A Prospective Study of Renal Blood Flow during Retrograde Intrarenal Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Regression Analysis
2.2.2. Statistical Environment
2.3. Characteristic of the Sample
3. Results
3.1. Regression Models for the Time-Point Predictor before RIRS–0 h after RIRS (Mod1–Mod9)
3.1.1. Resistive Index as Focal Variable
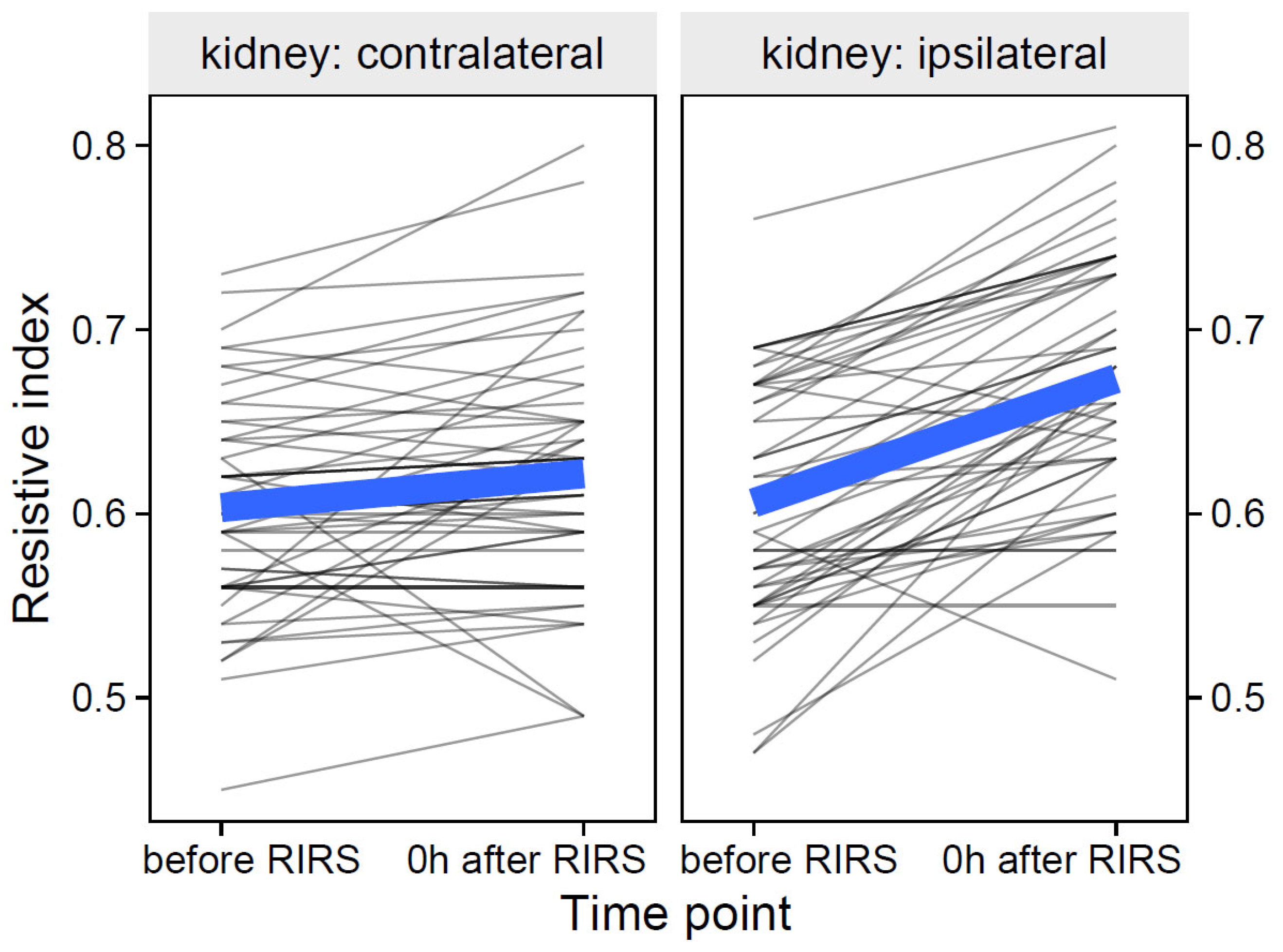
3.1.1.1. Estimation of the Effects of the Volume of the Mean Stone, Kidney Side and Time on the Resistive Index (Mod1)
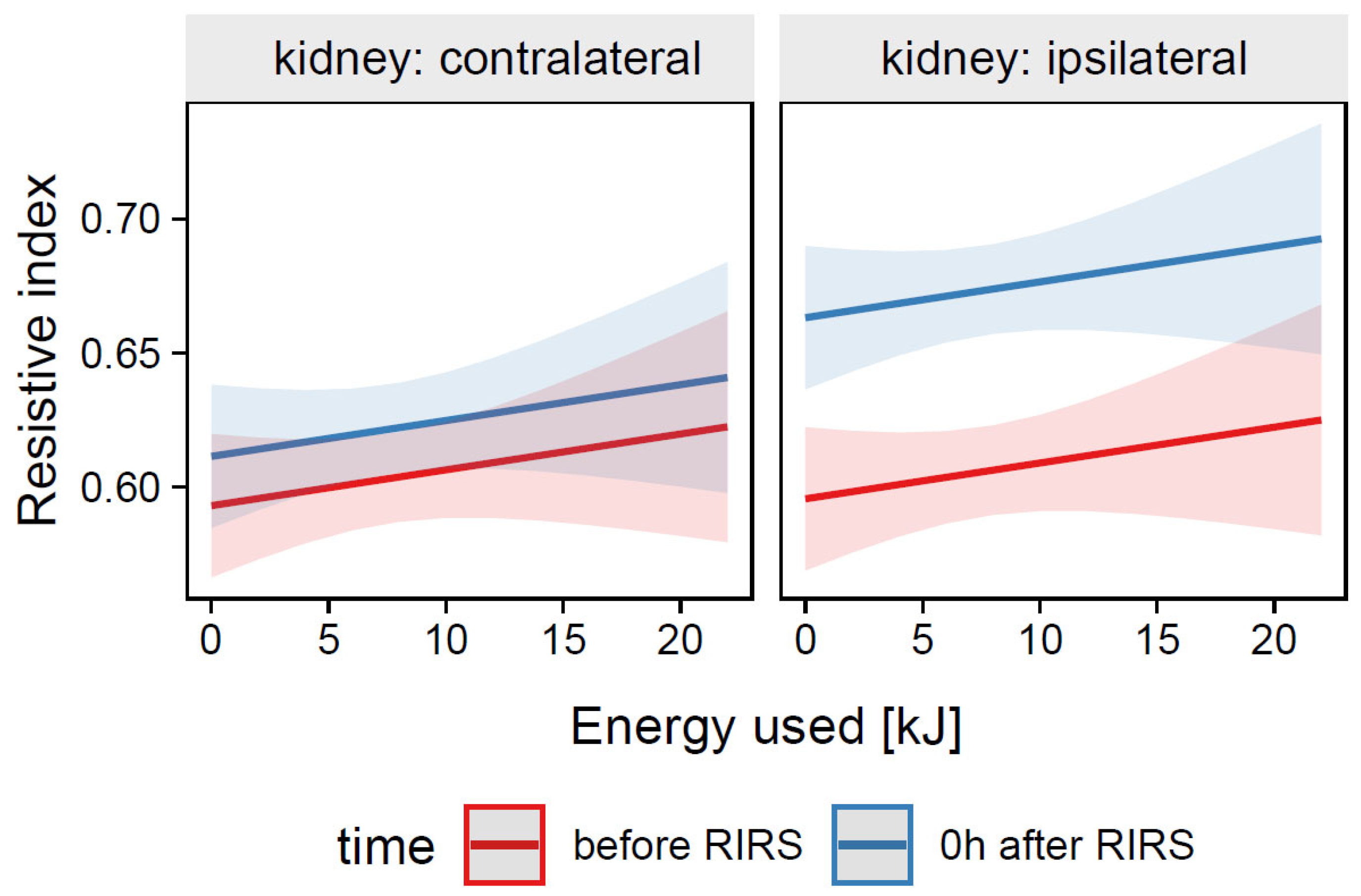
3.1.1.2. Estimation of the Effects of Energy Used, Kidney Side, and Time on the Resistive Index (Mod2)
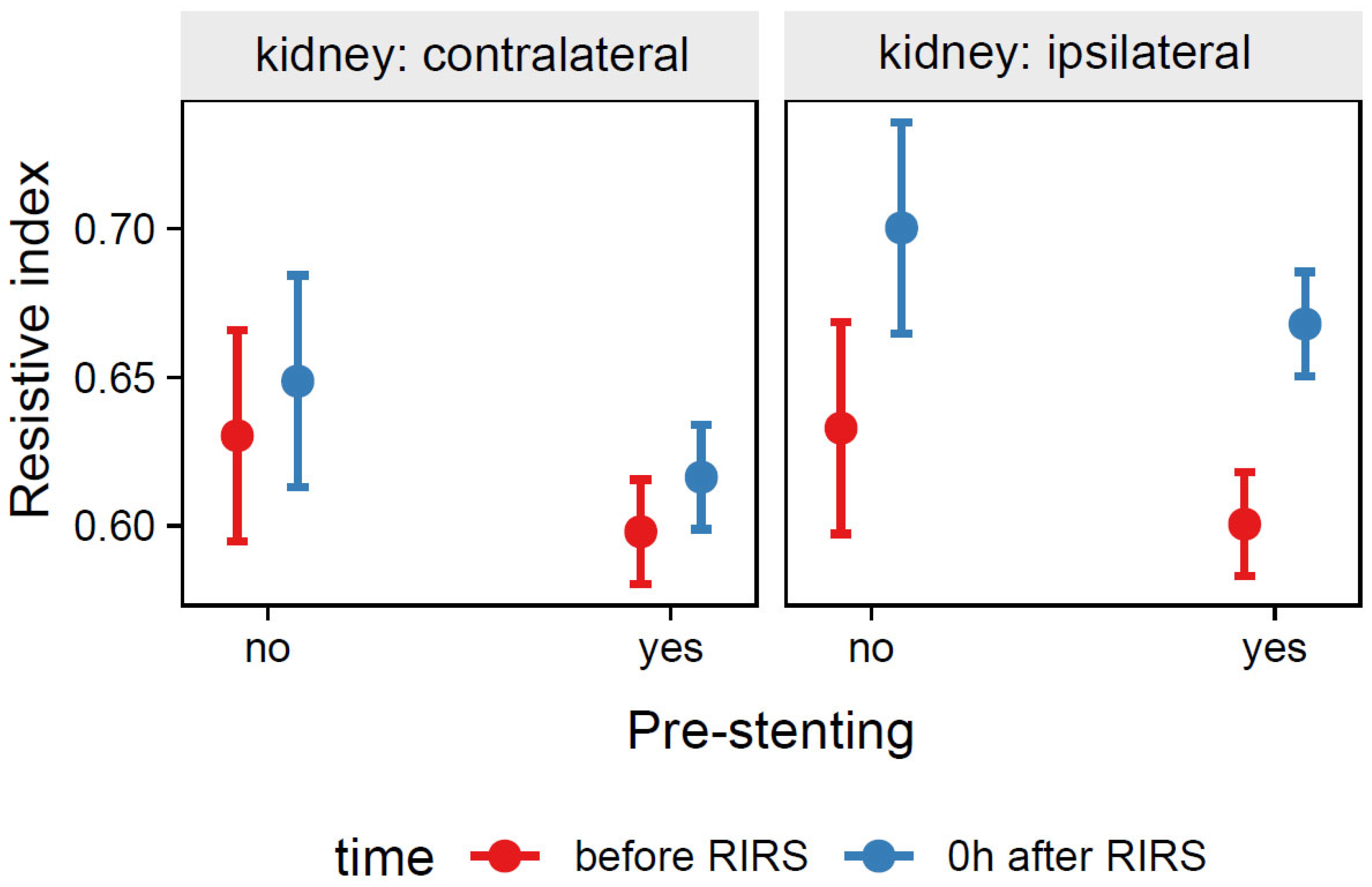
3.1.1.3. Estimation of the Effects of Pre-Stenting, Kidney Side, and Time on the Resistive Index (Mod3)
3.1.2. Pulsatility Index as Focal Variable
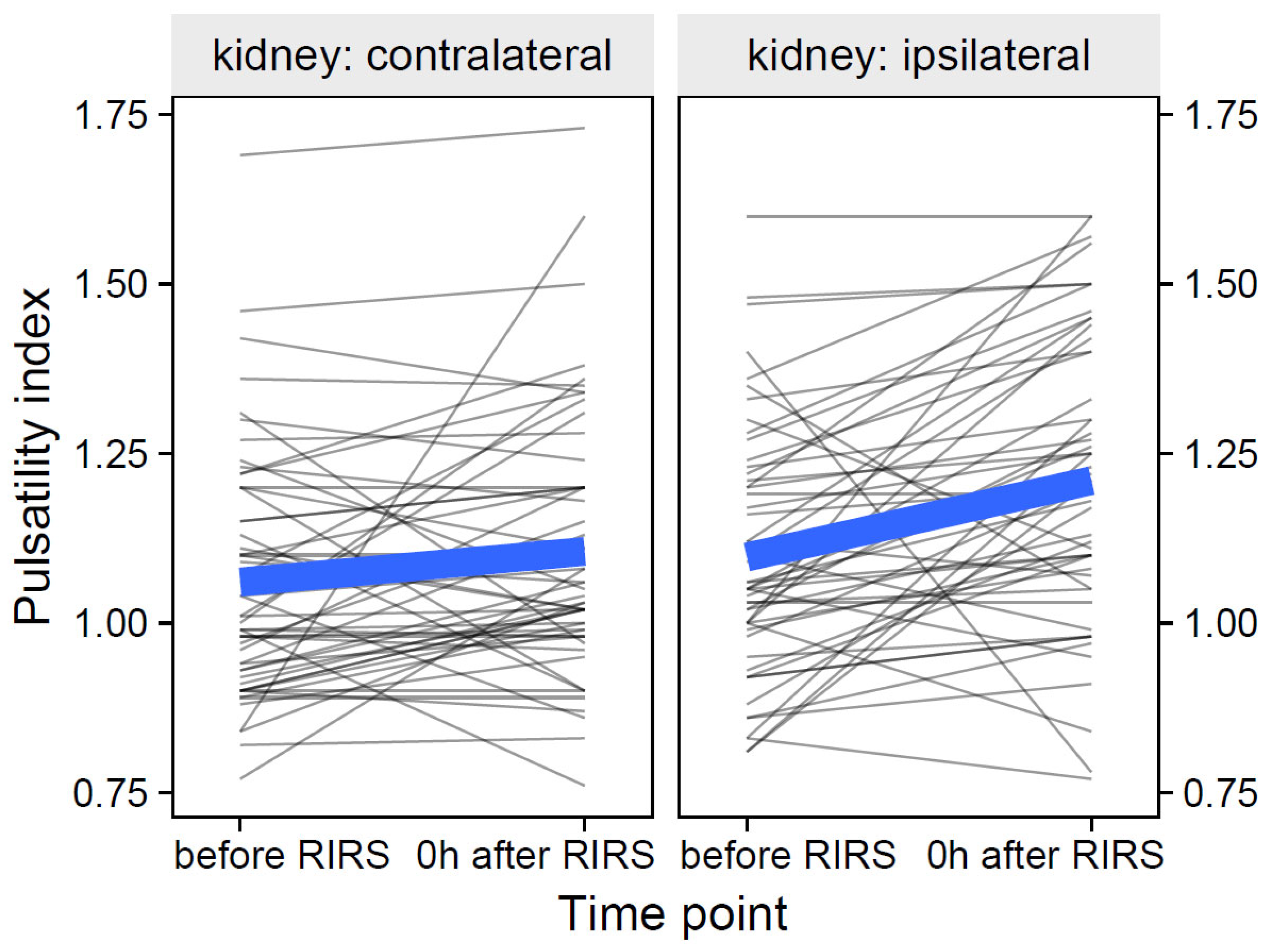
3.1.2.1. Estimation of the Effects of Mean Stone Volume, Kidney Side, and Time on Pulsatility Index (Mod4)
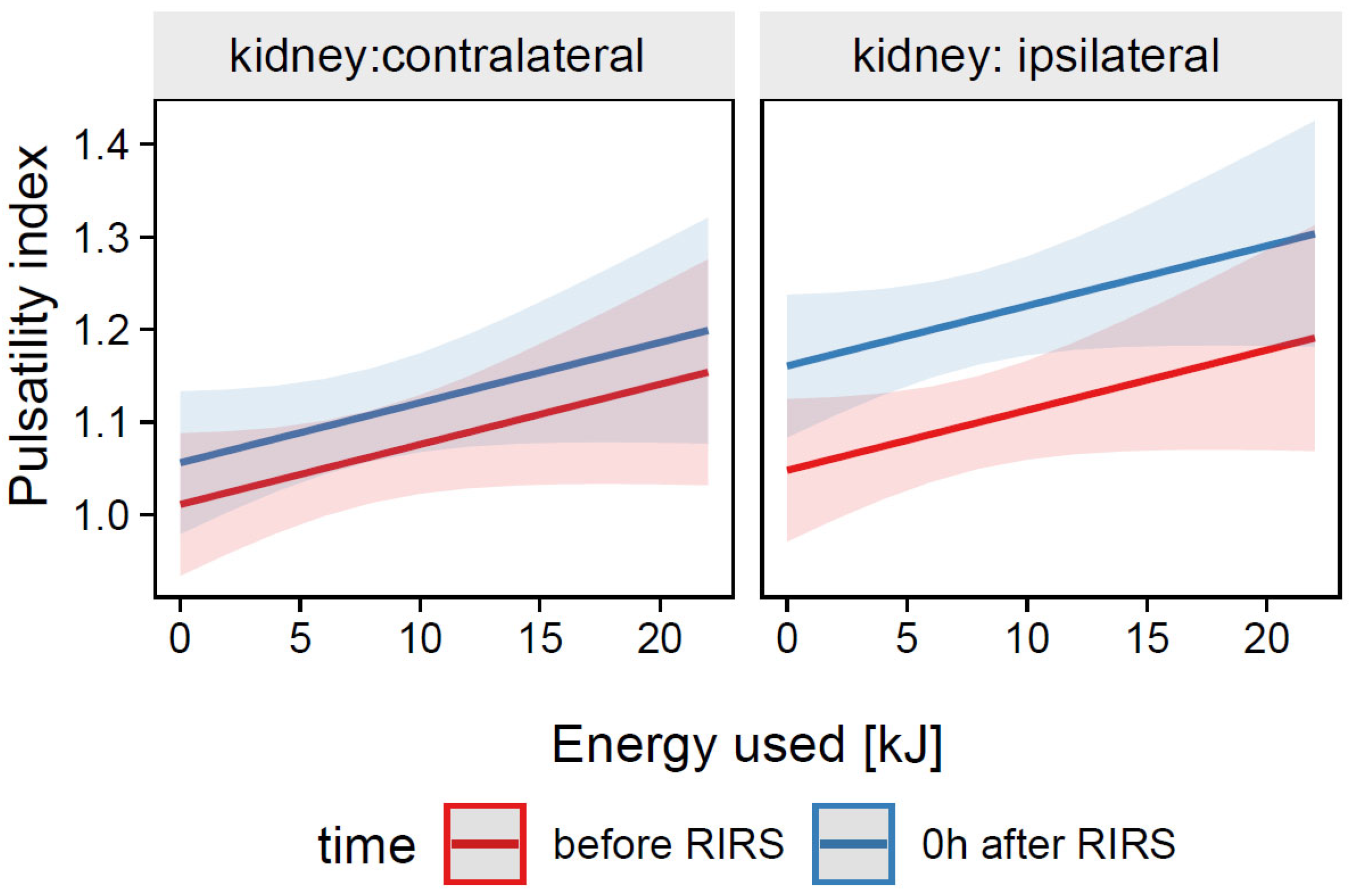
3.1.2.2. Estimation of the Effects of Energy Used, Kidney Side, and Time on the Resistive Index (Mod5)
3.1.2.3. Estimation of the Effects of Pre-Stenting, Kidney Side, and Time on Resistive Index (Mod6)
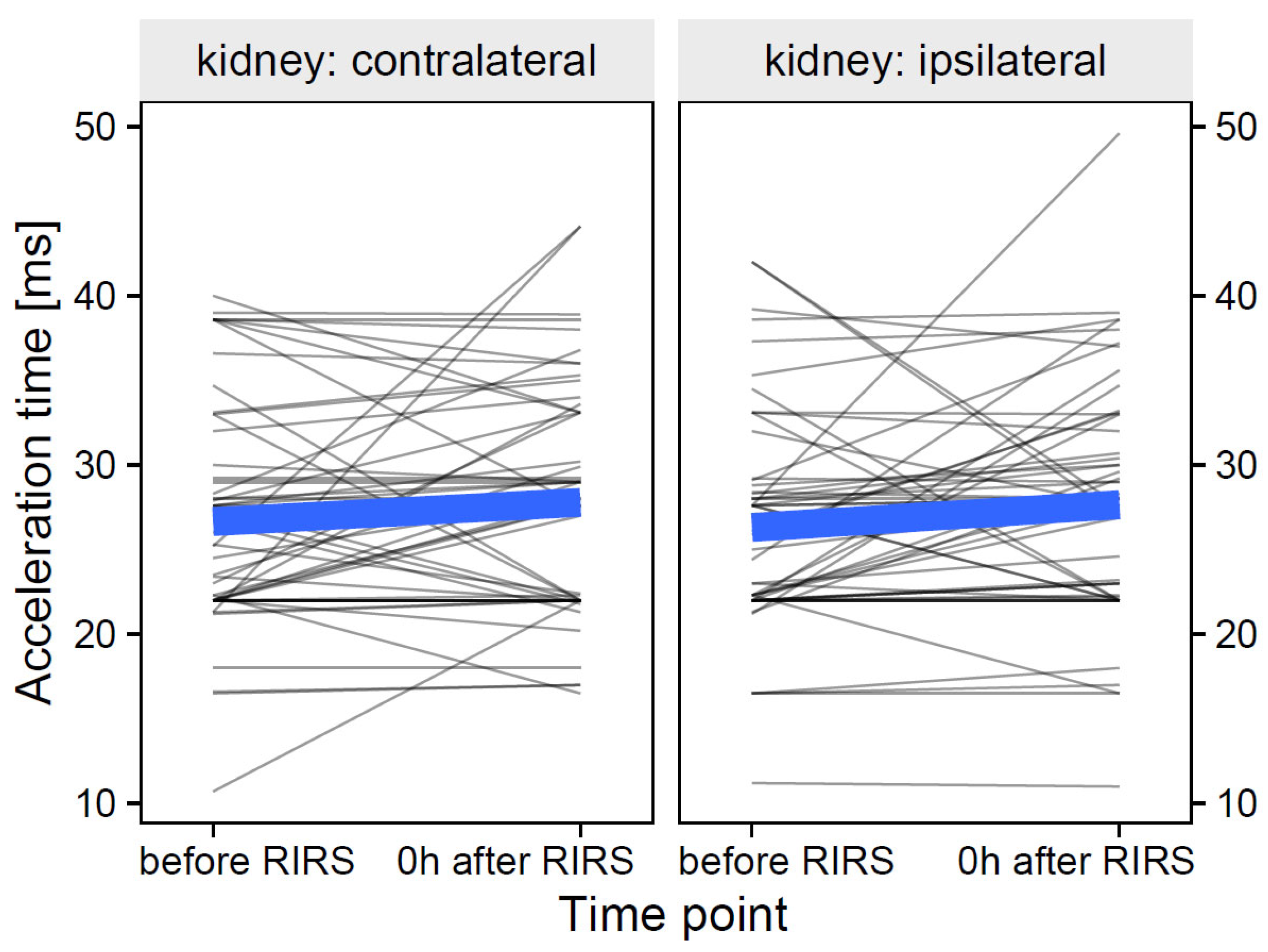
3.1.3. Acceleration Time as Focal Variable
3.1.3.1. Estimation of the Effects of Mean Stone Volume, Kidney Side, and Time on Acceleration Time (Mod7)
3.1.3.2. Estimation of the Effects of Energy used, Kidney Side, and Time on Acceleration Time (Mod8)
3.1.3.3. Estimation of the Effects of Pre-Stenting, Kidney Side, and Time on Acceleration Time (Mod9)
3.2. Regression Models for the Time Point Predictor 0 h after RIRS-24 h after RIRS (Mod10–Mod12)
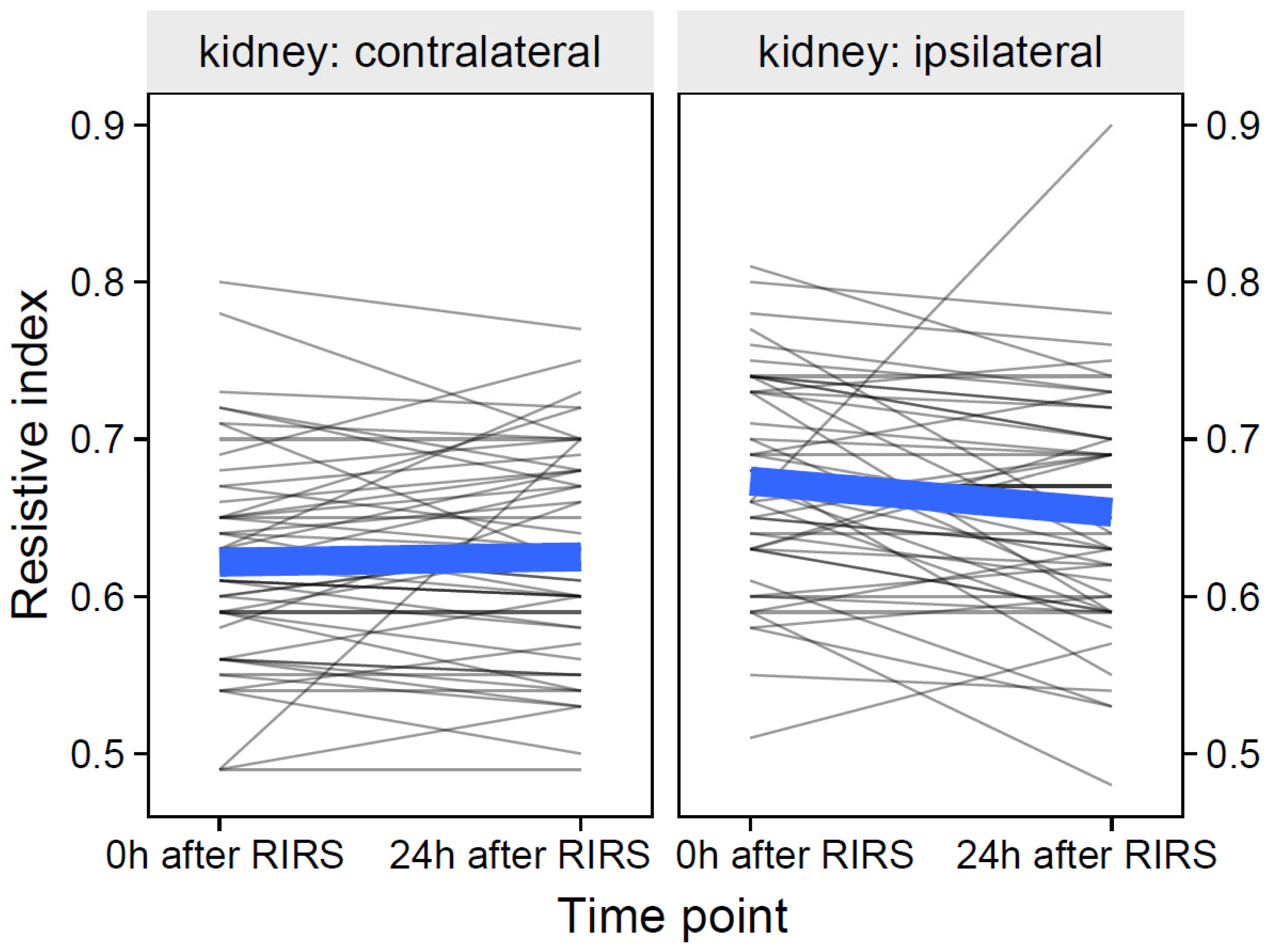
3.2.1. Resistive Index as Focal Variable
3.2.1.1. Estimation of the effects of mean stone volume, kidney side, and time on resistive index (Mod10)
3.2.1.2. Estimation of the Effects of Energy Used, Kidney Side, and Time on the Resistive Index (Mod11)
3.2.1.3. Estimation of the Effects of Pre-Stenting, Kidney Side, and Time on the Resistive Index (Mod12)
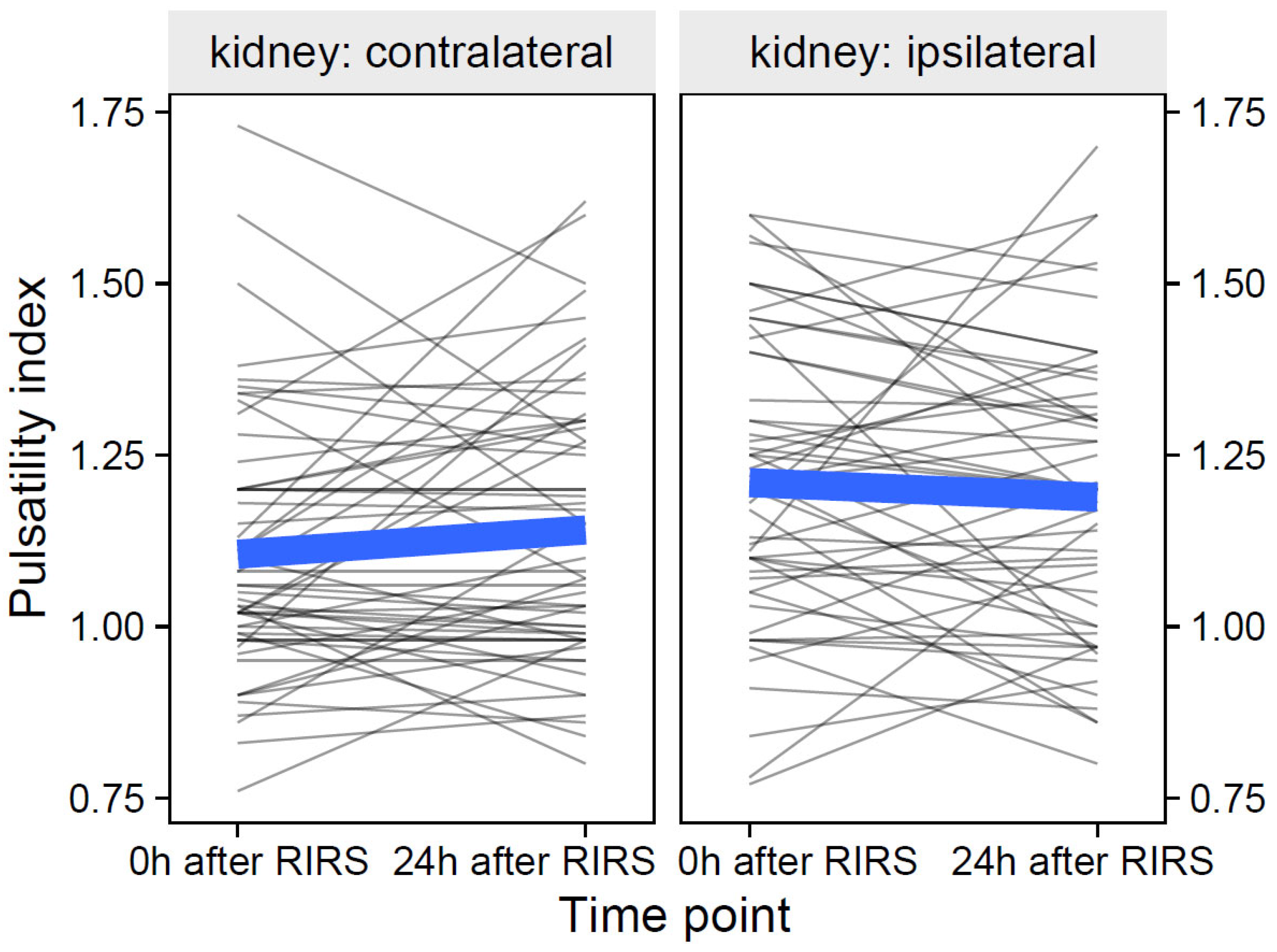
3.2.2. Pulsatility Index as Focal Variable
3.2.2.1. Estimation of the Effects of Mean Stone Volume, Kidney Side, and Time on Pulsatility Index (Mod13)
3.2.2.2. Estimation of the Effects of Energy Used, Kidney Side, and Time on the Pulsatility Index (Mod14)
3.2.2.3. Estimation of the Effects of Pre-Stenting, Kidney Side, and Time on the Pulsatility Index (Mod15)
3.2.3. Acceleration Time Index as Focal Variable
3.2.3.1. Estimation of the Effects of the Volume of the Mean Stone, the Kidney Side and the Time on the Acceleration Time (Mod16)
3.2.3.2. Estimation of the Effects of Energy Used, Kidney Side, and Time on Acceleration Time (Mod17)
3.2.3.3. Estimation of the Effects of Pre-Stenting, Kidney Side, and Time on Acceleration Time (Mod18)
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perri, D.; Berti, L.; Pacchetti, A.; Morini, E.; Maltagliati, M.; Besana, U.; Pastore, A.L.; Romero-Otero, J.; Saredi, G.; Centrella, D.; et al. A comparison among RIRS and MiniPerc for renal stones between 10 and 20 mm using thulium fiber laser (Fiber Dust): A randomized controlled trial. World J. Urol. 2022, 40, 2555–2560. (In English) [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Yang, H.M.; Liu, X.M.; Qi, H.G.; Weng, G.B. Retrograde intrarenal surgery vs miniaturized percutaneous nephrolithotomy to treat lower pole renal stones 1.5-2.5 cm in diameter. World J. Clin. Cases 2018, 6, 931–935. (In English) [Google Scholar] [CrossRef] [PubMed]
- Ucer, O.; Erbatu, O.; Albaz, A.C.; Temeltas, G.; Gumus, B.; Muezzinoglu, T. Comparison stone-free rate and effects on quality of life of percutaneous nephrolithotomy and retrograde intrarenal surgery for treatment of renal pelvis stone (2–4 cm): A prospective controlled study. Curr. Urol. 2022, 16, 5–8. (In English) [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Osther, P.J. Intraluminal pressure profiles during flexible ureterorenoscopy. Springerplus 2015, 4, 373. (In English) [Google Scholar] [CrossRef]
- Pauchard, F.; Ventimiglia, E.; Corrales, M.; Traxer, O. A Practical Guide for Intra-Renal Temperature and Pressure Management during Rirs: What Is the Evidence Telling Us. J. Clin. Med. 2022, 11, 3429. (In English) [Google Scholar] [CrossRef]
- Sierra, A.; Corrales, M.; Kolvatzis, M.; Doizi, S.; Traxer, O. Real Time Intrarenal Pressure Control during Flexible Ureterorrenscopy Using a Vascular PressureWire: Pilot Study. J. Clin. Med. 2022, 12, 147. (In English) [Google Scholar] [CrossRef]
- Osther, P.J.S.; Pedersen, K.V.; Lildal, S.K.; Pless, M.S.; Andreassen, K.H.; Osther, S.S.; Jung, H.U. Pathophysiological aspects of ureterorenoscopic management of upper urinary tract calculi. Curr. Opin. Urol. 2016, 26, 63–69. (In English) [Google Scholar] [CrossRef]
- KIIL, F. Pressure recordings in the upper urinary tract. Scand. J. Clin. Lab. Investig. 1953, 5, 383–384. (In English) [Google Scholar] [CrossRef]
- Tokas, T.; Herrmann, T.R.W.; Skolarikos, A.; Nagele, U.; Training and Research in Urological Surgery and Technology (T.R.U.S.T.)-Group. Pressure matters: Intrarenal pressures during normal and pathological conditions, and impact of increased values to renal physiology. World J. Urol. 2019, 37, 125–131. (In English) [Google Scholar] [CrossRef]
- Doizi, S.; Letendre, J.; Cloutier, J.; Ploumidis, A.; Traxer, O. Continuous monitoring of intrapelvic pressure during flexible ureteroscopy using a sensor wire: A pilot study. World J. Urol. 2021, 39, 555–561. (In English) [Google Scholar] [CrossRef]
- Sorokin, I.; Cardona-Grau, D.K.; Rehfuss, A.; Birney, A.; Stavrakis, C.; Leinwand, G.; Herr, A.; Feustel, P.J.; White, M.D. Stone volume is best predictor of operative time required in retrograde intrarenal surgery for renal calculi: Implications for surgical planning and quality improvement. Urolithiasis 2016, 44, 545–550. (In English) [Google Scholar] [CrossRef] [PubMed]
- Boddi, M. Renal Ultrasound (and Doppler Sonography) in Hypertension: An Update. Adv. Exp. Med. Biol. 2017, 956, 191–208. (In English) [Google Scholar] [CrossRef] [PubMed]
- Sener, T.E.; Tanidir, Y.; Bin Hamri, S.; Sever, I.H.; Ozdemir, B.; Al-Humam, A.; Traxer, O. Effects of flexible ureteroscopy on renal blood flow: A prospective evaluation. Scand. J. Urol. 2018, 52, 213–218. (In English) [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral-Sciences. In Perceptual and Motor Skills; Lawrence Erlbaum Associates Inc.: Mahwah, NY, USA, 1988; Volume 67, p. 1007. (In English) [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research (vol 15, pg 155, 2016). J. Chiropr. Med. 2017, 16, 346. (In English) [Google Scholar] [CrossRef]
- Yazici, C.M.; Akgul, M.; Ozcaglayan, O.; Dogru, M.; Dogan, C.; Altın, E. Prospective Evaluation of Ipsilateral and Contralateral Renal Blood Flow During Retrograde Intrarenal Surgery. Urology 2021, 154, 77–82. (In English) [Google Scholar] [CrossRef]
- Tokas, T.; Tzanaki, E.; Nagele, U.; Somani, B.K. Role of Intrarenal Pressure in Modern Day Endourology (Mini-PCNL and Flexible URS): A Systematic Review of Literature. Curr. Urol. Rep. 2021, 22, 52. (In English) [Google Scholar] [CrossRef]
- Schwalb, D.M.; Eshghi, M.; Davidian, M.; Franco, I. Morphological and physiological changes in the urinary tract associated with ureteral dilation and ureteropyeloscopy: An experimental study. J. Urol. 1993, 149, 1576–1585. (In English) [Google Scholar] [CrossRef]
- Patel, R.M.; Jefferson, F.A.; Owyong, M.; Hofmann, M.; Ayad, M.L.; Osann, K.; Okhunov, Z.; Landman, J.; Clayman, R.V. Characterization of intracalyceal pressure during ureteroscopy. World J. Urol. 2021, 39, 883–889. (In English) [Google Scholar] [CrossRef] [PubMed]
- Balawender, K.; Orkisz, S. Evaluation of selected Doppler parameters of renal blood flow in patients undergoing extracorporeal shock wave lithotripsy. Cent. Eur. J. Urol. 2017, 70, 264–269. (In English) [Google Scholar] [CrossRef]
- Aoki, Y.; Ishitoya, S.; Okubo, K.; Okada, T.; Maekawa, S.; Maeda, H.; Arai, Y. Changes in resistive index following extracorporeal shock wave lithotripsy. Int. J. Urol. 1999, 6, 483–492. (In English) [Google Scholar] [CrossRef]
- Yilmaz, E.; Mert, C.; Keskil, Z.; Tuglu, D.; Batislam, E. Effect of SWL on renal hemodynamics: Could a change in renal artery contraction-relaxation responses be the cause? Urol. Res. 2012, 40, 775–780. (In English) [Google Scholar] [CrossRef] [PubMed]




| Parameter | Distribution, Mdn (Q1–Q3) | p | |
|---|---|---|---|
| Women (n = 22) | Men (n = 34) | ||
| Age | 52.5 (44.5–62.0) | 49.0 (42.0–57.0) | 0.419 |
| Weight | 74.0 (64.0–82.8) | 85.0 (78.0–89.0) | 0.034 |
| Height | 164.0 (160.0–166.8) | 176.0 (170.0–180.0) | <0.001 |
| BMI | 28.2 (24.6–31.9) | 26.8 (25.11–29.4) | 0.390 |
| Parameter | Measure | Distribution |
|---|---|---|
| Laterality: | - | |
| Right | 27 (49.1%) | |
| Left | 28 (50.9%) | |
| Pre-stenting: | - | |
| No | 9 (16.3%) | |
| Yes | 46 (83.7%) | |
| Mean stone volume | mm3 | 589.0 (426.0–706.5) |
| Energy used | kJ | 6.8 (3.0–10.2) |
| Parameter | Kidney | Time | Distribution, Mdn (Q1–Q3) |
|---|---|---|---|
| Resistive index | ipsilateral | before RIRS | 0.61 (0.56–0.66) |
| 0 h after RIRS | 0.67 (0.63–0.73) | ||
| 24 h after RIRS | 0.65 (0.60–0.70) | ||
| contralateral | before RIRS | 0.60 (0.56–0.64) | |
| 0 h after RIRS | 0.62 (0.58–0.65) | ||
| 24 h after RIRS | 0.63 (0.58–0.68) | ||
| Pulsatility index | ipsilateral | before RIRS | 1.06 (1.00–1.21) |
| 0 h after RIRS | 1.20 (1.08–1.36) | ||
| 24 h after RIRS | 1.20 (1.00–1.31) | ||
| contralateral | before RIRS | 1.01 (0.92–1.17) | |
| 0 h after RIRS | 1.05 (0.98–1.20) | ||
| 24 h after RIRS | 1.08 (0.98–1.29) | ||
| Acceleration time | ipsilateral | before RIRS | 25.90 (22.00–28.60) |
| 0 h after RIRS | 27.60 (22.15–31.02) | ||
| 24 h after RIRS | 27.60 (22.10–31.35) | ||
| contralateral | before RIRS | 25.30 (22.00–29.60) | |
| 0 h after RIRS | 27.60 (22.00–33.10) | ||
| 24 h after RIRS | 26.30 (22.00–30.00) |
| Doppler Parameter | Time Period | p | Predictor | p |
|---|---|---|---|---|
| Resistive index | Before RIRS–0 h after RIRS | Mean stone volume | 0.142 | |
| 0.010 | Energy used | 0.343 | ||
| Pre-stenting | 0.096 | |||
| Pulsatility index | Before RIRS–0 h after RIRS | Mean stone volume | 0.170 | |
| <0.010 | Energy used | 0.101 | ||
| Pre-stenting | 0.194 | |||
| Acceleration time | Before RIRS–0 h after RIRS | Mean stone volume | 0.380 | |
| 0.206 | Energy used | 0.626 | ||
| Pre-stenting | 0.240 | |||
| Resistive index | 0 h after RIRS–24 h after RIRS | Mean stone volume | 0.261 | |
| 0.672 | Energy used | 0.484 | ||
| Pre-stenting | 0.140 | |||
| Pulsatility index | 0 h after RIRS–24 h after RIRS | Mean stone volume | 0.112 | |
| 0.116 | Energy used | 0.351 | ||
| Pre-stenting | 0.398 | |||
| Acceleration time | 0 h after RIRS–24 h after RIRS | Mean stone volume | 0.533 | |
| 0.057 | Energy used | 0.685 | ||
| Pre-stenting | 0.312 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balawender, K. A Prospective Study of Renal Blood Flow during Retrograde Intrarenal Surgery. J. Clin. Med. 2023, 12, 3030. https://doi.org/10.3390/jcm12083030
Balawender K. A Prospective Study of Renal Blood Flow during Retrograde Intrarenal Surgery. Journal of Clinical Medicine. 2023; 12(8):3030. https://doi.org/10.3390/jcm12083030
Chicago/Turabian StyleBalawender, Krzysztof. 2023. "A Prospective Study of Renal Blood Flow during Retrograde Intrarenal Surgery" Journal of Clinical Medicine 12, no. 8: 3030. https://doi.org/10.3390/jcm12083030
APA StyleBalawender, K. (2023). A Prospective Study of Renal Blood Flow during Retrograde Intrarenal Surgery. Journal of Clinical Medicine, 12(8), 3030. https://doi.org/10.3390/jcm12083030






