Diagnosis and Treatment for Gastric Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma
Abstract
:1. Introduction
2. Epidemiology of Gastric MALT Lymphoma
3. Pathogenesis of Gastric MALT Lymphoma
3.1. Helicobacter pylori
3.2. Genetic Aberrations
4. Diagnosis of Gastric MALT Lymphoma
4.1. Endoscopic/Macroscopic Findings
4.2. Histopathology of Gastric MALT Lymphomas
4.3. Clinical Staging
5. Treatments for Gastric MALT Lymphoma
5.1. Helicobacter pylori Eradication
5.2. Treatment Strategies for Patients with Gastric MALT Lymphoma Who Do Not Respond to Helicobacter pylori Eradication
6. Risk for Other Malignancies
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Isaacson, P.; Wright, D.H. Extranodal malignant lymphoma arising from mucosa-associated lymphoid tissue. Cancer 1984, 53, 2515–2524. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.R.; Isaacson, P.G.; Chott, A.; Nakamura, S.; Muller-Hermelink, H.K.; Harris, N.L.; Swerdlow, S.H. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; Swerdlow, S.H., Campo, E., Harris, N.L., Eds.; IARC: Lyon, France, 2008; pp. 214–217. [Google Scholar]
- Nakamura, S.; Müller-Hermelink, H.K.; Delabe, J. Lymphoma of the stomach. In WHO Classification of Tumours of the Digestive System, 4th ed.; Bosman, F.T., Carnerio, F., Hruban, R.H., Theise, N.D., Eds.; IARC: Lyon, France, 2010; pp. 69–73. [Google Scholar]
- Wotherspoon, A.C.; Ortiz-Hidalgo, C.; Falzon, M.R.; Isaacson, P.G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991, 338, 1175–1176. [Google Scholar] [CrossRef] [PubMed]
- Wotherspoon, A.; Diss, T.; Pan, L.; Isaacson, P.; Doglioni, C.; Moschini, A.; de Boni, M. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 1993, 342, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Akazawa, K.; Yao, T.; Tsuneyoshi, M. Primary gastric lymphoma. A clinicopathologic study of 233 cases with special reference to evaluation with the MIB-1 index. Cancer 1995, 76, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Yao, T.; Aoyagi, K.; Iida, M.; Fujishima, M.; Tsuneyoshi, M. Helicobacter pylori and primary gastric lymphoma. A histopathologic and immunohistochemical analysis of 237 patients. Cancer 1997, 79, 3–11. [Google Scholar] [CrossRef]
- Bayerdorffer, E.; Miehlke, S.; Neubauer, A.; Stolte, M. Gastric MALT-lymphoma and Helicobacter pylori infection. Aliment. Pharmacol. Ther. 1997, 11, 89–94. [Google Scholar] [CrossRef]
- Ryu, K.D.; Kim, G.H.; Park, S.O.; Lee, K.J.; Moon, J.Y.; Jeon, H.K.; Baek, D.H.; Lee, B.E.; Song, G.A. Treatment Outcome for gastric mucosa-associated jymphoid tissue lymphoma according to Helicobacter pylori infection status: A single-center experience. Gut Liver 2014, 8, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Matsumoto, T.; Iida, M.; Yao, T.; Tsuneyoshi, M. Primary gastrointestinal lymphoma in Japan: A clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer 2003, 97, 2462–2473. [Google Scholar] [CrossRef]
- Nakamura, S.; Matsumoto, T. Gastrointestinal lymphoma: Recent advances in diagnosis and treatment. Digestion 2013, 87, 182–188. [Google Scholar] [CrossRef]
- Nakamura, S.; Matsumoto, T. Gastrointestinal lymphomas: Recent topics in diagnosis and treatment. J. Jpn Soc. Gastroenterol. 2017, 114, 1833–1938. (In Japanese) [Google Scholar]
- Nakamura, S.; Matsumoto, T.; Suekane, H.; Nakamura, S.; Matsumoto, H.; Esaki, M.; Yao, T.; Iida, M. Long-term clinical outcome of Helicobacter pylori eradication for gastric mucosa-associated lymphoid tissue lymphoma with a reference to second-line treatment. Cancer 2005, 104, 532–540. [Google Scholar] [CrossRef]
- Nakamura, S.; Matsumoto, T.; Ye, H.; Nakamura, S.; Suekane, H.; Matsumoto, H.; Yao, T.; Tsuneyoshi, M.; Du, M.-Q.; Iida, M. Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma: A clinicopathologic and molecular study with reference to antibiotic treatment. Cancer 2006, 107, 2770–2778. [Google Scholar] [CrossRef]
- Capelle, L.; de Vries, A.; Looman, C.; Casparie, M.; Boot, H.; Meijer, G.; Kuipers, E. Gastric MALT lymphoma: Epidemiology and high adenocarcinoma risk in a nation-wide study. Eur. J. Cancer 2008, 44, 2470–2476. [Google Scholar] [CrossRef]
- Palmela, C.; Fonseca, C.; Faria, R.; Baptista, R.B.; Ribeiro, S.; Ferreira, A.O. Increased risk for metachronous gastric adenocarcinoma following gastric MALT lymphoma—A US population-based study. United Eur. Gastroenterol. J. 2016, 5, 473–478. [Google Scholar] [CrossRef] [Green Version]
- Blosse, A.; Peru, S.; Levy, M.; Marteyn, B.; Floch, P.; Sifré, E.; Giese, A.; Prochazkova-Carlotti, M.; Martin, L.A.; Dubus, P.; et al. APRIL-producing eosinophils are involved in gastric MALT lymphomagenesis induced by Helicobacter sp infection. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Shi, F.; Xue, R.; Zhou, X.; Shen, P.; Wang, S.; Yang, Y. Teliacicept as a BLyS/APRIL dual inhibitor for autoimmune disease. Immunopharmacol. Immunotoxicol. 2021, 43, 666–673. [Google Scholar] [CrossRef]
- Ye, H.; Liu, H.; Raderer, M.; Chott, A.; Ruskone-Fourmestraux, A.; Wotherspoon, A.; Dyer, M.; Chuang, S.-S.; Dogan, A.; Isaacson, P.G.; et al. High incidence of t(11;18)(q21;q21) in Helicobacter pylori-negative gastric MALT lymphoma. Blood 2002, 101, 2547–2550. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Z.; Liu, H.; Ye, H.; Chuang, S.-S.; Wang, J.; Lin, S.; Gao, Z.; Du, M.-Q. T(11;18)(q21;q21) in gastric MALT lymphoma and diffuse large B-cell lymphoma of Chinese patients. Hematol. J. 2003, 4, 342–345. [Google Scholar] [CrossRef]
- Chuang, S.-S.; Lee, C.; Hamoudi, R.A.; Liu, H.; Lee, P.-S.; Ye, H.; Diss, T.C.; Dogan, A.; Isaacson, P.G.; Du, M.-Q. High frequency of t(11;18) in gastric mucosa-associated lymphoid tissue lymphomas in Taiwan, including one patient with high-grade transformation. Br. J. Haematol. 2002, 120, 97–100. [Google Scholar] [CrossRef]
- Nakamura, S.; Ye, H.; Bacon, C.; Goatly, A.; Liu, H.; Banham, A.; Ventura, R.; Matsumoto, T.; Iida, M.; Ohji, Y.; et al. Clinical impact of genetic aberrations in gastric MALT lymphoma: A comprehensive analysis using interphase fluorescence in situ hybridisation. Gut 2007, 56, 1358–1363. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Matsuno, Y.; Torisu, T.; Shibata, H.; Hirano, A.; Umeno, J.; Kawasaki, K.; Fujioka, S.; Fuyuno, Y.; Moriyama, T.; et al. Gastric microbiota in patients with Helicobacter pylori-negative gastric MALT lymphoma. Medicine 2021, 100, e27287. [Google Scholar] [CrossRef] [PubMed]
- Sagaert, X.; Van Cutsem, E.; De Hertogh, G.; Geboes, K.; Tousseyn, T. Gastric MALT lymphoma: A model of chronic inflammation-induced tumor development. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Du, M.Q. MALT lymphoma: A paradigm of NF-κB dysregulation. Semin. Cancer Biol. 2016, 39, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, M.-Q. MALT lymphoma: Genetic abnormalities, immunological stimulation and molecular mechanism. Best Pract. Res. Clin. Haematol. 2017, 30, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Chanudet, E.; Huang, Y.; Ichimura, K.; Dong, G.; Hamoudi, R.; Radford, J.; Wotherspoon, A.C.; Isaacson, P.G.; Ferry, J.; Du, M.-Q. A20 is targeted by promoter methylation, deletion and inactivating mutation in MALT lymphoma. Leukemia 2009, 24, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Lee, D.H.; Ahn, B.K.; Hwang, J.J.; Yoon, H.; Park, Y.S.; Shin, C.M.; Kim, N. Correlation of endoscopic findings of gastric mucosa-associated lymphoid tissue lymphoma with recurrence after complete remission. Clin. Endosc. 2017, 50, 51–57. [Google Scholar] [CrossRef]
- Ruskone-Fourmestraux, A.; Fischbach, W.; Aleman, B.M.P.; Boot, H.; Du, M.Q.; Megraud, F.; Montalban, C.; Raderer, M.; Savio, A.; Wotherspoon, A.; et al. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut 2011, 60, 747–758. [Google Scholar] [CrossRef] [Green Version]
- In Proceedings of the 5th International Conference on Malignant Lymphoma, Part Proceedings, Lugano, Switzerland, 9–12 June 1994; Volume 5, pp. 1–163.
- Cohen, D.; Perry, C.; Hazut-Krauthammer, S.; Kesler, M.; Herishanu, Y.; Luttwak, E.; Even-Sapir, E.; Avivi, I. Is there a role for [18F]FDG PET-CT in staging MALT lymphoma? Cancers 2022, 14, 750. [Google Scholar] [CrossRef]
- Stathis, A.; Chini, C.; Bertoni, F.; Proserpio, I.; Capella, C.; Mazzucchelli, L.; Pedrinis, E.; Cavalli, F.; Pinotti, G.; Zucca, E. Long-term outcome following Helicobacter pylori eradication in a retrospective study of 105 patients with localized gastric marginal zone B-cell lymphoma of MALT type. Ann. Oncol. 2009, 20, 1086–1093. [Google Scholar] [CrossRef]
- Zullo, A.; Hassan, C.; Cristofari, F.; Andriani, A.; De Francesco, V.; Ierardi, E.; Tomao, S.; Stolte, M.; Morini, S.; Vaira, D. Effects of Helicobacter pylori eradication on early stage gastric mucosa–associated lymphoid tissue lymphoma. Clin. Gastroenterol. Hepatol. 2010, 8, 105–110. [Google Scholar] [CrossRef]
- Zullo, A.; Hassan, C.; Andriani, A.; Cristofari, F.; Bassanelli, C.; Spinelli, G.P.; Tomao, S.; Morini, S. Treatment of low-grade gastric MALT-lymphoma unresponsive to Helicobacter pylori therapy: A pooled-data analysis. Med. Oncol. 2010, 27, 291–295. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Calvet, X. Review article: Common misconceptions in the management of Helicobacter pylori-associated gastric MALT-lymphoma. Aliment. Pharmacol. Ther. 2011, 34, 1047–1062. [Google Scholar] [CrossRef]
- Nakamura, S.; Sugiyama, T.; Matsumoto, T.; Iijima, K.; Ono, S.; Tajika, M.; Tari, A.; Kitadai, Y.; Matsumoto, H.; Nagaya, T.; et al. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: A multicentre cohort follow-up study of 420 patients in Japan. Gut 2011, 61, 507–513. [Google Scholar] [CrossRef]
- Nakamura, S.; Matsumoto, T. Helicobacter pylori and gastric mucosa-associated lymphoid tissue lymphoma: Recent progress in pathogenesis and management. World J. Gastroenterol. 2013, 19, 8181–8187. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, S.; Zhang, Y. Treatment of gastric MALT lymphoma with a focus on Helicobacter pylori eradication. Int. J. Hematol. 2013, 97, 735–742. [Google Scholar] [CrossRef]
- Zucca, E.; Bergman, C.C.; Ricardi, U.; Thieblemont, C.; Raderer, M.; Ladetto, M. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi144–vi148. [Google Scholar] [CrossRef]
- Xie, Y.-L.; He, C.-Y.; Wei, S.-Q.; Guan, W.-J.; Jiang, Z. Clinical efficacy of the modified Helicobacter pylori eradication therapy for Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma: A meta analysis. Chin. Med. J. 2020, 133, 1337–1346. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, N.; Paik, J.H.; Kim, J.M.; Lee, S.H.; Park, Y.S.; Hwang, J.-H.; Kim, J.-W.; Jeong, S.-H.; Lee, D.H.; et al. Characteristics of Helicobacter pylori-positive and Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma and their influence on clinical outcome. Helicobacter 2013, 18, 197–205. [Google Scholar] [CrossRef]
- Bergman, C.C.; Wotherspoon, A.C.; Capella, C.; Motta, T.; Pedrinis, E.; Pileri, S.A.; Bertoni, F.; Conconi, A.; Zucca, E.; Ponzoni, M.; et al. Gela histological scoring system for post-treatment biopsies of patients with gastric MALT lymphoma is feasible and reliable in routine practice. Br. J. Haematol. 2012, 160, 47–52. [Google Scholar] [CrossRef]
- Zucca, E.; Conconi, A.; Martinelli, G.; Bouabdallah, R.; Tucci, A.; Vitolo, U.; Martelli, M.; Pettengell, R.; Salles, G.; Sebban, C.; et al. Final results of the IELSG-19 randomized trial of mucosa-associated lymphoid tissue lymphoma: Improved event-free and progression-free survival with rituximab plus chlorambucil versus either chlorambucil or rituximab monotherapy. J. Clin. Oncol. 2017, 35, 1905–1912. [Google Scholar] [CrossRef]
- Yahalom, J.; Xu, A.J.; Noy, A.; Lobaugh, S.; Chelius, M.; Chau, K.; Portlock, C.; Hajj, C.; Imber, B.S.; Straus, D.J.; et al. Involved-site radiotherapy for Helicobacter pylori–independent gastric MALT lymphoma: 26 years of experience with 178 patients. Blood Adv. 2021, 5, 1830–1836. [Google Scholar] [CrossRef] [PubMed]
- Sugizaki, K.; Tari, A.; Kitadai, Y.; Oda, I.; Nakamura, S.; Yoshino, T.; Sugiyama, T. Anti-Helicobacter pylori therapy in localized gastric mucosa-associated lymphoid tissue lymphoma: A prospective, nationwide, multicenter study in Japan. Helicobacter 2018, 23, e12474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levitt, M.; Gharibo, M.; Strair, R.; Schaar, D.; Rubin, A.; Bertino, J. Accelerated R-COP: A Pilot Study for the Treatment of Advanced Low Grade Lymphomas that Has a High Complete Response Rate. J. Chemother. 2009, 21, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, W. Gastric MALT lymphoma—Update on diagnosis and treatment. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 1069–1077. [Google Scholar] [CrossRef]
- Cencini, E.; Fabbri, A.; Lauria, F.; Bocchia, M. Long-term efficacy and toxicity of rituximab plus fludarabine and mitoxantrone (R-FM) for gastric marginal zone lymphoma: A single-center experience and literature review. Ann. Hematol. 2018, 97, 821–829. [Google Scholar] [CrossRef]
- Morigi, A.; Argnani, L.; Lolli, G.; Broccoli, A.; Pellegrini, C.; Nanni, L.; Stefoni, V.; Coppola, P.E.; Carella, M.; Casadei, B.; et al. Bendamustine-rituximab regimen in untreated indolent marginal zone lymphoma: Experience on 65 patients. Hematol. Oncol. 2020, 38, 487–492. [Google Scholar] [CrossRef]
- Alderuccio, J.P.; Arcaini, L.; Watkins, M.P.; Beaven, A.W.; Shouse, G.; Epperla, N.; Spina, M.; Stefanovic, A.; Sandoval-Sus, J.; Torka, P.; et al. An international analysis evaluating frontline bendamustine with rituximab in extranodal marginal zone lymphoma. Blood Adv. 2022, 6, 2035–2044. [Google Scholar] [CrossRef]
- Zucca, E.; Arcaini, L.; Buske, C.; Johnson, P.; Ponzoni, M.; Raderer, M.; Ricardi, U.; Salar, A.; Stamatopoulos, K.; Thieblemont, C.; et al. Marginal zone lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 17–29. [Google Scholar] [CrossRef]
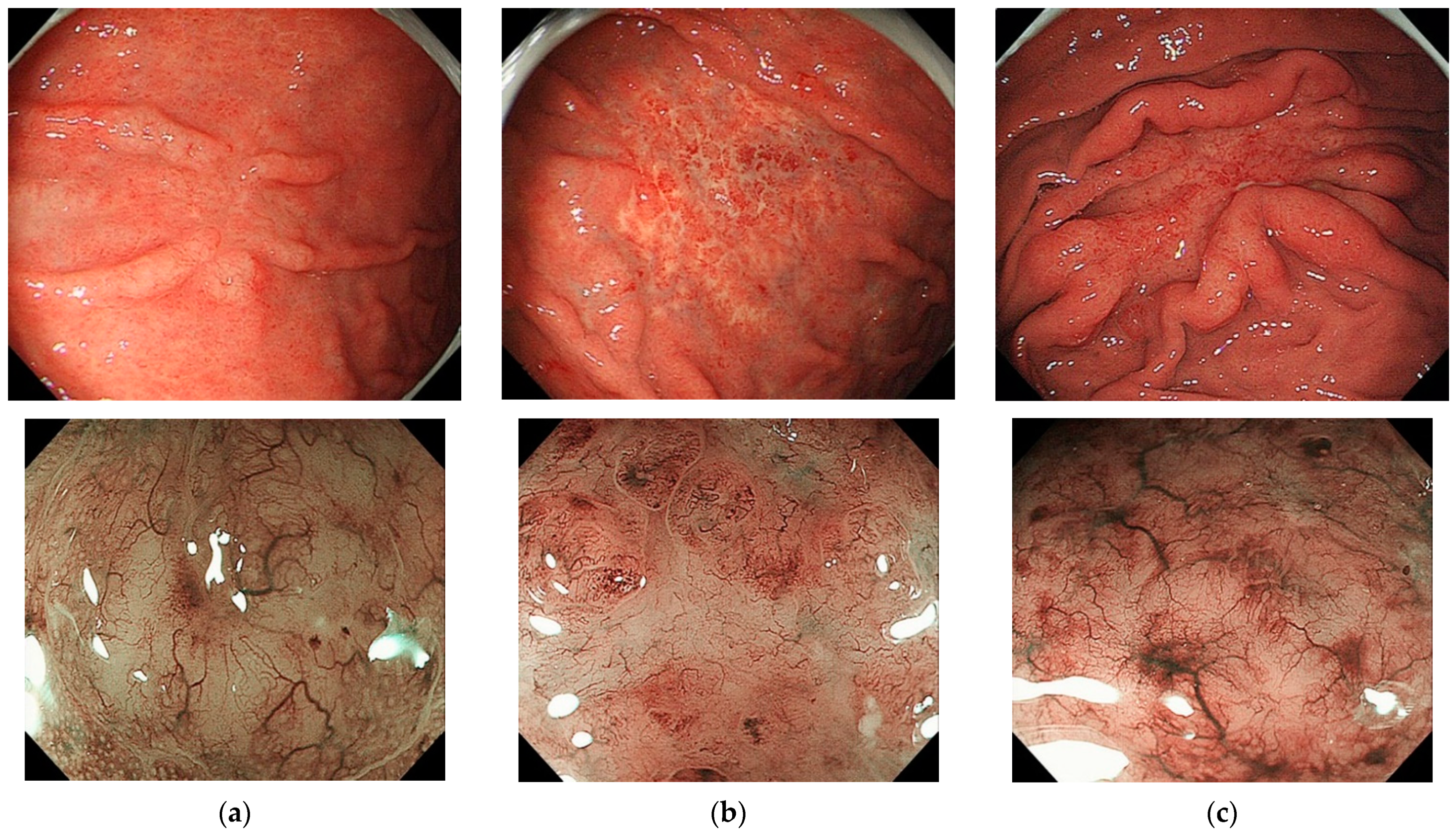
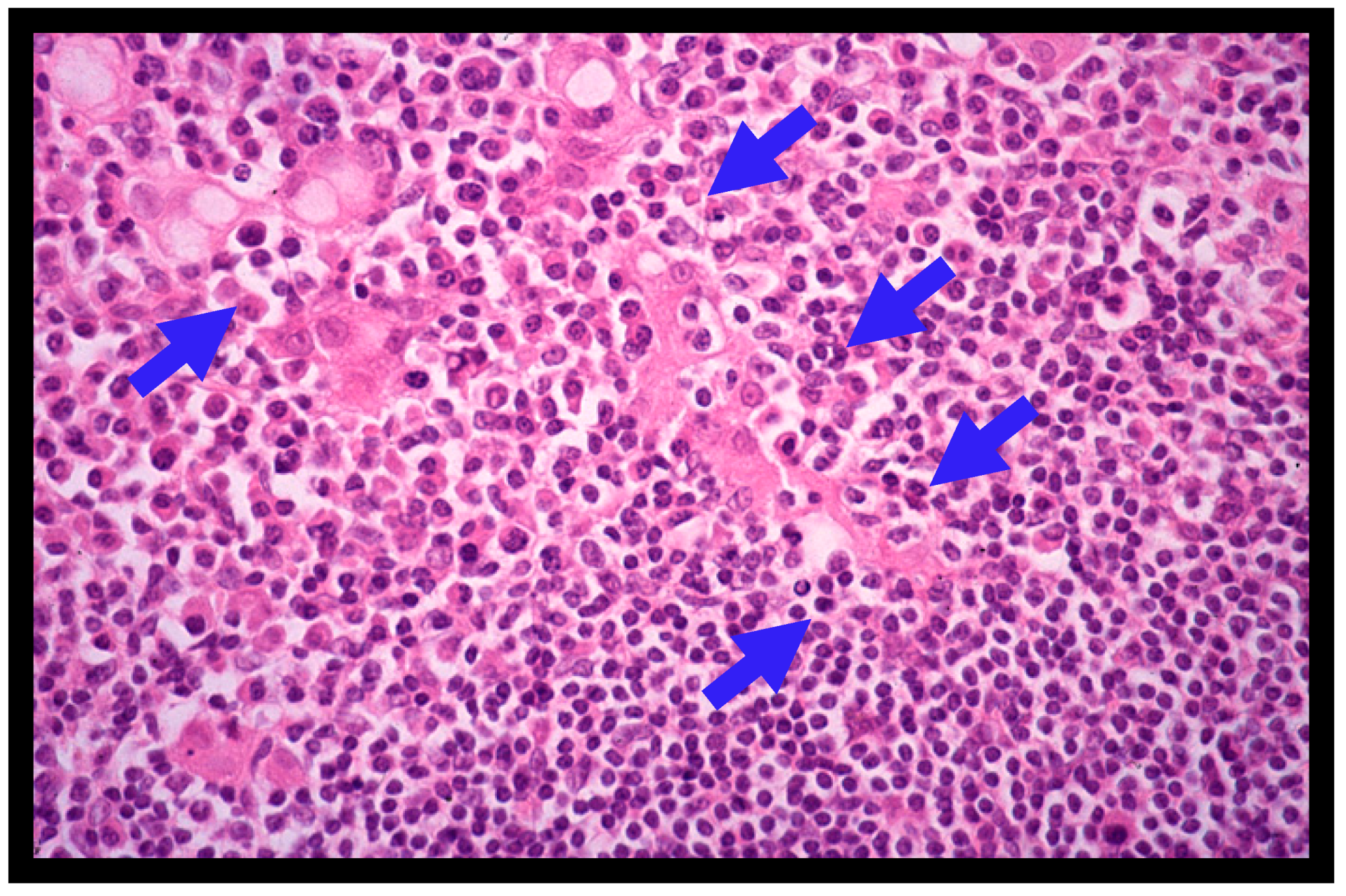
| Grade | Description | Histologic Features |
|---|---|---|
| 0 | Normal | Scattered plasma cells in mucosa; no lymphoid follicles. |
| 1 | Chronic active gastritis | Small clusters of lymphocytes in mucosa; no lymphoid follicles; no lymphoepithelial lesions. |
| 2 | Chronic active gastritis with florid lymphoid follicle formation | Prominent lymphoid follicles with surrounding mantle zone and plasma cells; no lymphoepithelial lesions. |
| 3 | Suspicious lymphoid infiltrate in mucosa, probably reactive | Lymphoid follicles surrounded by small lymphocytes that have infiltrated diffusely in the mucosa and occasionally into epithelium. |
| 4 | Suspicious lymphoid infiltrate in mucosa, probably lymphoma | Lymphoid follicles surrounded by centrocyte-like cells that have infiltrated diffusely in the mucosa and epithelium in small groups. |
| 5 | MALT lymphoma | Presence of dense diffuse infiltrate of centrocyte-like cells in the mucosa with prominent lymphoepithelial lesions. |
| Stage | Lugano System | Paris System | Tumor Extension |
|---|---|---|---|
| I | Tumor confined to GI tract (single, primary site or multiple, noncontiguous lesions) | T1m N0 M0 | Mucosa |
| T1sm N0 M0 | Submucosa | ||
| T2 N0 M0 | Muscularis propria | ||
| T3 N0 M0 | Serosa | ||
| II | Tumor extending into abdomen | NA | NA |
| II1 | Local nodal involvement | T1–3 N1 M0 | Perigastric lymph nodes |
| II2 | Distant nodal involvement | T1–3 N2 M0 | More distant regional lymph nodes |
| IIE | Perforation of serosa to involve adjacent organs or tissues | T4 N0–2 M0 | Invasion of adjacent structures with or without invasion of abdominal lymph nodes |
| IV | Disseminated extranodal involvement or concomitant supradiaphragmatic nodal involvement | T1–4 N3 M0 | Extra-abdominal lymph nodes |
| T1–4 N0–3 M1 | and/or additional distant GI/non-GI sites | ||
| T1–4 N0–3 M2 | BM not assessed | ||
| T1–4 N0–3 M0–2 BX | BM not involved | ||
| T1–4 N0–3 M2 B1 | BM involvement |
| Score | Lymphoid Infiltrate | LEL | Stromal Changes | Clinical Significance |
|---|---|---|---|---|
| Complete histologic response (ChR) | Absent or scattered plasma cells and small lymphoid cells in LP | Absent | Normal or empty LP and/or fibrosis | Complete remission |
| Probable minimal residual disease (pMRD) | Aggregates of lymphoid cells or lymphoid nodules in LP/MM and/or SM | Absent | Empty LP and/or fibrosis | Complete remission |
| Responding residual disease (rRD) | Dense, diffuse, or nodular, extending around glands in LP | Focal or absent | Focal empty LP and/or absent | Partial remission |
| No change (NC) | Dense, diffuse, or nodular | Present, may be absent | No changes | Stable or progressive disease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, S.; Hojo, M. Diagnosis and Treatment for Gastric Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma. J. Clin. Med. 2023, 12, 120. https://doi.org/10.3390/jcm12010120
Nakamura S, Hojo M. Diagnosis and Treatment for Gastric Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma. Journal of Clinical Medicine. 2023; 12(1):120. https://doi.org/10.3390/jcm12010120
Chicago/Turabian StyleNakamura, Shotaro, and Mariko Hojo. 2023. "Diagnosis and Treatment for Gastric Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma" Journal of Clinical Medicine 12, no. 1: 120. https://doi.org/10.3390/jcm12010120
APA StyleNakamura, S., & Hojo, M. (2023). Diagnosis and Treatment for Gastric Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma. Journal of Clinical Medicine, 12(1), 120. https://doi.org/10.3390/jcm12010120






