sensitivity to AMOX is decreased by CAM.
However, if the patient is infected with CAM-sensitive strains, there is no problem because they can be killed by CAM. However, in cases infected with CAM-resistant strains of H. pylori, there might be some problems. MIC ranges of CAM-resistant strains of H. pylori are very wide (e.g., from 1 µg/mL to >32 µg/mL). In cases infected with CAM-weakly resistant strains of H. pylori, CAM will work halfway. Although H. pylori strains cannot be killed by CAM, the inhibitory effect of CAM on rRNA may affect the subsequent protein synthesis including PBP, leading to the reduced sensitivity to AMOX [53]. This seems to be the reason why the eradication rates attained by a PPI, AMOX, and CAM were reportedly very low in patients infected with CAM-resistant strains of H. pylori [54,55], although AMOX-resistant strains of H. pylori are rare.
7. Strategy for Patients Allergic to Penicillin
The dual therapy with VPZ and AMOX cannot be used for patients allergic to penicillin. Gao et al. [56] performed dual therapy with VPZ 20 mg bid and tetracycline 500 mg tid (body weight < 70 kg) or 500 mg qid (body weight ≥ 70 kg) for 14 days in patients who were allergic to penicillin or who had failed before. The eradication rate was 93.5%. Therefore, it is suggested that if one antibacterial agent with high susceptibility is selected besides AMOX, H. pylori eradication can be achieved with dual therapy with VPZ plus single effective antibacterial agent.
8. Conclusions
As mentioned above, the greatest merit of the dual VPZ/AMOX therapy is to reduce the risk of adverse events without lowering the eradication rates. In this regimen, CAM is not used, indicating the risk reduction of drug–drug interactions, arrhythmia, and other adverse events caused by CAM. The regimen is simple. There is also a report of the effective dual therapy with VPZ and single antimicrobial agent other than AMOX, such as tetracycline.
Now that we have vonoprazan in our hands, it is expected that eradication therapy will be reconstructed into a simpler regimen, such as dual therapy with VPZ and one antibacterial agent with high susceptibility (i.e., AMOX) in the optimal dosing scheme. It is also expected that we can break away from the stereotype of using multiple antibiotics to eradicate H. pylori.
Unfortunately, the use of VPZ is not widespread worldwide. It is hoped that VPZ will become widely available around the world in the future, and new drugs with similar effects will be developed, leading to the simplification of eradication regimens in many countries.
Author Contributions
Conceptualization, T.F., M.Y., T.H., S.T. (Satoru Takahashi). Validation, S.O., M.I., Y.H. Formal analysis, S.T. (Satoshi Tamura), S.T. (Shinya Tani), N.I. Investigation, T.F., M.Y., T.H., S.T. (Satoru Takahashi). Resources T.F.; data curation Writing—original draft preparation: T.F. Writing—review and editing: M.Y., T.H., S.T. (Satoru Takahashi), S.O., M.I., S.T. (Shinya Tani), S.T. (Satoshi Tamura). Visualization, T.F. Supervision K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
We have used the published data.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AMOX = amoxicillin, CAM = clarithromycin, CYP = cytochrome P450, EPZ = esomeprazole, RPZ = rabeprazole, T>MIC = time above MIC, VPZ = vonoprazan.
References
- Sasaki, M.; Ogasawara, N.; Utsumi, K.; Kawamura, N.; Kamiya, T.; Kataoka, H.; Tanida, S.; Mizoshita, T.; Kasugai, K.; Joh, T. Changes in 12-Year First-Line Eradication Rate of Helicobacter pylori Based on Triple Therapy with Proton Pump Inhibitor, Amoxicillin and Clarithromycin. J. Clin. Biochem. Nutr. 2010, 47, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Fasciana, T.; Calà, C.; Bonura, C.; Di Carlo, E.; Matranga, D.; Scarpulla, G.; Manganaro, M.; Camilleri, S.; Giammanco, A. Resistance to clarithromycin and genotypes in Helicobacter pylori strains isolated in Sicily. J. Med. Microbiol. 2015, 64, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Watanabe, Y.; Oikawa, R.; Watanabe, R.; Higashino, M.; Kubo, K.; Yamamoto, H.; Itoh, F.; Kato, M. Clinical evaluation of a novel molecular diagnosis kit for detecting Helicobacter pylori and clarithromycin-resistant using intragastric fluid. Helicobacter 2022, 27, e12933. [Google Scholar] [CrossRef] [PubMed]
- Sue, S.; Ogushi, M.; Arima, I.; Kuwashima, H.; Nakao, S.; Naito, M.; Komatsu, K.; Kaneko, H.; Tamura, T.; Sasaki, T.; et al. Vonoprazan- vs. proton-pump inhibitor-based first-line 7-day triple therapy for clarithromycin-susceptible Helicobacter pylori: A multicenter, prospective, randomized trial. Helicobacter 2018, 23, e12456. [Google Scholar] [CrossRef]
- Furuta, T.; Shirai, N.; Takashima, M.; Xiao, F.; Hanai, H.; Nakagawa, K.; Sugimura, H.; Ohashi, K.; Ishizaki, T. Effects of genotypic differences in CYP2C19 status on cure rates for Helicobacter pylori infection by dual therapy with rabeprazole plus amoxicillin. Pharmacogenetics 2001, 11, 341–348. [Google Scholar] [CrossRef]
- Furuta, T.; Shirai, N.; Takashima, M.; Xiao, F.; Hanai, H.; Sugimura, H.; Ohashi, K.; Ishizaki, T.; Kaneko, E. Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin. Pharmacol. Ther. 2001, 69, 158–168. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Furuta, T.; Shirai, N.; Xiao, F.; Takashita, M.; Sugimoto, M.; Kajimura, M.; Ohashi, K.; Ishizaki, T. High-dose rabeprazole/amoxicillin therapy as the second-line regimen after failure to eradicate H. pylori by triple therapy with the usual doses of a proton pump inhibitor, clarithromycin and amoxicillin. Hepatogastroenterology 2003, 50, 2274–2278. [Google Scholar]
- Tai, W.-C.; Liang, C.-M.; Kuo, C.-M.; Huang, P.-Y.; Wu, C.-K.; Yang, S.-C.; Kuo, Y.-H.; Lin, M.-T.; Lee, C.-H.; Hsu, C.-N.; et al. A 14 day esomeprazole- and amoxicillin-containing high-dose dual therapy regimen achieves a high eradication rate as first-line anti-Helicobacter pylori treatment in Taiwan: A prospective randomized trial. J. Antimicrob. Chemother. 2019, 74, 1718–1724. [Google Scholar] [CrossRef]
- Shirai, N.; Sugimoto, M.; Kodaira, C.; Nishino, M.; Ikuma, M.; Kajimura, M.; Ohashi, K.; Ishizaki, T.; Hishida, A.; Furuta, T. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. Eur. J. Clin. Pharmacol. 2007, 63, 743–749. [Google Scholar] [CrossRef]
- Bayerdörffer, E.; Miehlke, S.; Mannes, G.A.; Sommer, A.; Höchter, W.; Weingart, J.; Heldwein, W.; Klann, H.; Simon, T.; Schmitt, W.; et al. Double-blind trial of omeprazole and amoxicillin to cure Helicobacter pylori infection in patients with duodenal ulcers. Gastroenterology 1995, 108, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Sugimoto, M.; Kodaira, C.; Nishino, M.; Yamade, M.; Uotani, T.; Ikuma, M.; Shirai, N. The dual therapy with 4 times daily dosing of rabeprazole and amoxicillin as the 3rd rescue regimen for eradication of H. pylori. Hepatogastroenterology 2010, 57, 1314–1319. [Google Scholar] [PubMed]
- Miehlke, S.; Kirsch, C.; Schneider-Brachert, W.; Haferland, C.; Neumeyer, M.; Bastlein, E.; Papke, J.; Jacobs, E.; Vieth, M.; Stolte, M.; et al. A Prospective, Randomized Study of Quadruple Therapy and High-Dose Dual Therapy for Treatment of Helicobacter pylori Resistant to Both Metronidazole and Clarithromycin. Helicobacter 2003, 8, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, H.; Krause, R.; Sahba, B.; Haber, M.; Weissfeld, A.; Rose, P.; Siepman, N.; Freston, J. Triple versus dual therapy for eradicating Helicobacter pylori and preventing ulcer recurrence: A randomized, double-blind, multicenter study of lansoprazole, clarithromycin, and/or amoxicillin in different dosing regimens. Am. J. Gastroenterol. 1998, 93, 584–590. [Google Scholar] [CrossRef]
- Miehlke, S.; Hansky, K.; Schneider-Brachert, W.; Kirsch, C.; Morgner, A.; Madisch, A.; Kuhlisch, E.; Bästlein, E.; Jacobs, E.; Bayerdörffer, E.; et al. Randomized trial of rifabutin-based triple therapy and high-dose dual therapy for rescue treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Aliment. Pharmacol. Ther. 2006, 24, 395–403. [Google Scholar] [CrossRef]
- Miyoshi, M.; Mizuno, M.; Ishiki, K.; Nagahara, Y.; Maga, T.; Torigoe, T.; Nasu, J.; Okada, H.; Yokota, K.; Oguma, K.; et al. A randomized open trial for comparison of proton pump inhibitors, omeprazole versus rabeprazole, in dual therapy for Helicobacter pylori infection in relation to CYP2C19 genetic polymorphism. J. Gastroenterol. Hepatol. 2001, 16, 723–728. [Google Scholar] [CrossRef]
- Nishizawa, T.; Suzuki, H.; Maekawa, T.; Harada, N.; Toyokawa, T.; Kuwai, T.; Ohara, M.; Suzuki, T.; Kawanishi, M.; Noguchi, K.; et al. Dual therapy for third-line Helicobacter pylori eradication and urea breath test prediction. World J. Gastroenterol. 2012, 18, 2735–2738. [Google Scholar] [CrossRef]
- Isomoto, H.; Inoue, K.; Furusu, H.; Enjoji, A.; Fujimoto, C.; Yamakawa, M.; Hirakata, Y.; Omagari, K.; Mizuta, Y.; Murase, K.; et al. High-dose rabeprazole-amoxicillin versus rabeprazole-amoxicillin-metronidazole as second-line treatment after failure of the Japanese standard regimen for Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2003, 18, 101–107. [Google Scholar] [CrossRef]
- Wong, B.; Xiao, S.; Hu, P.; Qian, S.; Huang, N.; Li, Y.; Manan, C.; Lesmana, L.; Carpio, R.; Perez, J.Y.; et al. Comparison of lansoprazole-based triple and dual therapy for treatment of Helicobacter pylori-related duodenal ulcer: An Asian multicentre double-blind randomized placebo controlled study. Aliment. Pharmacol. Ther. 2000, 14, 217–224. [Google Scholar] [CrossRef]
- Attumi, T.A.; Graham, D.Y. High-Dose Extended-Release Lansoprazole (Dexlansoprazole) and Amoxicillin Dual Therapy for Helicobacter pylori Infections. Helicobacter 2014, 19, 319–322. [Google Scholar] [CrossRef]
- Koizumi, W.; Tanabe, S.; Hibi, K.; Imaizumi, H.; Ohida, M.; Okabe, H.; Saigenji, K.; Okayasu, I. A prospective randomized study of amoxycillin and omeprazole with and without metronidazole in the eradication treatment of Helicobacter pylori. J. Gastroenterol. Hepatol. 1998, 13, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Ohashi, K.; Kamata, T.; Takashima, M.; Kosuge, K.; Kawasaki, T.; Hanai, H.; Kubota, T.; Ishizaki, T.; Kaneko, E. Effect of genetic differences in omeprazole metabolism on cure rates for Helicobacter pylori infection and peptic ulcer. Ann. Intern. Med. 1998, 129, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.D.; Bate, C.M.; Axon, A.T.R.; Tildesley, G.; Kerr, G.D.; Green, J.R.B.; Emmas, C.E.; Taylor, M.D. Addition of metronidazole to omeprazole/amoxycillin dual therapy increases the rate of Helicobacter pylori eradication: A double-blind, randomized trial. Aliment. Pharmacol. Ther. 1995, 9, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Cottrill, M.R.B.; Kinnon, C.M.C.; Mason, I.; Chesters, S.A.; Slatcher, G.; Copeman, M.B.; Turbitt, M.L. Two omeprazole-based Helicobacter pylori eradication regimens for the treatment of duodenal ulcer disease in general practice. Aliment. Pharmacol. Ther. 1997, 11, 919–927. [Google Scholar] [CrossRef]
- Kagaya, H.; Kato, M.; Komatsu, Y.; Mizushima, T.; Sukegawa, M.; Nishikawa, K.; Hokari, K.; Takeda, H.; Sugiyama, T.; Asaka, M. High-dose ecabet sodium improves the eradication rate of Helicobacter pylori in dual therapy with lansoprazole and amoxicillin. Aliment. Pharmacol. Ther. 2000, 14, 1523–1527. [Google Scholar] [CrossRef]
- Sakurai, Y.; Mori, Y.; Okamoto, H.; Nishimura, A.; Komura, E.; Araki, T.; Shiramoto, M. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects—A randomised open-label cross-over study. Aliment. Pharmacol. Ther. 2015, 42, 719–730. [Google Scholar] [CrossRef]
- Murakami, K.; Sakurai, Y.; Shiino, M.; Funao, N.; Nishimura, A.; Asaka, M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: A phase III, randomised, double-blind study. Gut 2016, 65, 1439–1446. [Google Scholar] [CrossRef]
- Marcus, E.A.; Inatomi, N.; Nagami, G.T.; Sachs, G.; Scott, D.R. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment. Pharmacol. Ther. 2012, 36, 972–979. [Google Scholar] [CrossRef]
- Furuta, T.; Graham, D.Y. Pharmacologic Aspects of Eradication Therapy for Helicobacter pylori Infection. Gastroenterol. Clin. N. Am. 2010, 39, 465–480. [Google Scholar] [CrossRef]
- Sugimoto, M.; Furuta, T.; Shirai, N.; Kodaira, C.; Nishino, M.; Ikuma, M.; Ishizaki, T.; Hishida, A. Evidence that the Degree and Duration of Acid Suppression are Related to Helicobacter pylori Eradication by Triple Therapy. Helicobacter 2007, 12, 317–323. [Google Scholar] [CrossRef]
- Sugimoto, M.; Furuta, T.; Shirai, N.; Kajimura, M.; Hishida, A.; Sakurai, M.; Ohashi, K.; Ishizaki, T. Different dosage regimens of rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotype status. Clin. Pharmacol. Ther. 2004, 76, 290–301. [Google Scholar] [CrossRef]
- Kagami, T.; Sahara, S.; Ichikawa, H.; Uotani, T.; Yamade, M.; Sugimoto, M.; Hamaya, Y.; Iwaizumi, M.; Osawa, S.; Miyajima, H.; et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment. Pharmacol. Ther. 2016, 43, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. State-of-the-Art Clinical Article: Pharmacokinetic/Pharmacodynamic Parameters: Rationale for Antibacterial Dosing of Mice and Men. Clin. Infect. Dis. 1998, 26, 1–10, quiz 11–12. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 1995, 22, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. Choosing an antibiotic on the basis of pharmacodynamics. Ear Nose Throat J. 1998, 77 (Suppl. S6), 7–11. [Google Scholar] [PubMed]
- Furuta, T.; Yamade, M.; Kagami, T.; Uotani, T.; Suzuki, T.; Higuchi, T.; Tani, S.; Hamaya, Y.; Iwaizumi, M.; Miyajima, H.; et al. Dual Therapy with Vonoprazan and Amoxicillin Is as Effective as Triple Therapy with Vonoprazan, Amoxicillin and Clarithromycin for Eradication of Helicobacter pylori. Digestion 2019, 101, 743–751. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, X.; Liu, X.-S.; He, C.; Ouyang, Y.-B.; Li, N.-S.; Xie, C.; Peng, C.; Zhu, Z.-H.; Xie, Y.; et al. Fourteen-day vonoprazan and low- or high-dose amoxicillin dual therapy for eradicating Helicobacter pylori infection: A prospective, open-labeled, randomized non-inferiority clinical study. Front. Immunol. 2022, 13, 1049908. [Google Scholar] [CrossRef]
- Suzuki, S.; Gotoda, T.; Kusano, C.; Ikehara, H.; Ichijima, R.; Ohyauchi, M.; Ito, H.; Kawamura, M.; Ogata, Y.; Ohtaka, M.; et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: A multicentre randomised trial in Japan. Gut 2020, 69, 1019–1026. [Google Scholar] [CrossRef]
- Gotoda, T.; Kusano, C.; Suzuki, S.; Horii, T.; Ichijima, R.; Ikehara, H. Clinical impact of vonoprazan-based dual therapy with amoxicillin for H. pylori infection in a treatment-naive cohort of junior high school students in Japan. J. Gastroenterol. 2020, 55, 969–976. [Google Scholar] [CrossRef]
- Sue, S.; Kondo, M.; Sato, T.; Oka, H.; Sanga, K.; Ogashiwa, T.; Matsubayashi, M.; Kaneko, H.; Irie, K.; Maeda, S. Vonoprazan and high-dose amoxicillin dual therapy for Helicobacter pylori first-line eradication: A single-arm, interventional study. JGH Open 2023, 7, 55–60. [Google Scholar] [CrossRef]
- Qian, H.-S.; Li, W.-J.; Dang, Y.-N.; Li, L.-R.; Xu, X.-B.; Yuan, L.; Zhang, W.-F.; Yang, Z.; Gao, X.; Zhang, M.; et al. Ten-Day Vonoprazan-Amoxicillin Dual Therapy as a First-Line Treatment of Helicobacter pylori Infection Compared with Bismuth-Containing Quadruple Therapy. Am. J. Gastroenterol. 2022, 118, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, H.; Yun, J.; Yu, X.; Shi, Y.; Zhang, D. The efficacy of vonoprazan combined with different dose amoxicillin on eradication of Helicobacter pylori: An open, multicenter, randomized clinical study. Ann. Transl. Med. 2022, 10, 987. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Teng, G.; Wang, C.; Xu, Y.; Li, Y.; Cheng, H. Eradication rate and safety of a “simplified rescue therapy”: 14-day vonoprazan and amoxicillin dual regimen as rescue therapy on treatment of Helicobacter pylori infection previously failed in eradication: A real-world, retrospective clinical study in China. Helicobacter 2022, 27, e12918. [Google Scholar] [PubMed]
- Zuberi, B.F.; Ali, F.S.; Rasheed, T.; Bader, N.; Hussain, S.M.; Saleem, A. Comparison of Vonoprazan and Amoxicillin Dual Therapy with Standard Triple Therapy with Proton Pump Inhibitor for Helicobacter pylori eradication: A Randomized Control Trial. Pak. J. Med. Sci. 2022, 38, 965–969. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, X.; Ouyang, Y.B.; He, C.; Li, N.S.; Xie, C.; Peng, C.; Zhu, Z.H.; Xie, Y.; Shu, X.; et al. Optimization of vonoprazan-amoxicillin dual therapy for eradicating Helicobacter pylori infection in China: A prospective, randomized clinical pilot study. Helicobacter 2022, 27, e12896. [Google Scholar] [CrossRef]
- Chey, W.D.; Mégraud, F.; Laine, L.; López, L.J.; Hunt, B.J.; Howden, C.W. Vonoprazan Triple and Dual Therapy for Helicobacter pylori Infection in the United States and Europe: Randomized Clinical Trial. Gastroenterology 2022, 163, 608–619. [Google Scholar] [CrossRef]
- Zhou, S.; Chan, S.Y.; Goh, B.C.; Chan, E.; Duan, W.; Huang, M.; McLeod, H.L. Mechanism-Based Inhibition of Cytochrome P450 3A4 by Therapeutic Drugs. Clin. Pharmacokinet. 2005, 44, 279–304. [Google Scholar] [CrossRef]
- Kagami, T.; Yamade, M.; Suzuki, T.; Uotani, T.; Hamaya, Y.; Iwaizumi, M.; Osawa, S.; Sugimoto, K.; Umemura, K.; Miyajima, H.; et al. Comparative study of effects of vonoprazan and esomeprazole on anti-platelet function of clopidogrel or prasugrel in relation to CYP2C19 genotype. Clin. Pharmacol. Ther. 2017, 103, 906–913. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Wang, S.; Zhou, Q.; Dai, D.; Shi, J.; Xu, X.; Luo, Q. Cytochrome P450-Based Drug-Drug Interactions of Vonoprazan In Vitro and In Vivo. Front. Pharmacol. 2020, 11, 53. [Google Scholar] [CrossRef]
- Kang, J.; Kim, Y.-J.; Shim, T.S.; Jo, K.-W. Risk for cardiovascular disease in patients with nontuberculous mycobacteria treated with macrolide. J. Thorac. Dis. 2018, 10, 5784–5795. [Google Scholar] [CrossRef]
- Drago, L.; Nicola, L.; Rodighiero, V.; Larosa, M.; Mattina, R.; De Vecchi, E. Comparative evaluation of synergy of combinations of beta-lactams with fluoroquinolones or a macrolide in Streptococcus pneumoniae. J. Antimicrob. Chemother. 2011, 66, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Medscape. Drug Interaction Checker. Available online: https://reference.medscape.com/drug-interactionchecker (accessed on 3 March 2023).
- Furuta, T.; Yamade, M.; Kagami, T.; Suzuki, T.; Higuchi, T.; Tani, S.; Hamaya, Y.; Iwaizumi, M.; Miyajima, H.; Umemura, K.; et al. Influence of clarithromycin on the bactericidal effect of amoxicillin in patients infected with clarithromycin-resistant strains of H. pylori. Gut 2020, 69, 2056. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Sato, R.; Okimoto, T.; Nasu, M.; Fujioka, T.; Kodama, M.; Kagawa, J.; Sato, S.; Abe, H.; Arita, T. Eradication rates of clarithromycin-resistant Helicobacter pylori using either rabeprazole or lansoprazole plus amoxicillin and clarithromycin. Aliment. Pharmacol. Ther. 2002, 16, 1933–1938. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, H.; Habu, Y.; Tomioka, H.; Kutsumi, H.; Kobayashi, M.; Oyasu, K.; Hayakumo, T.; Mizuno, S.; Kiyota, K.; Nakajima, M.; et al. Effect of different proton pump inhibitors, differences in CYP2C19 genotype and antibiotic resistance on the eradication rate of Helicobacter pylori infection by a 1-week regimen of proton pump inhibitor, amoxicillin and clarithromycin. Aliment. Pharmacol. Ther. 2003, 17, 259–264. [Google Scholar] [CrossRef]
- Gao, W.; Xu, Y.; Liu, J.; Wang, X.; Dong, X.; Teng, G.; Liu, B.; Dong, J.; Ge, C.; Ye, H.; et al. A real-world exploratory study on the feasibility of vonoprazan and tetracycline dual therapy for the treatment of Helicobacter pylori infection in special populations with penicillin allergy or failed in previous amoxicillin-containing therapies. Helicobacter 2023, 28, e12947. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).