Unexplained Hyperthyrotropinemia: A Biochemical and Clinical Challenge
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
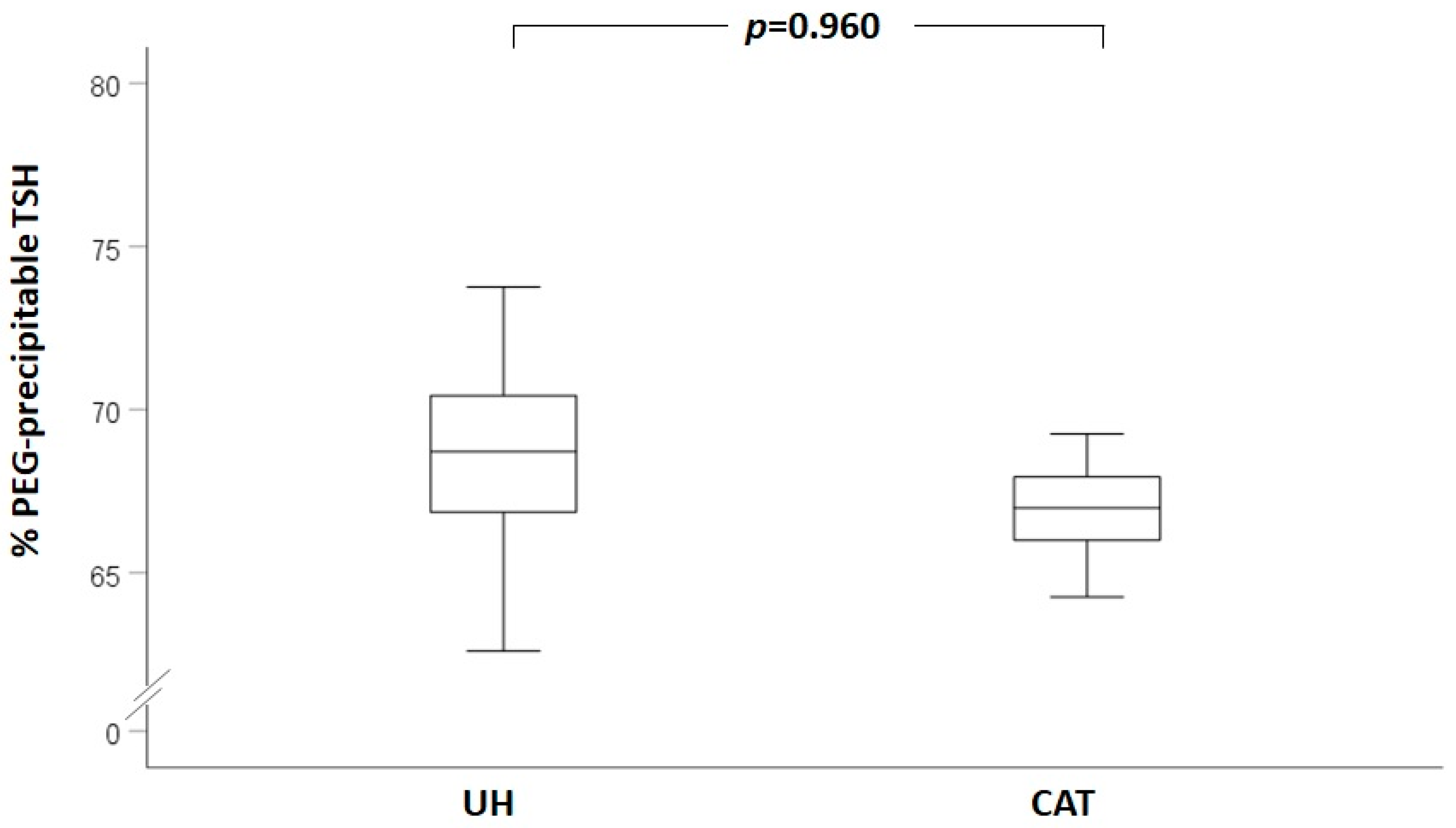
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magri, F.; Chiovato, L.; Croce, L.; Rotondi, M. Thyroid hormone therapy for subclinical hypothyroidism. Endocrine 2019, 66, 27–34. [Google Scholar] [CrossRef]
- Croce, L.; De Martinis, L.; Pinto, S.; Coperchini, F.; Dito, G.; Bendotti, G.; Pasquali, D.; Cappelli, C.; Latrofa, F.; Magri, F.; et al. Compared with classic Hashimoto’s thyroiditis, chronic autoimmune serum-negative thyroiditis requires a lower substitution dose of L-thyroxine to correct hypothyroidism. J. Endocrinol. Invest. 2020, 43, 1631–1636. [Google Scholar] [CrossRef]
- Rotondi, M.; Leporati, P.; La Manna, A.; Pirali, B.; Mondello, T.; Fonte, R.; Magri, F.; Chiovato, L. Raised serum TSH levels in patients with morbid obesity: Is it enough to diagnose subclinical hypothyroidism? Eur. J. Endocrinol. 2009, 160, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Burlacu, M.C.; Maiter, D.; Gruson, D. Interferences With Thyroid Function Immunoassays: Clinical Implications and Detection Algorithm. Endocr. Rev. 2018, 39, 830–850. [Google Scholar] [CrossRef] [PubMed]
- Boscato, L.M.; Stuart, M.C. Heterophilic antibodies: A problem for all immunoassays. Clin. Chem. 1988, 34, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.P.; Pin, Y.C. Heterophile antibody interference with thyroid assay. Intern. Med. 2008, 47, 2033–2037. [Google Scholar] [CrossRef] [PubMed]
- Sapin, R.; Agin, A.; Gasser, F. Efficacy of a new blocker against anti-ruthenium antibody interference in the Elecsys free triiodothyronine assay. Clin. Chem. Lab. Med. 2007, 45, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Yasui, J.; Inokuchi, N.; Usa, T.; Ashizawa, K.; Kamihara, S.; Eguchi, K. Non-specific activities against ruthenium crosslinker as a new cause of assay interference in an electrochemilluminescent immunoassay. Intern. Med. 2007, 46, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Buijs, M.M.; Gorgels, J.P.; Endert, E. Interference by antiruthenium antibodies in the Roche thyroid-stimulating hormone assay. Ann. Clin. Biochem. 2011, 48, 276–281. [Google Scholar] [CrossRef]
- Öncül, Ü.; Eminoğlu, F.T.; Köse, E.; Doğan, Ö.; Özsu, E.; Aycan, Z. Serum biotin interference: A troublemaker in hormone immunoassays. Clin. Biochem. 2022, 99, 97–102. [Google Scholar] [CrossRef]
- Pappa, T.; Johannesen, J.; Scherberg, N.; Torrent, M.; Dumitrescu, A.; Refetoff, S. A TSHβ Variant with Impaired Immunoreactivity but Intact Biological Activity and Its Clinical Implications. Thyroid 2015, 25, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Mills, F.; Jeffery, J.; Mackenzie, P.; Cranfield, A.; Ayling, R.M. An immunoglobulin G complexed form of thyroid-stimulating hormone (macro thyroid-stimulating hormone) is a cause of elevated serum thyroid-stimulating hormone concentration. Ann. Clin. Biochem. 2013, 50, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Ishihara, T.; Matsuoka, N.; Saito, T.; Shimatsu, A. Anti-Thyrotropin Autoantibodies in Patients with Macro-Thyrotropin and Long-Term Changes in Macro-Thyrotropin and Serum Thyrotropin Levels. Thyroid 2017, 27, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Ishihara, T.; Yamagami, K.; Shimatsu, A. Macro TSH in patients with subclinical hypothyroidism. Clin. Endocrinol. 2015, 83, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.P.; Suliman, A.M.; Fahie-Wilson, M.N.; McKenna, T.J. Gross variability in the detection of prolactin in sera containing big big prolactin (macroprolactin) by commercial immunoassays. J. Clin. Endocrinol. Metab. 2002, 87, 5410–5415. [Google Scholar] [CrossRef]
- Hattori, N.; Ishihara, T.; Shimatsu, A. Variability in the detection of macro TSH in different immunoassay systems. Eur. J. Endocrinol. 2016, 174, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.B.; Petersen, E.R.B.; Overgaard, M.; Bonnema, S.J. Macro-TSH: A Diagnostic Challenge. Eur. Thyroid J. 2021, 10, 93–97. [Google Scholar] [CrossRef]
- Van der Spoel, E.; Roelfsema, F.; van Heemst, D. Within-Person Variation in Serum Thyrotropin Concentrations: Main Sources, Potential Underlying Biological Mechanisms, and Clinical Implications. Front. Endocrinol. 2021, 12, 619568. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Brigante, G.; Setti, M.; Tagliavini, S.; Trenti, T.; Simoni, M. Semi-annual seasonal pattern of serum thyrotropin in adults. Sci. Rep. 2019, 9, 10786. [Google Scholar] [CrossRef]
- Church, D.; Cardoso, L.; Bradbury, S.; Clarke, C.; Stears, A.; Dover, A.; Halsall, D.; Semple, R. Diagnosis of insulin autoimmune syndrome using polyethylene glycol precipitation and gel filtration chromatography with ex vivo insulin exchange. Clin. Endocrinol. 2017, 86, 347–353. [Google Scholar] [CrossRef]
- Overgaard, M.; Pedersen, S.M. Serum prolactin revisited: Parametric reference intervals and cross platform evaluation of polyethylene glycol precipitation-based methods for discrimination between hyperprolactinemia and macroprolactinemia. Clin. Chem. Lab. Med. 2017, 55, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Aisaka, K.; Yamada, A.; Matsuda, T.; Shimatsu, A. Prevalence and pathogenesis of macro-TSH in neonates: Analysis of umbilical cord blood from 939 neonates and their mothers. Thyroid 2022, 33, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Loh, T.P.; Kao, S.L.; Halsall, D.J.; Toh, S.A.; Chan, E.; Ho, S.C.; Tai, E.S.; Khoo, C.M. Macro-thyrotropin: A case report and review of literature. J. Clin. Endocrinol. Metab. 2012, 97, 1823–1828. [Google Scholar] [CrossRef]
- Biondi, B.; Cappola, A.R.; Cooper, D.S. Subclinical Hypothyroidism: A Review. JAMA 2019, 322, 153–160. [Google Scholar] [CrossRef]
- Mendoza, H.; Connacher, A.; Srivastava, R. Unexplained high thyroid stimulating hormone: A “BIG” problem. BMJ Case Rep. 2009, 2009, bcr0120091474. [Google Scholar] [CrossRef] [PubMed]
- Tonacchera, M.; Perri, A.; De Marco, G.; Agretti, P.; Banco, M.E.; Di Cosmo, C.; Grasso, L.; Vitti, P.; Chiovato, L.; Pinchera, A. Low prevalence of thyrotropin receptor mutations in a large series of subjects with sporadic and familial nonautoimmune subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2004, 89, 5787–5793. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Aisaka, K.; Chihara, K.; Shimatsu, A. Current Thyrotropin Immunoassays Recognize Macro-Thyrotropin Leading to Hyperthyrotropinemia in Females of Reproductive Age. Thyroid 2018, 28, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
| Study Group (UH) | Control Group (CAT) | p Value | |
|---|---|---|---|
| N | 36 | 14 | |
| Sex (F/M; % of females) | 28/8 (77.8%) | 9/5 (64.3%) | 0.329 |
| Age (years, mean ± SD) | 45.4 ± 19.8 | 46.1 ± 16.9 | 0.913 |
| TSH at first evaluation (µU/mL, median (interquartile range)) | 5.65 (5.21–6.37) | 5.62 (5.17–8.50) | 0.489 |
| FT4 at first evaluation (ng/dl, mean ± SD) | 1.02 ± 0.20 | 1.00 ± 0.20 | 0.789 |
| Patients previously treated with LT4 (N, %) | 8/36 (22.2%) | 6/14 (42.9%) | 0.145 |
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Group | CAT | CAT | UH |
| Sex | Male | Female | Female |
| Age (years) | 68 | 21 | 74 |
| BMI (kg/m2) | 24.2 | 32.4 | 28.9 |
| TSH—Alinity I system, Abbott (uUI/mL) | 5.3 | 5.1 | 5.5 |
| TSH—ADVIA Centaur® XPT system, Siemens (uUI/mL) | 3.80 | 5.50 | 3.50 |
| FT4 (ng/dL) | 1.14 | 0.73 | 0.90 |
| TSH % precipitable post-PEG | 89.28 | 79.05 | 77.73 |
| AbTPO | Positive | Positive | Negative |
| AbTG | Positive | Positive | Negative |
| Sonographic thyroid features | Markedly hypoechoic and inhomogeneous structure; Normal vascularization. | Markedly hypoechoic and inhomogeneous structure; Slightly increased vascularization. | Normoechoic and homogeneous structure; Normal vascularization. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croce, L.; Chytiris, S.; Coperchini, F.; Ferraro, G.; Minelli, L.; Navarra, A.; Magri, F.; Chiovato, L.; Trimboli, P.; Rotondi, M. Unexplained Hyperthyrotropinemia: A Biochemical and Clinical Challenge. J. Clin. Med. 2023, 12, 2934. https://doi.org/10.3390/jcm12082934
Croce L, Chytiris S, Coperchini F, Ferraro G, Minelli L, Navarra A, Magri F, Chiovato L, Trimboli P, Rotondi M. Unexplained Hyperthyrotropinemia: A Biochemical and Clinical Challenge. Journal of Clinical Medicine. 2023; 12(8):2934. https://doi.org/10.3390/jcm12082934
Chicago/Turabian StyleCroce, Laura, Spyridon Chytiris, Francesca Coperchini, Giovanni Ferraro, Linda Minelli, Antonella Navarra, Flavia Magri, Luca Chiovato, Pierpaolo Trimboli, and Mario Rotondi. 2023. "Unexplained Hyperthyrotropinemia: A Biochemical and Clinical Challenge" Journal of Clinical Medicine 12, no. 8: 2934. https://doi.org/10.3390/jcm12082934
APA StyleCroce, L., Chytiris, S., Coperchini, F., Ferraro, G., Minelli, L., Navarra, A., Magri, F., Chiovato, L., Trimboli, P., & Rotondi, M. (2023). Unexplained Hyperthyrotropinemia: A Biochemical and Clinical Challenge. Journal of Clinical Medicine, 12(8), 2934. https://doi.org/10.3390/jcm12082934






