Mortality Risks after Two Years in Frail and Pre-Frail Older Adults Admitted to Hospital
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Statistical Methods
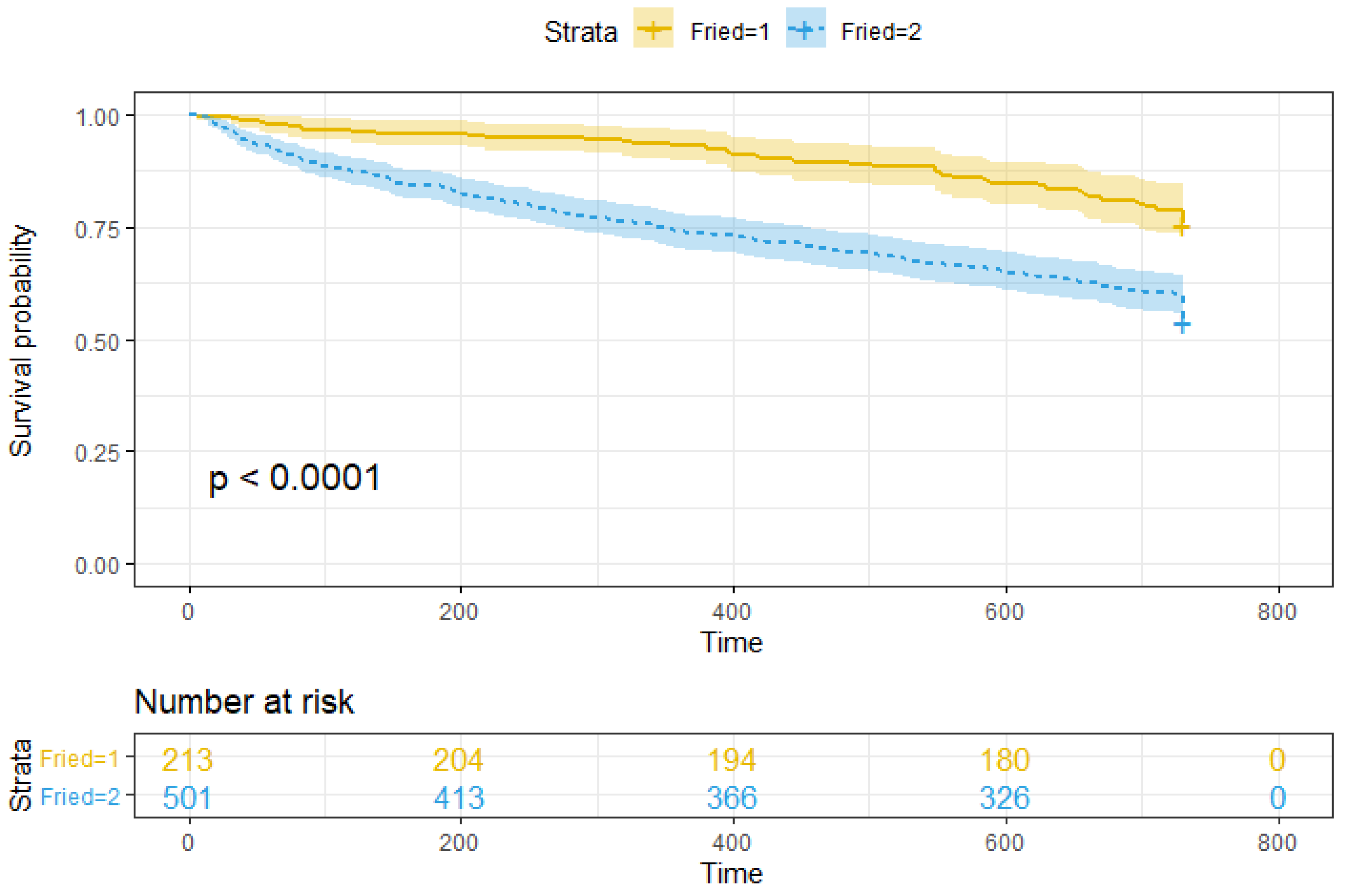
3. Results
4. Discussion
Limitations
5. Conclusions and Future Work
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.L.; Bandeen-Roche, K.; Tian, J.; Kasper, J.D.; Fried, L.P. Progression of Physical Frailty and the Risk of All-Cause Mortality: Is There a Point of No Return? J. Am. Geriatr. Soc. 2021, 69, 908–915. [Google Scholar] [CrossRef]
- Muscedere, J.; Waters, B.; Varambally, A.; Bagshaw, S.M.; Boyd, J.G.; Maslove, D.; Sibley, S.; Rockwood, K. The impact of frailty on intensive care unit outcomes: A systematic review and meta-analysis. Intensive Care Med. 2017, 43, 1105–1122. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Iliffe, S.; Walters, K. Frailty index as a predictor of mortality: A systematic review and meta-analysis. Age Ageing 2017, 47, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.; Tangen, C.; Walston, J.; Newman, A.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Loecker, C.; Schmaderer, M.; Zimmerman, L. Frailty in Young and Middle-Aged Adults: An Integrative Review. J. Frailty Aging 2021, 10, 327–333. [Google Scholar] [CrossRef]
- Blodgett, J.M.; Theou, O.; Howlett, S.E.; Rockwood, K. A frailty index from common clinical and laboratory tests predicts increased risk of death across the life course. GeroScience 2017, 39, 447–455. [Google Scholar] [CrossRef]
- Campbell, A.J.; Buchner, D.M. Unstable disability and the fluctuations of frailty. Age Ageing 1997, 26, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.; Bock, J.O.; Saum, K.U.; Matschinger, H.; Brenner, H.; Holleczek, B.; Haefeli, W.E.; Heider, D.; König, H.H. Frailty and healthcare costs—Longitudinal results of a prospective cohort study. Age Ageing 2017, 47, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Apóstolo, J.; Cooke, R.; Bobrowicz-Campos, E.; Santana, S.; Marcucci, M.; Cano, A.; Vollenbroek-Hutten, M.; Germini, F.; D’Avanzo, B.; Gwyther, H.; et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: A systematic review. JBI Database Syst. Rev. Implement Rep. 2018, 16, 140–232. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Custodero, C.; Maggi, S.; Polidori, M.C.; Veronese, N.; Ferrucci, L. A multidimensional approach to frailty in older people. Ageing Res. Rev. 2020, 60, 101047. [Google Scholar] [CrossRef]
- Faller, J.W.; Pereira, D.d.N.; de Souza, S.; Nampo, F.K.; Orlandi, F.d.S.; Matumoto, S. Instruments for the detection of frailty syndrome in older adults: A systematic review. PLoS ONE 2019, 14, e0216166. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Jiang, L.; Xue, L. Comparison of three frailty measures for 90-day outcomes of elderly patients undergoing elective abdominal surgery. ANZ J. Surg. 2021, 91, 335–340. [Google Scholar] [CrossRef]
- Fried, L.; Walston, J. Frailty and failure to thrive. In Principles of Geriatric Medicine and Gerontology; Hazzard, W., Blass, J., Ettinger, W.J., Halter, J., Ouslander, J., Eds.; McGraw-Hill: New York, NY, USA, 1998; pp. 1387–1402. [Google Scholar]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Cesari, M.; Gambassi, G.; van Kan, G.A.; Vellas, B. The frailty phenotype and the frailty index: Different instruments for different purposes. Age Ageing 2014, 43, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Zugasti Murillo, A.; Casas Herrero, Á. Frailty syndrome and nutritional status: Assessment, prevention and treatment. Nutr. Hosp. 2019, 36, 26–37. [Google Scholar] [CrossRef]
- Rezaei-Shahsavarloo, Z.; Atashzadeh-Shoorideh, F.; Gobbens, R.J.J.; Ebadi, A.; Ghaedamini Harouni, G. The impact of interventions on management of frailty in hospitalized frail older adults: A systematic review and meta-analysis. BMC Geriatr. 2020, 20, 526. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, J.; Lund, H.; Aadahl, M.; Sørensen, E.E. The experience of daily life of acutely admitted frail elderly patients one week after discharge from the hospital. Int. J. Q. Stud. Health Well-Being 2015, 10, 27370. [Google Scholar] [CrossRef] [PubMed]
- Aw, D.; Woodrow, L.; Ogliari, G.; Harwood, R. Association of frailty with mortality in older inpatients with COVID-19: A cohort study. Age Ageing 2020, 49, 915–922. [Google Scholar] [CrossRef]
- Blomaard, L.C.; van der Linden, C.M.J.; van der Bol, J.M.; Jansen, S.W.M.; Polinder-Bos, H.A.; Willems, H.C.; Festen, J.; Barten, D.G.; Borgers, A.J.; Bos, J.C.; et al. Frailty is associated with in-hospital mortality in older hospitalised COVID-19 patients in the Netherlands: The COVID-OLD study. Age Ageing 2021, 50, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Maynou, L.; Owen, R.; Konstant-Hambling, R.; Imam, T.; Arkill, S.; Bertfield, D.; Street, A.; Abrams, K.R.; Conroy, S. The association between frailty risk and COVID-19-associated all-mortality in hospitalised older people: A national cohort study. Eur. Geriatr. Med. 2022, 13, 1149–1157. [Google Scholar] [CrossRef]
- Pagliuca, R.; Cupido, M.G.; Mantovani, G.; Bugada, M.; Matteucci, G.; Caffarelli, A.; Bellotti, F.; Cocchieri, R.; Dentale, A.; Lozzi, F.; et al. Absence of negativization of nasal swab test and frailty as risk factors for mortality in elderly COVID-19 patients admitted in long-term care facilities. Eur. Geriatr. Med. 2022, 13, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Rebora, P.; Focà, E.; Salvatori, A.; Zucchelli, A.; Ceravolo, I.; Ornago, A.M.; Finazzi, A.; Arsuffi, S.; Bonfanti, P.; Citerio, G.; et al. The effect of frailty on in-hospital and medium-term mortality of patients with COronaVIrus Disease-19: The FRACOVID study. Panminerva Med. 2022, 64, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A.; Shekar, K.; Afroz, A.; Ashwin, S.; Billah, B.; Brown, H.; Kundi, H.; Lim, Z.J.; Ponnapa Reddy, M.; Curtis, J.R. Frailty and mortality associations in patients with COVID-19: A systematic review and meta-analysis. Inter. Med. J. 2022, 52, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Miles, A.; Webb, T.E.; Mcloughlin, B.C.; Mannan, I.; Rather, A.; Knopp, P.; Davis, D. Outcomes from COVID-19 across the range of frailty: Excess mortality in fitter older people. Eur. Geriatr. Med. 2020, 11, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, F.; Branje, K.E.; Hladkowicz, E.S.; Lalu, M.; McIsaac, D.I. Association of frailty with outcomes in individuals with COVID-19: A living review and meta-analysis. J. Am. Geriatr. Soc. 2021, 69, 2419–2429. [Google Scholar] [CrossRef] [PubMed]
- Cosco, T.D.; Best, J.; Davis, D.; Bryden, D.; Arkill, S.; van Oppen, J.; Riadi, I.; Wagner, K.R.; Conroy, S. What is the relationship between validated frailty scores and mortality for adults with COVID-19 in acute hospital care? A systematic review. Age Ageing 2021, 50, 608–616. [Google Scholar] [CrossRef]
- Chang, S.F.; Lin, P.L. Frail phenotype and mortality prediction: A systematic review and meta-analysis of prospective cohort studies. Int. J. Nurs. Stud. 2015, 52, 1362–1374. [Google Scholar] [CrossRef]
- Graña, M.; Besga, A. Fragility and Delirium Data from UHA. zenodo.org. 2021. Available online: https://doi.org/10.5281/zenodo.5803233 (accessed on 1 January 2023).
- O’Bryant, S.E.; Humphreys, J.D.; Smith, G.E.; Ivnik, R.J.; Graff-Radford, N.R.; Petersen, R.C.; Lucas, J.A. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch. Neurol. 2008, 65, 963–967. [Google Scholar] [CrossRef]
- Shigemori, K.; Ohgi, S.; Okuyama, E.; Shimura, T.; Schneider, E. The factorial structure of the mini mental state examination (MMSE) in Japanese dementia patients. BMC Geriatr. 2010, 10, 36. [Google Scholar] [CrossRef]
- Creavin, S.T.; Wisniewski, S.; Noel-Storr, A.H.; Trevelyan, C.M.; Hampton, T.; Rayment, D.; Thom, V.M.; Nash, K.J.E.; Elhamoui, H.; Milligan, R.; et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst. Rev. 2016, 2016, CD011145. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association With Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Mahoney, F.; Barthel, D. Functional evaluation: The barthel index. Md. State. Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.L. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. JNHA J. Nutr. Health Aging 2009, 13, 782. [Google Scholar] [CrossRef]
- Erkinjuntti, T.; Sulkava, R.; Wikström, J.; Autio, L. Short Portable Mental Status Questionnaire as a Screening Test for Dementia and Delirium Among the Elderly. J. Am. Geriatr. Soc. 1987, 35, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.T.; Neely, J.G.; Paniello, R.C.; Voelker, C.C.J.; Nussenbaum, B.; Wang, E.W. A practical guide to understanding Kaplan-Meier curves. Otolaryngol.—Head Neck Surg. 2010, 143, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Mittal, A.; Ricketts, D.; Rogers, B.A. Understanding survival analysis: Actuarial life tables and the Kaplan–Meier plot. Br. J. Hosp. Med. 2019, 80, 642–646. [Google Scholar] [CrossRef]
- Everitt, B.; Hothorn, T. A Handbook of Statistical Analyses Using R; CRC Press: Boca Raton, MA, USA, 2010. [Google Scholar]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B (Methodological) 1972, 34, 187–220. [Google Scholar] [CrossRef]
- Kidd, T.; Mold, F.; Jones, C.; Ream, E.; Grosvenor, W.; Sund-Levander, M.; Tingström, P.; Carey, N. What are the most effective interventions to improve physical performance in pre-frail and frail adults? A systematic review of randomised control trials. BMC Geriatr. 2019, 19, 184. [Google Scholar] [CrossRef]
- Hanlon, P.; Nicholl, B.I.; Jani, B.D.; Lee, D.; McQueenie, R.; Mair, F.S. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: A prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 2018, 3, e323–e332. [Google Scholar] [CrossRef] [PubMed]
- Prudham, D.; Evans, J.G. Factors associated with falls in the elderly: A community study. Age Ageing 1981, 10, 141–146. [Google Scholar] [CrossRef] [PubMed]
- WHO. Falls; Technical Report; WHO: Geneva, Switzerland, 2021.
- Berková, M.; Berka, Z. Falls: A significant cause of morbidity and mortality in elderly people. Vnitr. Lek. 2018, 64, 1076–1083. [Google Scholar] [CrossRef]
- Gary, R. Evaluation of frailty in older adults with cardiovascular disease: Incorporating physical performance measures. J. Cardiovasc. Nurs. 2012, 27, 120–131. [Google Scholar] [CrossRef]
- Topinková, E. Aging, disability and frailty. Ann. Nutr. Metab. 2008, 52 (Suppl. 1), 6–11. [Google Scholar] [CrossRef]
- Xue, Q.L.; Bandeen-Roche, K.; Varadhan, R.; Zhou, J.; Fried, L.P. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J. Gerontol. Biol. Sci. Med. Sci. 2008, 63, 984–990. [Google Scholar] [CrossRef]
- Vermeiren, S.; Vella-Azzopardi, R.; Beckwée, D.; Habbig, A.K.; Scafoglieri, A.; Jansen, B.; Bautmans, I. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1163.e1–1163.e17. [Google Scholar] [CrossRef]
- Downing, L.J.; Caprio, T.V.; Lyness, J.M. Geriatric psychiatry review: Differential diagnosis and treatment of the 3 D’s—Delirium, dementia, and depression. Curr. Psychiatry Rep. 2013, 15, 365. [Google Scholar] [CrossRef] [PubMed]
- Schuerch, M.; Farag, L.; Deom, S. [Delirium, depression, dementia: Solving the 3D’s]. Rev. Med. Liege 2012, 67, 26–34. [Google Scholar] [PubMed]
- Kim, D.J.; Massa, M.S.; Potter, C.M.; Clarke, R.; Bennett, D.A. Systematic review of the utility of the frailty index and frailty phenotype to predict all-cause mortality in older people. Syst. Rev. 2022, 11, 187. [Google Scholar] [CrossRef]
- Ekram, A.R.M.S.; Woods, R.L.; Britt, C.; Espinoza, S.; Ernst, M.E.; Ryan, J. The Association between Frailty and All-Cause Mortality in Community-Dwelling Older Individuals: An Umbrella Review. J. Frailty Aging 2021, 10, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.M.; Olivieri-Mui, B.; McCarthy, E.P.; Kim, D.H. Changes in a Frailty Index and Association with Mortality. J. Am. Geriatr. Soc. 2021, 69, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Stolz, E.; Mayerl, H.; Hoogendijk, E.O. Frailty in the oldest old: Is the current level or the rate of change more predictive of mortality? Age Ageing 2022, 51. [Google Scholar] [CrossRef]
- Stolz, E.; Hoogendijk, E.O.; Mayerl, H.; Freidl, W. Frailty Changes Predict Mortality in 4 Longitudinal Studies of Aging. J. Gerontol. Biol. Sci. Med. Sci. 2021, 76, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Lu, Q.; Lu, Z.; Xu, F.; Cao, Z.; Li, S.; Yang, H.; Zhao, Y.; Wang, Y. Frailty Phenotype and Mortality: A Prospective Cohort Study. J. Am. Med. Dir. Assoc. 2022, 23, 182–185. [Google Scholar] [CrossRef]
- Lohman, M.C.; Sonnega, A.J.; Resciniti, N.V.; Leggett, A.N. Frailty Phenotype and Cause-Specific Mortality in the United States. J. Gerontol. Biol. Sci. Med. Sci. 2020, 75, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Adebusoye, L.A.; Cadmus, E.O.; Owolabi, M.O.; Ogunniyi, A. Frailty and mortality among older patients in a tertiary hospital in Nigeria. Ghana Med. J. 2019, 53, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Lujic, S.; Randall, D.A.; Simpson, J.M.; Falster, M.O.; Jorm, L.R. Interaction effects of multimorbidity and frailty on adverse health outcomes in elderly hospitalised patients. Sci. Rep. 2022, 12, 14139. [Google Scholar] [CrossRef]
- Rosas, C.; Oliveira, H.C.; Neri, A.L.; Ceolim, M.F. Stressful events, depressive symptoms, and frailty associated to older adults’ survival and mortality. Geriatr. Nurs. 2022, 46, 62–68. [Google Scholar] [CrossRef]
- Kane, A.E.; Howlett, S.E. Sex differences in frailty: Comparisons between humans and preclinical models. Mech. Ageing Dev. 2021, 198, 111546. [Google Scholar] [CrossRef]
- Núñez, J.; Palau, P.; Sastre, C.; D’Ascoli, G.; Ruiz, V.; Bonanad, C.; Miñana, G.; Núñez, E.; Sanchis, J. Sex-differential effect of frailty on long-term mortality in elderly patients after an acute coronary syndrome. Int. J. Cardiol. 2020, 302, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Dallmeier, D.; Braisch, U.; Rapp, K.; Klenk, J.; Rothenbacher, D.; Denkinger, M. Frailty Index and Sex-Specific 6-Year Mortality in Community-Dwelling Older People: The ActiFE Study. J. Gerontol. Biol. Sci. Med. Sci. 2020, 75, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.Y.; Streiter, S.; O’Mara, L.; Sison, S.M.; Theou, O.; Bernacki, R.; Orkaby, A. Frailty and Survival After In-Hospital Cardiopulmonary Resuscitation. J. Gen. Intern. Med. 2022, 37, 3554–3561. [Google Scholar] [CrossRef]
- Sánchez Arteaga, A.; Tinoco González, J.; Tallón Aguilar, L.; Anguiano Díaz, G.; Jiménez-Rodriguez, R.M.; Rovira Liarde, A.; Pareja Ciuró, F.; Padillo Ruíz, J. Long-term influence of frailty in elderly patients after surgical emergencies. Eur. J. Trauma Emerg. Surg. 2022, 48, 3855–3862. [Google Scholar] [CrossRef] [PubMed]
- Venado, A.; Kolaitis, N.A.; Huang, C.Y.; Gao, Y.; Glidden, D.V.; Soong, A.; Sutter, N.; Katz, P.P.; Greenland, J.R.; Calabrese, D.R.; et al. Frailty after lung transplantation is associated with impaired health-related quality of life and mortality. Thorax 2020, 75, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Cotton, J.; Cabot, J.; Buckner, J.; Field, A.; Pounds, L.; Quint, C. Increased Frailty Associated with Higher Long-Term Mortality after Major Lower Extremity Amputation. Ann. Vasc. Surg. 2022, 86, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tian, R.; Ye, P.; Luo, Y. Frailty in Older Patients Undergoing Hemodialysis and Its Association with All-Cause Mortality: A Prospective Cohort Study. Clin. Interv. Aging 2022, 17, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.; Ma, Q.; Diao, Z.; Liu, S.; Shi, X. The Impact of Frailty on Prognosis in Elderly Hemodialysis Patients: A Prospective Cohort Study. Clin. Interv. Aging 2021, 16, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Zhang, A.; Ma, L.; Jia, L.; Chhetri, J.K.; Chan, P. Severity of frailty as a significant predictor of mortality for hemodialysis patients: A prospective study in China. Int. J. Med. Sci. 2021, 18, 3309–3317. [Google Scholar] [CrossRef]
- Burton, J.K.; Stewart, J.; Blair, M.; Oxley, S.; Wass, A.; Taylor-Rowan, M.; Quinn, T.J. Prevalence and implications of frailty in acute stroke: Systematic review & meta-analysis. Age Ageing 2022, 51, afac064. [Google Scholar] [CrossRef]
- Faye, A.S.; Wen, T.; Soroush, A.; Ananthakrishnan, A.N.; Ungaro, R.; Lawlor, G.; Attenello, F.J.; Mack, W.J.; Colombel, J.F.; Lebwohl, B. Increasing Prevalence of Frailty and Its Association with Readmission and Mortality Among Hospitalized Patients with IBD. Dig. Dis. Sci. 2021, 66, 4178–4190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Mobley, E.M.; Manini, T.M.; Leeuwenburgh, C.; Anton, S.D.; Washington, C.J.; Zhou, D.; Parker, A.S.; Okunieff, P.G.; Bian, J.; et al. Frailty and risk of mortality in older cancer survivors and adults without a cancer history: Evidence from the National Health and Nutrition Examination Survey, 1999–2014. Cancer 2022, 128, 2978–2987. [Google Scholar] [CrossRef] [PubMed]
- McMillan, S.; Kim, S.J.; Tin, A.L.; Downey, R.J.; Vickers, A.J.; Korc-Grodzicki, B.; Shahrokni, A. Association of frailty with 90-day postoperative mortality & geriatric comanagement among older adults with cancer. Eur. J. Surg. Oncol. 2022, 48, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Kremer, K.M.; Braisch, U.; Rothenbacher, D.; Denkinger, M.; Dallmeier, D. Systolic Blood Pressure and Mortality in Community-Dwelling Older Adults: Frailty as an Effect Modifier. Hypertension 2022, 79, 24–32. [Google Scholar] [CrossRef]
- Ruiz-Grao, M.C.; Sánchez-Jurado, P.M.; Molina-Alarcón, M.; Hernández-Martínez, A.; Avendaño Céspedes, A.; Abizanda, P. Frailty, depression risk, and 10-year mortality in older adults: The FRADEA study. Int. Psychogeriatrics 2021, 33, 803–812. [Google Scholar] [CrossRef]
- Arts, M.H.L.; van den Berg, K.S.; Marijnissen, R.M.; de Jonge, L.; Hegeman, A.J.M.; Collard, R.M.; Comijs, H.C.; Aprahamian, I.; Naarding, P.; Oude Voshaar, R.C. Frailty as a Predictor of Mortality in Late-Life Depression: A Prospective Clinical Cohort Study. J. Clin. Psychiatry 2021, 82. [Google Scholar] [CrossRef] [PubMed]
- Laur, C.V.; McNicholl, T.; Valaitis, R.; Keller, H.H. Malnutrition or frailty? Overlap and evidence gaps in the diagnosis and treatment of frailty and malnutrition. Appl. Physiol. Nutr. Metab. 2017, 42, 449–458. [Google Scholar] [CrossRef]
- Jayanama, K.; Theou, O.; Blodgett, J.M.; Cahill, L.; Rockwood, K. Frailty, nutrition-related parameters, and mortality across the adult age spectrum. BMC Med. 2018, 16, 188. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; Guerra, V.; Donghia, R.; Bortone, I.; Griseta, C.; Lampignano, L.; Dibello, V.; Lozupone, M.; Coelho-Júnior, H.J.; et al. Associations between nutritional frailty and 8-year all-cause mortality in older adults: The Salus in Apulia Study. J. Intern. Med. 2021, 290, 1071–1082. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Kline, K.A.; Bowdish, D.M.E. Infection in an aging population. Curr. Opin. Microbiol. 2016, 29, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.S.; Abdo, Z.; Forney, L.J. Caring about trees in the forest: Incorporating frailty in risk analysis for personalized medicine. Per. Med. 2011, 8, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Ince, C. Personalized physiological medicine. Crit. Care 2017, 21, 308. [Google Scholar] [CrossRef]
- Picca, A.; Coelho-Junior, H.J.; Cesari, M.; Marini, F.; Miccheli, A.; Gervasoni, J.; Bossola, M.; Landi, F.; Bernabei, R.; Marzetti, E.; et al. The metabolomics side of frailty: Toward personalized medicine for the aged. Exp. Gerontol. 2019, 126, 110692. [Google Scholar] [CrossRef]
- García-Giménez, J.; Mena-Molla, S.; Tarazona-Santabalbina, F.J.; Viña, J.; Gomez-Cabrera, M.C.; Pallardó, F.V. Implementing Precision Medicine in Human Frailty through Epigenetic Biomarkers. Int. J. Environ. Res. Public Health 2021, 18, 1883. [Google Scholar] [CrossRef] [PubMed]
- Pavasini, R.; Guralnik, J.; Brown, J.C.; di Bari, M.; Cesari, M.; Landi, F.; Vaes, B.; Legrand, D.; Verghese, J.; Wang, C.; et al. Short Physical Performance Battery and all-cause mortality: Systematic review and meta-analysis. BMC Med. 2016, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Dharmarajan, K.; Rich, M.W. Epidemiology, Pathophysiology, and Prognosis of Heart Failure in Older Adults. Heart Fail. Clin. 2017, 13, 417–426. [Google Scholar] [CrossRef]
- Uchmanowicz, I.; Lee, C.S.; Vitale, C.; Manulik, S.; Denfeld, Q.E.; Uchmanowicz, B.; Rosińczuk, J.; Drozd, M.; Jaroch, J.; Jankowska, E.A. Frailty and the risk of all-cause mortality and hospitalization in chronic heart failure: A meta-analysis. ESC Heart Fail. 2020, 7, 3427–3437. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lupón, J.; Vidán, M.T.; Ferguson, C.; Gastelurrutia, P.; Newton, P.J.; Macdonald, P.S.; Bueno, H.; Bayés-Genís, A.; Woo, J.; et al. Impact of Frailty on Mortality and Hospitalization in Chronic Heart Failure: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008251. [Google Scholar] [CrossRef] [PubMed]
- Jujo, K.; Kagiyama, N.; Saito, K.; Kamiya, K.; Saito, H.; Ogasahara, Y.; Maekawa, E.; Konishi, M.; Kitai, T.; Iwata, K.; et al. Impact of Social Frailty in Hospitalized Elderly Patients With Heart Failure: A FRAGILE-HF Registry Subanalysis. J. Am. Heart Assoc. 2021, 10, e019954. [Google Scholar] [CrossRef]
- Wehling, M. Morbus diureticus in the elderly: Epidemic overuse of a widely applied group of drugs. J. Am. Med. Dir. Assoc. 2013, 14, 437–442. [Google Scholar] [CrossRef]
- Folsom, A.R.; Boland, L.L.; Cushman, M.; Heckbert, S.R.; Rosamond, W.D.; Walston, J.D. Frailty and risk of venous thromboembolism in older adults. J. Gerontol. Biol. Sci. Med. Sci. 2007, 62, 79–82. [Google Scholar] [CrossRef]
- de Souza Ramos, J.T.G.; Ferrari, F.S.; Andrade, M.F.; de Melo, C.S.; Boas, P.J.V.; Costa, N.A.; Pereira, A.G.; Dorna, M.S.; Azevedo, P.S.; Banerjee, J.; et al. Association between frailty and C-terminal agrin fragment with 3-month mortality following ST-elevation myocardial infarction. Exp. Gerontol. 2022, 158, 111658. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.; Wang, W.; Wang, H.; Ma, Y.; Hai, S.; Lin, X.; Liu, Y.; Zhang, X.; Wu, J.; Dong, B. Prognostic value of frailty in elderly patients with acute coronary syndrome: A systematic review and meta-analysis. BMC Geriatr. 2019, 19, 222. [Google Scholar] [CrossRef] [PubMed]
- São Romão Preto, L.; do Carmo Dias Conceição, M.; Soeiro Amaral, S.I.; Martins Figueiredo, T.; Ramos Sánchez, A.; Fernandes-Ribeiro, A.S. Fragilidad en ancianos que viven en la comunidad con y sin enfermedad cerebrovascular previa. Rev. Cient. Soc. Esp. Enferm. Neurol. 2017, 46, 11–17. [Google Scholar] [CrossRef]
- Elf, M.; Eriksson, G.; Johansson, S.; von Koch, L.; Ytterberg, C. Self-Reported Fatigue and Associated Factors Six Years after Stroke. PLoS ONE 2016, 11, e0161942. [Google Scholar] [CrossRef] [PubMed]
- Assar, M.E.; Laosa, O.; Rodríguez Mañas, L. Diabetes and frailty. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J. Management of hypoglycemia in older adults with type 2 diabetes. Postgrad. Med. 2019, 131, 241–250. [Google Scholar] [CrossRef]
- Gómez, C.; Vega-Quiroga, S.; Bermejo-Pareja, F.; Medrano, M.J.; Louis, E.D.; Benito-León, J. Polypharmacy in the Elderly: A Marker of Increased Risk of Mortality in a Population-Based Prospective Study (NEDICES). Gerontology 2015, 61, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.; Arthur, A.; Savva, G.M. How do potentially inappropriate medications and polypharmacy affect mortality in frail and non-frail cognitively impaired older adults? A cohort study. BMJ Open 2019, 9, e026171. [Google Scholar] [CrossRef]
- Xie, Y.; Bowe, B.; Yan, Y.; Xian, H.; Li, T.; Al-Aly, Z. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: Cohort study. BMJ 2019, 365. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, R.; Piscione, F.; Galasso, G.; De Servi, S.; Savonitto, S. Antiplatelet therapy in very elderly and comorbid patients with acute coronary syndromes. J. Geriatr. Cardiol. 2019, 16, 103–113. [Google Scholar] [CrossRef]
- Schneider, L.S.; Dagerman, K.S.; Insel, P. Risk of death with atypical antipsychotic drug treatment for dementia: Meta-analysis of randomized placebo-controlled trials. JAMA 2005, 294, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Lee, J.; Jung, S.J.; Shin, A.; Lee, Y.J. Use of Sedative-Hypnotics and Mortality: A Population-Based Retrospective Cohort Study. J. Clin. Sleep Med. 2018, 14, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.Y.; Huang, S.T.; Chen, L.K.; Hsiao, F.Y. Development of frailty index using ICD-10 codes to predict mortality and rehospitalization of older adults: An update of the multimorbidity frailty index. Arch. Gerontol. Geriatr. 2022, 100, 104646. [Google Scholar] [CrossRef] [PubMed]
- Lekan, D.; McCoy, T.P.; Jenkins, M.; Mohanty, S.; Manda, P. Frailty and In-Hospital Mortality Risk Using EHR Nursing Data. Biol. Res. Nurs. 2022, 24, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Aalberg, J.J.; Soriano, R.P.; Divino, C.M. The 5-Factor Modified Frailty Index in the Geriatric Surgical Population. Am. Surg. 2021, 87, 1420–1425. [Google Scholar] [CrossRef] [PubMed]

| Estimate | Standard Error | Standardized | Odds Ratio | z | Wald Statistic | df | p | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|
| Needs Walking Stick | 0.842 | 0.318 | 0.392 | 2.321 | 2.649 | 7.019 | 1 | 0.008 | (0.219, 1.465) |
| MNA Weight Loss 3 months | 0.683 | 0.197 | 0.812 | 1.980 | 3.467 | 12.019 | 1 | <0.001 | (0.297, 1.070) |
| MNA Mobility | 2.165 | 0.599 | 1.029 | 8.717 | 3.612 | 13.046 | 1 | <0.001 | (0.990, 3.340) |
| SPPB March 4 m | 0.590 | 0.210 | 0.590 | 1.804 | 2.810 | 7.897 | 1 | 0.005 | (0.179, 1.002) |
| Falls | −0.618 | 0.319 | −0.296 | 0.539 | −1.936 | 3.749 | 1 | 0.053 | (−1.243, 0.008) |
| Dementia | 2.146 | 0.965 | 0.436 | 8.552 | 2.225 | 4.950 | 1 | 0.026 | (0.256, 4.037 |
| Depression | 1.641 | 0.780 | 0.465 | 5.162 | 2.104 | 4.427 | 1 | 0.035 | (0.112, 3.171) |
| Delirium | −0.734 | 0.370 | −0.325 | 0.480 | −1.987 | 3.947 | 1 | 0.047 | (−1.459, −0.010) |
| 2 Years | ||||||
|---|---|---|---|---|---|---|
| Variable | Coefficients | Exp(Coef) | Se(Coef) | z | HR | p |
| Age | 0.048 | 1.049 | 0.019 | 2.545 | 1.05 (1.01–1.09) | 0.011 |
| Sex | −0.817 | 0.442 | 0.207 | −3.945 | 0.44 (0.29–0.66) | <0.001 |
| Weight | −0.019 | 0.980 | 0.009 | −2.218 | 0.98 (0.96–1.00) | 0.027 |
| R30 | −0.703 | 0.495 | 0.203 | −3.463 | 0.50 (0.33–0.74) | <0.001 |
| SPPB-SUG | −0.482 | 0.618 | 0.162 | −2.980 | 0.62 (0.45–0.85) | 0.003 |
| Congestive Heart Failure | −0.398 | 0.671 | 0.193 | −2.062 | 0.67 (0.46–0.98) | 0.039 |
| PPIs | −0.512 | 0.599 | 0.188 | −2.722 | 0.60 (0.41–0.87) | 0.006 |
| Antiplatelet | 0.588 | 1.800 | 0.209 | 2.808 | 1.80 (1.19–2.71) | 0.005 |
| Quetiapine | −1.166 | 0.312 | 0.488 | −2.387 | 0.31 (0.12–0.81) | 0.017 |
| Paracetamol | 0.368 | 1.445 | 0.187 | 1.973 | 1.45 (1.00–2.08) | 0.049 |
| 2 Years | ||||||
|---|---|---|---|---|---|---|
| Variable | Coefficients | Exp(Coef) | Se(Coef) | z | HR | p |
| Age | 0.518 | 1.679 | 0.131 | 3.945 | 1.7 (1.3–2.2) | <0.001 |
| Sex | −5.295 | 0.005 | 1.945 | −2.723 | 0.005 ( 0.00011–0.23) | 0.006 |
| Weight | 0.251 | 1.286 | 0.066 | 3.806 | 1.3 (1.1–1.5) | <0.001 |
| Own-home | 4.824 | 124.4 | 2.065 | 2.336 | 120 (2.2–7100) | 0.019 |
| Barthel | 4.748 | 115.3 | 1.383 | 3.434 | 120 (7.7–1700) | <0.001 |
| Pfeiffer | 2.131 | 8.426 | 0.669 | 3.184 | 8.4 (2.3–31) | 0.001 |
| MNA | 7.127 | 1246 | 2.174 | 3.278 | 1200 (18–88,000) | 0.001 |
| MNA Weight Loss 3 months | 2.410 | 11.14 | 0.815 | 2.957 | 11 (2.3–55) | 0.003 |
| MNA Mobility | 6.378 | 588.7 | 2.043 | 3.123 | 590 (11–32,000) | 0.002 |
| MNA Acute Disease 3 months | −7.656 | <0.001 | 2.038 | −3.757 | 0.00047 (0.0000087–0.026) | <0.001 |
| Vision Loss | −3.160 | 0.042 | 1.247 | −2.534 | 0.042 (0.0037–0.49) | 0.011 |
| Constipation | −3.687 | 0.025 | 1.143 | −3.226 | 0.025 (0.0027–0.24) | 0.001 |
| Falls | 9.797 | 1798 | 2.444 | 4.009 | 18,000 (150–2,200,000) | <0.001 |
| Congestive Heart Failure | 3.739 | 42.07 | 1.761 | 2.124 | 42 (1.3–1300) | 0.034 |
| Deep Venous Thrombosis | 9.038 | 8421 | 3.098 | 2.917 | 8400 (19–3,700,000) | 0.004 |
| Cerebrovascular Disease | −4.585 | 0.010 | 1.409 | −3.255 | 0.01 (0.00064–0.16) | 0.001 |
| Diabetes | 5.899 | 364.8 | 2.385 | 2.474 | 360 (3.4–39,000) | 0.013 |
| Thyroid Disease | −6.913 | <0.001 | 2.271 | −3.045 | 0.00099 (0.000012–0.085) | 0.002 |
| Drug Oligopharma | −3.410 | 0.033 | 1.537 | −2.219 | 0.033 (0.0016–0.67) | 0.026 |
| PPIs | −2.780 | 0.062 | 1.101 | −2.524 | 0.062 (0.0072–0.54) | 0.012 |
| Zolpidem | −11.19 | <0.001 | 4.488 | −2.493 | 0.000014 (0.0000000021–0.092) | 0.013 |
| Antidiabetics | −8.830 | <0.001 | <0.001 | −2.953 | 0.00015 (0.00000042–0.051) | 0.003 |
| Diuretics | −8.113 | <0.001 | 2.196 | −3.694 | 0.0003 (0.000004–0.022) | <0.001 |
| Opiates | −9.585 | <0.001 | 2.691 | −3.562 | 0.000069 (0.00000035–0.013) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Escalera, G.; Graña, M.; Irazusta, J.; Labayen, I.; Gonzalez-Pinto, A.; Besga, A. Mortality Risks after Two Years in Frail and Pre-Frail Older Adults Admitted to Hospital. J. Clin. Med. 2023, 12, 3103. https://doi.org/10.3390/jcm12093103
Cano-Escalera G, Graña M, Irazusta J, Labayen I, Gonzalez-Pinto A, Besga A. Mortality Risks after Two Years in Frail and Pre-Frail Older Adults Admitted to Hospital. Journal of Clinical Medicine. 2023; 12(9):3103. https://doi.org/10.3390/jcm12093103
Chicago/Turabian StyleCano-Escalera, Guillermo, Manuel Graña, Jon Irazusta, Idoia Labayen, Ana Gonzalez-Pinto, and Ariadna Besga. 2023. "Mortality Risks after Two Years in Frail and Pre-Frail Older Adults Admitted to Hospital" Journal of Clinical Medicine 12, no. 9: 3103. https://doi.org/10.3390/jcm12093103
APA StyleCano-Escalera, G., Graña, M., Irazusta, J., Labayen, I., Gonzalez-Pinto, A., & Besga, A. (2023). Mortality Risks after Two Years in Frail and Pre-Frail Older Adults Admitted to Hospital. Journal of Clinical Medicine, 12(9), 3103. https://doi.org/10.3390/jcm12093103









