The Role of Peroxisome Proliferator-Activated Receptors in Polycystic Ovary Syndrome
Abstract
1. Introduction
2. Methodology
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. PPAR Expression in PCOS (-Induced) Animal Models and Patients
3.1. PPAR
3.2. PPARα
3.3. PPARγ
4. The Role of PPAR Polymorphisms in PCOS
4.1. PPAR
4.2. PPARγ
4.3. PPAR Polymorphisms Depending on the Ethnic Background
5. PPAR Expression in PCOS Organ Tissues
5.1. Cardiac Tissue
5.2. Skeletal Tissue
5.3. Adipose Tissue
6. The Influence of Natural Agents on PPAR Expression in PCOS
6.1. PPARα
6.2. PPARγ
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Prim. 2016, 2, 16057. [Google Scholar] [CrossRef]
- Gleicher, N.; Darmon, S.; Patrizio, P.; Barad, D.H. Reconsidering the Polycystic Ovary Syndrome (PCOS). Biomedicines 2022, 10, 1505. [Google Scholar] [CrossRef] [PubMed]
- Al Wattar, B.H.; Fisher, M.; Bevington, L.; Talaulikar, V.; Davies, M.; Conway, G.; Yasmin, E. Clinical Practice Guidelines on the Diagnosis and Management of Polycystic Ovary Syndrome: A Systematic Review and Quality Assessment Study. J. Clin. Endocrinol. Metab. 2021, 106, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K.; Endocrine, S. Diagnosis and Treatment of Polycystic Ovary Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, Y.; Guo, Y.; Lai, Z. The Effect of Low Carbohydrate Diet on Polycystic Ovary Syndrome: A Meta-Analysis of Randomized Controlled Trials. Int. J. Endocrinol. 2019, 2019, 4386401. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Teede, H.; Skouteris, H.; Linardon, J.; Hill, B.; Moran, L. Lifestyle and Behavioral Management of Polycystic Ovary Syndrome. J. Women’s Health 2017, 26, 836–848. [Google Scholar] [CrossRef]
- Hakimi, O.; Cameron, L.-C. Effect of Exercise on Ovulation: A Systematic Review. Sport. Med. 2017, 47, 1555–1567. [Google Scholar] [CrossRef]
- Sadeghi, H.M.; Adeli, I.; Calina, D.; Docea, A.O.; Mousavi, T.; Daniali, M.; Nikfar, S.; Tsatsakis, A.; Abdollahi, M. Polycystic Ovary Syndrome: A Comprehensive Review of Pathogenesis, Management, and Drug Repurposing. Int. J. Mol. Sci. 2022, 23, 583. [Google Scholar] [CrossRef]
- Hoeger, K.M.; Dokras, A.; Piltonen, T. Update on PCOS: Consequences, Challenges, and Guiding Treatment. J. Clin. Endocrinol. Metab. 2021, 106, e1071–e1083. [Google Scholar] [CrossRef]
- Chen, W.; Pang, Y. Metabolic Syndrome and PCOS: Pathogenesis and the Role of Metabolites. Metabolites 2021, 11, 869. [Google Scholar] [CrossRef] [PubMed]
- Harada, M. Pathophysiology of polycystic ovary syndrome revisited: Current understanding and perspectives regarding future research. Reprod. Med. Biol. 2022, 21, e12487. [Google Scholar] [CrossRef] [PubMed]
- Burt Solorzano, C.M.; McCartney, C.R.; Blank, S.K.; Knudsen, K.L.; Marshall, J.C. Hyperandrogenaemia in adolescent girls: Origins of abnormal gonadotropin-releasing hormone secretion. BJOG 2010, 117, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.E.; McCourt, B.; Martin, K.A.; Anderson, E.J.; Adams, J.M.; Schoenfeld, D.; Hall, J. Determinants of Abnormal Gonadotropin Secretion in Clinically Defined Women with Polycystic Ovary Syndrome1. J. Clin. Endocrinol. Metab. 1997, 82, 2248–2256. [Google Scholar] [CrossRef]
- Visser, J.A.; Durlinger, A.L.; Peters, I.J.; van den Heuvel, E.R.; Rose, U.M.; Kramer, P.; de Jong, F.H.; Themmen, A.P. Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Mullerian hormone null mice. Endocrinology 2007, 148, 2301–2308. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Akopians, A.L.; Madrigal, V.K.; Ramirez, E.; Margolis, D.J.; Sarma, M.K.; Thomas, A.M.; Grogan, T.R.; Haykal, R.; Schooler, T.A.; et al. Hyperandrogenism Accompanies Increased Intra-Abdominal Fat Storage in Normal Weight Polycystic Ovary Syndrome Women. J. Clin. Endocrinol. Metab. 2016, 101, 4178–4188. [Google Scholar] [CrossRef] [PubMed]
- Thong, E.P.; Codner, E.; Laven, J.S.E.; Teede, H. Diabetes: A metabolic and reproductive disorder in women. Lancet Diabetes Endocrinol. 2020, 8, 134–149. [Google Scholar] [CrossRef]
- Nestler, J.E.; Powers, L.P.; Matt, D.W.; Steingold, K.A.; Plymate, S.R.; Rittmaster, R.S.; Clore, J.N.; Blackard, W.G. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1991, 72, 83–89. [Google Scholar] [CrossRef]
- Nuclear Receptors Nomenclature Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell 1999, 97, 161–163. [Google Scholar] [CrossRef]
- Han, L.; Shen, W.J.; Bittner, S.; Kraemer, F.B.; Azhar, S. PPARs: Regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-beta/delta and PPAR-gamma. Future Cardiol. 2017, 13, 279–296. [Google Scholar] [CrossRef]
- Wagner, K.D.; Wagner, N. Peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) acts as regulator of metabolism linked to multiple cellular functions. Pharmacol. Ther. 2010, 125, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Moller, D.E. The Mechanisms of Action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Górniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Giaginis, C.; Giagini, A.; Theocharis, S. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) ligands as potential therapeutic agents to treat arthritis. Pharmacol. Res. 2009, 60, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Giaginis, C.; Margeli, A.; Theocharis, S. Peroxisome proliferator-activated receptor-gamma ligands as investigational modulators of angiogenesis. Expert Opin. Investig. Drugs 2007, 16, 1561–1572. [Google Scholar] [CrossRef]
- Giaginis, C.; Theocharis, S.; Tsantili-Kakoulidou, A. A consideration of PPAR-gamma ligands with respect to lipophilicity: Current trends and perspectives. Expert Opin. Investig. Drugs 2007, 16, 413–417. [Google Scholar] [CrossRef]
- Giaginis, C.; Theocharis, S.; Tsantili-Kakoulidou, A. Investigation of the lipophilic behaviour of some thiazolidinediones: Relationships with PPAR-γ activity. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 857, 181–187. [Google Scholar] [CrossRef]
- Giaginis, C.; Theocharis, S.; Tsantili-Kakoulidou, A. Quantitative structure-activity relationships for PPAR-gamma binding and gene transactivation of tyrosine-based agonists using multivariate statistics. Chem. Biol. Drug Des. 2008, 72, 257–264. [Google Scholar] [CrossRef]
- Giaginis, C.; Theocharis, S.; Tsantili-Kakoulidou, A. Structural basis for the design of PPAR-gamma ligands: A survey on quantitative structure-activity relationships. Mini Rev. Med. Chem. 2009, 9, 1075–1083. [Google Scholar] [CrossRef]
- Giaginis, C.; Tsantili-Kakoulidou, A.; Theocharis, S. Peroxisome proliferator-activated receptor-gamma ligands as bone turnover modulators. Expert Opin. Investig. Drugs. 2007, 16, 195–207. [Google Scholar] [CrossRef]
- Giaginis, C.; Tsantili-Kakoulidou, A.; Theocharis, S. Peroxisome Proliferator-Activated Receptor-gamma Ligands: Potential Pharmacological Agents for Targeting the Angiogenesis Signaling Cascade in Cancer. PPAR Res. 2008, 2008, 431763. [Google Scholar] [CrossRef]
- Giaginis, C.; Tsourouflis, G.; Theocharis, S. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) ligands: Novel pharmacological agents in the treatment of ischemia reperfusion injury. Curr. Mol. Med. 2008, 8, 562–579. [Google Scholar] [CrossRef]
- Psilopatis, I.; Vrettou, K.; Fleckenstein, F.N.; Theocharis, S. The Role of Peroxisome Proliferator-Activated Receptors in Preeclampsia. Cells 2023, 12, 647. [Google Scholar] [CrossRef] [PubMed]
- Brunmeir, R.; Xu, F. Functional Regulation of PPARs through Post-Translational Modifications. Int. J. Mol. Sci. 2018, 19, 1738. [Google Scholar] [CrossRef] [PubMed]
- Bensinger, S.J.; Tontonoz, P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 2008, 454, 470–477. [Google Scholar] [CrossRef]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism 2021, 114, 154338. [Google Scholar] [CrossRef]
- Francque, S.; Szabo, G.; Abdelmalek, M.F.; Byrne, C.D.; Cusi, K.; Dufour, J.-F.; Roden, M.; Sacks, F.; Tacke, F. Nonalcoholic steatohepatitis: The role of peroxisome proliferator-activated receptors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 24–39. [Google Scholar] [CrossRef]
- Shi, Y.; Zou, Y.; Shen, Z.; Xiong, Y.; Zhang, W.; Liu, C.; Chen, S. Trace Elements, PPARs, and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2612. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cai, Y.; Jian, L.; Cheung, C.W.; Zhang, L.; Xia, Z. Impact of peroxisome proliferator-activated receptor-α on diabetic cardiomyopathy. Cardiovasc. Diabetol. 2021, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Przybycień, P.; Gąsior-Perczak, D.; Placha, W. Cannabinoids and PPAR Ligands: The Future in Treatment of Polycystic Ovary Syndrome Women with Obesity and Reduced Fertility. Cells 2022, 11, 2569. [Google Scholar] [CrossRef]
- Bai, L.; Gong, J.; Guo, Y.; Li, Y.; Huang, H.; Liu, X. Construction of a ceRNA network in polycystic ovary syndrome (PCOS) driven by exosomal lncRNA. Front. Genet. 2022, 13, 979924. [Google Scholar] [CrossRef]
- Morsy, M.A.; El-Hussieny, M.; Zenhom, N.M.; Nair, A.B.; Venugopala, K.N.; Refaie, M.M.M. Fenofibrate ameliorates letrozole-induced polycystic ovary in rats via modulation of PPARalpha and TNFalpha/CD95 pathway. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 7359–7370. [Google Scholar]
- Tao, T.; Wang, Y.; Xu, B.; Mao, X.; Sun, Y.; Liu, W. Role of adiponectin/peroxisome proliferator-activated receptor alpha signaling in human chorionic gonadotropin-induced estradiol synthesis in human luteinized granulosa cells. Mol. Cell Endocrinol. 2019, 493, 110450. [Google Scholar] [CrossRef]
- Amalfi, S.; Velez, L.M.; Heber, M.F.; Vighi, S.; Ferreira, S.R.; Orozco, A.V.; Pignataro, O.; Motta, A.B. Prenatal hyperandrogenization induces metabolic and endocrine alterations which depend on the levels of testosterone exposure. PLoS ONE 2012, 7, e37658. [Google Scholar] [CrossRef]
- Di Emidio, G.; Placidi, M.; Rea, F.; Rossi, G.; Falone, S.; Cristiano, L.; Nottola, S.; D’alessandro, A.M.; Amicarelli, F.; Palmerini, M.G.; et al. Methylglyoxal-Dependent Glycative Stress and Deregulation of SIRT1 Functional Network in the Ovary of PCOS Mice. Cells 2020, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- El-Saka, M.H.; Barhoma, R.A.; Ibrahim, R.R.; Elsaadany, A.; Alghazaly, G.M.; Elshwaikh, S.; Marea, K.E.; Madi, N.M. Potential effect of adrenomedullin on metabolic and endocrinal dysfunctions in the experimentally induced polycystic ovary: Targeting implication of endoplasmic reticulum stress. J. Biochem. Mol. Toxicol. 2021, 35, e22725. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Maowulieti, G.; Yu, T. Effect of testosterone on the expression of PPARgamma mRNA in PCOS patients. Exp. Ther. Med. 2019, 17, 1761–1765. [Google Scholar]
- Lee, J.Y.; Tae, J.C.; Kim, C.H.; Hwang, D.; Kim, K.C.; Suh, C.S.; Kim, S.H. Expression of the genes for peroxisome proliferator-activated receptor-γ, cyclooxygenase-2, and proinflammatory cytokines in granulosa cells from women with polycystic ovary syndrome. Clin. Exp. Reprod. Med. 2017, 44, 146–151. [Google Scholar] [CrossRef]
- Qu, F.; Wang, F.-F.; Yin, R.; Ding, G.-L.; El-Prince, M.; Gao, Q.; Shi, B.-W.; Pan, H.-H.; Huang, Y.-T.; Jin, M.; et al. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: Hyperandrogenism induces epigenetic alterations in the granulosa cells. J. Mol. Med. 2012, 90, 911–923. [Google Scholar] [CrossRef]
- Skov, V.; Glintborg, D.; Knudsen, S.; Jensen, T.; Kruse, T.A.; Tan, Q.; Brusgaard, K.; Beck-Nielsen, H.; Højlund, K. Reduced Expression of Nuclear-Encoded Genes Involved in Mitochondrial Oxidative Metabolism in Skeletal Muscle of Insulin-Resistant Women with Polycystic Ovary Syndrome. Diabetes 2007, 56, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhai, J.; Chen, J.; Wang, X.; Wen, T. PGC-1α protects against oxidized low-density lipoprotein and luteinizing hormone-induced granulosa cells injury through ROS-p38 pathway. Hum. Cell 2019, 32, 285–296. [Google Scholar] [CrossRef]
- Hu, W.; Qiao, J. Expression and regulation of adipocyte fatty acid binding protein in granulosa cells and its relation with clinical characteristics of polycystic ovary syndrome. Endocrine 2011, 40, 196–202. [Google Scholar] [CrossRef]
- Jansen, E.; Laven, J.S.E.; Dommerholt, H.B.R.; Polman, J.; Rijt, C.V.D.W.-V.; Hurk, C.V.D.; Westland, J.; Mosselman, S.; Fauser, B.C.J.M. Abnormal Gene Expression Profiles in Human Ovaries from Polycystic Ovary Syndrome Patients. Mol. Endocrinol. 2004, 18, 3050–3063. [Google Scholar] [CrossRef]
- Kohan, K.; Carvajal, R.; Gabler, F.; Vantman, D.; Romero, C.; Vega, M. Role of the transcriptional factors FOXO1 and PPARG on gene expression of SLC2A4 in endometrial tissue from women with polycystic ovary syndrome. Reproduction 2010, 140, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, Y.; Ren, Y.; Li, M.; Li, T.; Li, R.; Yu, Y.; Qiao, J. Epigenetic regulation of an adverse metabolic phenotype in polycystic ovary syndrome: The impact of the leukocyte methylation of PPARGC1A promoter. Fertil. Steril. 2017, 107, 467–474.e5. [Google Scholar] [CrossRef]
- He, Y.; Wang, C.L. Effects of testosterone on PPARgamma and P450arom expression in polycystic ovary syndrome patients and related mechanisms. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1549–1553. [Google Scholar] [PubMed]
- Christopoulos, P.; Mastorakos, G.; Gazouli, M.; Deligeoroglou, E.; Katsikis, I.; Diamanti-Kandarakis, E.; Panidis, D.; Creatsas, G. Peroxisome proliferator-activated receptor-gamma and -delta polymorphisms in women with polycystic ovary syndrome. Ann. N. Y. Acad. Sci. 2010, 1205, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Knebel, B.; Janssen, O.E.; Hahn, S.; Jacob, S.; Gleich, J.; Kotzka, J.; Muller-Wieland, D. Increased low grade inflammatory serum markers in patients with Polycystic ovary syndrome (PCOS) and their relationship to PPARgamma gene variants. Exp. Clin. Endocrinol. Diabetes 2008, 116, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Antoine, H.J.; Pall, M.; Trader, B.C.; Chen, Y.-D.I.; Azziz, R.; Goodarzi, M.O. Genetic variants in peroxisome proliferator-activated receptor gamma influence insulin resistance and testosterone levels in normal women, but not those with polycystic ovary syndrome. Fertil. Steril. 2007, 87, 862–869. [Google Scholar] [CrossRef]
- Orio, F., Jr.; Matarese, G.; Di Biase, S.; Palomba, S.; Labella, D.; Sanna, V.; Savastano, S.; Zullo, F.; Colao, A.; Lombardi, G. Exon 6 and 2 peroxisome proliferator-activated receptor-gamma polymorphisms in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 5887–5892. [Google Scholar] [CrossRef]
- Orio, F., Jr.; Palomba, S.; Cascella, T.; Di Biase, S.; Labella, D.; Russo, T.; Savastano, S.; Zullo, F.; Colao, A.; Vettor, R.; et al. Lack of an association between peroxisome proliferator-activated receptor-gamma gene Pro12Ala polymorphism and adiponectin levels in the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 5110–5115. [Google Scholar] [CrossRef]
- Xita, N.; Lazaros, L.; Georgiou, I.; Tsatsoulis, A. The Pro12Ala polymorphism of the PPAR-gamma gene is not associated with the polycystic ovary syndrome. Hormones 2009, 8, 267–272. [Google Scholar] [CrossRef]
- Zaki, M.; Hassan, N.; Bassyouni, H.; Kamal, S.; Basha, W.; Azmy, O.; Amr, K. Association of the Pro12Ala Polymorphism with the Metabolic Parameters in Women with Polycystic Ovary Syndrome. Open Access Maced. J. Med. Sci. 2017, 5, 275–280. [Google Scholar] [CrossRef]
- Yilmaz, M.; Ergun, M.A.; Karakoc, A.; Yurtcu, E.; Yetkin, I.; Ayvaz, G.; Cakir, N.; Arslan, M. Pro12Ala polymorphism of the peroxisome proliferator-activated receptor-gamma gene in first-degree relatives of subjects with polycystic ovary syndrome. Gynecol. Endocrinol. 2005, 21, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Ergun, M.A.; Karakoc, A.; Yurtcu, E.; Cakir, N.; Arslan, M. Pro12Ala polymorphism of the peroxisome proliferator-activated receptor-gamma gene in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2006, 22, 336–342. [Google Scholar] [CrossRef]
- Bidzińska-Speichert, B.; Lenarcik, A.; Tworowska-Bardzińska, U.; Slezak, R.; Bednarek-Tupikowska, G.; Milewicz, A.; Krepuła, K. Pro12Ala PPAR gamma2 gene polymorphism in women with polycystic ovary syndrome. Ginekol. Pol. 2011, 82, 426–429. [Google Scholar] [PubMed]
- Bidzinska-Speichert, B.; Lenarcik, A.; Tworowska-Bardzinska, U.; Slezak, R.; Bednarek-Tupikowska, G.; Milewicz, A. Pro12Ala PPAR gamma2 gene polymorphism in PCOS women: The role of compounds regulating satiety. Gynecol. Endocrinol. 2012, 28, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Tok, E.C.; Aktas, A.; Ertunc, D.; Erdal, E.M.; Dilek, S. Evaluation of glucose metabolism and reproductive hormones in polycystic ovary syndrome on the basis of peroxisome proliferator-activated receptor (PPAR)-gamma2 Pro12Ala genotype. Hum. Reprod. 2005, 20, 1590–1595. [Google Scholar] [CrossRef]
- Hahn, S.; Fingerhut, A.; Khomtsiv, U.; Khomtsiv, L.; Tan, S.; Quadbeck, B.; Herrmann, B.L.; Knebel, B.; Muller-Wieland, D.; Mann, K.; et al. The peroxisome proliferator activated receptor gamma Pro12Ala polymorphism is associated with a lower hirsutism score and increased insulin sensitivity in women with polycystic ovary syndrome. Clin. Endocrinol. 2005, 62, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Koika, V.; Marioli, D.J.; Saltamavros, A.D.; Vervita, V.; Koufogiannis, K.D.; Adonakis, G.; Decavalas, G.; Georgopoulos, N.A. Association of the Pro12Ala polymorphism in peroxisome proliferator-activated receptor gamma2 with decreased basic metabolic rate in women with polycystic ovary syndrome. Eur. J. Endocrinol. 2009, 161, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, S.; Heinonen, S.; Hiltunen, M.; Helisalmi, S.; Hippeläinen, M.; Koivunen, R.; Tapanainen, J.; Laakso, M. Polymorphism in the peroxisome proliferator-activated receptor-gamma gene in women with polycystic ovary syndrome. Hum. Reprod. 2003, 18, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, Z.; Chamaie-Nejad, F.; Saeidi, S.; Rahimi, Z.; Ebrahimi, A.; Shakiba, E.; Vaisi-Raygani, A. The Association of PPARgamma Pro12Ala and C161T Polymorphisms with Polycystic Ovary Syndrome and Their Influence on Lipid and Lipoprotein Profiles. Int. J. Fertil. Steril. 2018, 12, 147–151. [Google Scholar] [PubMed]
- Shi, C.-Y.; Xu, J.-J.; Li, C.; Yu, J.-L.; Wu, Y.-T.; Huang, H.-F. A PPARG Splice Variant in Granulosa Cells Is Associated with Polycystic Ovary Syndrome. J. Clin. Med. 2022, 11, 7285. [Google Scholar] [CrossRef] [PubMed]
- Giandalia, A.; Pappalardo, M.A.; Russo, G.T.; Romeo, E.L.; Alibrandi, A.; Di Bari, F.; Vita, R.; Cucinotta, D.; Benvenga, S. Influence of peroxisome proliferator-activated receptor-gamma exon 2 and exon 6 and insulin receptor substrate (IRS)-1 Gly972Arg polymorphisms on insulin resistance and beta-cell function in southern mediterranean women with polycystic ovary syndrome. J. Clin. Transl. Endocrinol. 2018, 13, 1–8. [Google Scholar]
- Reddy, T.V.; Govatati, S.; Deenadayal, M.; Shivaji, S.; Bhanoori, M. Polymorphisms in the TFAM and PGC1-α genes and their association with polycystic ovary syndrome among South Indian women. Gene 2018, 641, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Baldani, D.P.; Skrgatic, L.; Cerne, J.Z.; Ferk, P.; Simunic, V.; Gersak, K. Association of PPARG Pro12Ala polymorphism with insulin sensitivity and body mass index in patients with polycystic ovary syndrome. Biomed. Rep. 2014, 2, 199–206. [Google Scholar] [CrossRef]
- Chae, S.J.; Kim, J.J.; Choi, Y.M.; Kim, J.M.; Cho, Y.M.; Moon, S.Y. Peroxisome proliferator-activated receptor-gamma and its coactivator-1alpha gene polymorphisms in Korean women with polycystic ovary syndrome. Gynecol. Obstet. Investig. 2010, 70, 1–7. [Google Scholar] [CrossRef]
- Gu, B.H.; Baek, K.H. Pro12Ala and His447His polymorphisms of PPAR-gamma are associated with polycystic ovary syndrome. Reprod. Biomed. Online 2009, 18, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, X.; Cao, Y.; Yi, L.; Fan, H.; Chen, J. Polymorphisms of the peroxisome proliferator-activated receptor-gamma and its coactivator-1alpha genes in Chinese women with polycystic ovary syndrome. Fertil. Steril. 2006, 85, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gong, H.; Liu, W.; Tao, T. The association of Pro12Ala polymorphism in the peroxisome proliferator-activated receptor-gamma2 gene with the metabolic characteristics in Chinese women with polycystic ovary syndrome. Int. J. Clin. Exp. Pathol. 2013, 6, 1894–1902. [Google Scholar] [PubMed]
- Dasgupta, S.; Sirisha, P.; Neelaveni, K.; Anuradha, K.; Sudhakar, G.; Reddy, B.M. Polymorphisms in the IRS-1 and PPAR-gamma genes and their association with polycystic ovary syndrome among South Indian women. Gene 2012, 503, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Mukherjee, A.; Shah, N.; Meherji, P.; Mukherjee, S. Peroxisome proliferator activated receptor gamma gene variants influence susceptibility and insulin related traits in Indian women with polycystic ovary syndrome. J. Assist. Reprod. Genet. 2013, 30, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, M.; Godla, U.R.; Paul Solomon, F.D.; Maddaly, R. Single-nucleotide polymorphism of INS, INSR, IRS1, IRS2, PPAR-G and CAPN10 genes in the pathogenesis of polycystic ovary syndrome. J. Genet. 2017, 96, 87–96. [Google Scholar] [CrossRef]
- Tepavčević, S.; Milutinović, D.V.; Macut, D.; Stojiljković, M.; Nikolić, M.; Božić-Antić, I.; Ćulafić, T.; Bjekić-Macut, J.; Matić, G.; Korićanac, G. Cardiac fatty acid uptake and metabolism in the rat model of polycystic ovary syndrome. Endocrine 2015, 50, 193–201. [Google Scholar] [CrossRef]
- Dantas, W.S.; Murai, I.H.; Perandini, L.A.; Azevedo, H.; Moreira-Filho, C.A.; Camara, N.O.S.; Roschel, H.; Gualano, B. Acute exercise elicits differential expression of insulin resistance genes in the skeletal muscle of patients with polycystic ovary syndrome. Clin. Endocrinol. 2017, 86, 688–697. [Google Scholar] [CrossRef]
- Keller, E.; Chazenbalk, G.D.; Aguilera, P.; Madrigal, V.; Grogan, T.; Elashoff, D.; Dumesic, D.A.; Abbott, D.H. Impaired Preadipocyte Differentiation Into Adipocytes in Subcutaneous Abdominal Adipose of PCOS-Like Female Rhesus Monkeys. Endocrinology 2014, 155, 2696–2703. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhu, W.J.; Xie, B.G. Expression of PPAR-gamma in adipose tissue of rats with polycystic ovary syndrome induced by DHEA. Mol. Med. Rep. 2014, 9, 889–893. [Google Scholar] [CrossRef]
- Nada, S.E.; Thompson, R.C.; Padmanabhan, V. Developmental Programming: Differential Effects of Prenatal Testosterone Excess on Insulin Target Tissues. Endocrinology 2010, 151, 5165–5173. [Google Scholar] [CrossRef]
- Siemienowicz, K.J.; Furmanska, K.; Filis, P.; Talia, C.; Thomas, J.; Fowler, P.A.; Rae, M.T.; Duncan, W.C. Pubertal FGF21 deficit is central in the metabolic pathophysiology of an ovine model of polycystic ovary syndrome. Mol. Cell Endocrinol. 2021, 525, 111196. [Google Scholar] [CrossRef]
- Maxel, T.; Svendsen, P.F.; Smidt, K.; Lauridsen, J.K.; Brock, B.; Pedersen, S.B.; Rungby, J.; Larsen, A. Expression Patterns and Correlations with Metabolic Markers of Zinc Transporters ZIP14 and ZNT1 in Obesity and Polycystic Ovary Syndrome. Front. Endocrinol. 2017, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Li, S.; Zhao, A.; Zhang, Y.; Liu, W. Expression of the CD11c gene in subcutaneous adipose tissue is associated with cytokine level and insulin resistance in women with polycystic ovary syndrome. Eur. J. Endocrinol. 2012, 167, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Zhao, A.; Tao, T.; Mao, X.; Zhang, P.; Liu, W. The expression of sex steroid synthesis and inactivation enzymes in subcutaneous adipose tissue of PCOS patients. J. Steroid Biochem. Mol. Biol. 2012, 132, 120–126. [Google Scholar] [CrossRef]
- Mlinar, B.; Pfeifer, M.; Vrtacnik-Bokal, E.; Jensterle, M.; Marc, J. Decreased lipin 1 beta expression in visceral adipose tissue is associated with insulin resistance in polycystic ovary syndrome. Eur. J. Endocrinol. 2008, 159, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Mlinar, B.; Marc, J.; Jensterle, M.; Bokal, E.V.; Jerin, A.; Pfeifer, M. Expression of 11beta-hydroxysteroid dehydrogenase type 1 in visceral and subcutaneous adipose tissues of patients with polycystic ovary syndrome is associated with adiposity. J. Steroid. Biochem. Mol. Biol. 2011, 123, 127–132. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Tulberg, A.; Leung, K.L.; Fisch, S.C.; Grogan, T.R.; Abbott, D.H.; Naik, R.; Chazenbalk, G.D. Accelerated subcutaneous abdominal stem cell adipogenesis predicts insulin sensitivity in normal-weight women with polycystic ovary syndrome. Fertil. Steril. 2021, 116, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Kokabiyan, Z.; Yaghmaei, P.; Jameie, S.B.; Hajebrahimi, Z. Therapeutic Effects of Eugenol in Polycystic Ovarian Rats Induced by Estradiol Valerate: A Histopathological and A Biochemical Study. Int. J. Fertil. Steril. 2022, 16, 184–191. [Google Scholar] [PubMed]
- Hai, Y.; Zuo, L.; Wang, M.; Zhang, R.; Wang, M.; Ren, L.; Yang, C.; Wang, J. Icariin Alleviates Nonalcoholic Fatty Liver Disease in Polycystic Ovary Syndrome by Improving Liver Fatty Acid Oxidation and Inhibiting Lipid Accumulation. Molecules 2023, 28, 517. [Google Scholar] [CrossRef] [PubMed]
- Mansor, F.; Gu, H.F.; Östenson, C.-G.; Mannerås-Holm, L.; Stener-Victorin, E.; Mohamud, W.N.W. Labisia pumila Upregulates Peroxisome Proliferator-Activated Receptor Gamma Expression in Rat Adipose Tissues and 3T3-L1 Adipocytes. Adv. Pharmacol. Sci. 2013, 2013, 808914. [Google Scholar] [CrossRef]
- Prabhu, Y.D.; Valsala Gopalakrishnan, A. Gamma-Linolenic acid ameliorates DHEA induced pro-inflammatory response in polycystic ovary syndrome via PPAR-gamma signaling in rats. Reprod. Biol. 2020, 20, 348–356. [Google Scholar] [CrossRef]
- Suriyakalaa, U.; Ramachandran, R.; Doulathunnisa, J.A.; Aseervatham, S.B.; Sankarganesh, D.; Kamalakkannan, S.; Kadalmani, B.; Angayarkanni, J.; Akbarsha, M.A.; Achiraman, S. Upregulation of Cyp19a1 and PPAR-gamma in ovarian steroidogenic pathway by Ficus religiosa: A potential cure for polycystic ovary syndrome. J. Ethnopharmacol. 2021, 267, 113540. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Chen, W.; Zhou, Q.; Dou, X. Astragaloside IV regulates autophagy-mediated proliferation and apoptosis in a rat model of PCOS by activating the PPARgamma pathway. Iran J. Basic Med. Sci. 2022, 25, 882–889. [Google Scholar] [PubMed]
- Zhang, Y.; Li, C.; Zhang, W.; Zheng, X.; Chen, X. Decreased Insulin Resistance by Myo-Inositol Is Associated with Suppressed Interleukin 6/Phospho-STAT3 Signaling in a Rat Polycystic Ovary Syndrome Model. J. Med. Food. 2020, 23, 375–387. [Google Scholar] [CrossRef]
- Safaei, Z.; Bakhshalizadeh, S.; Nasr-Esfahani, M.H.; Sene, A.A.; Najafzadeh, V.; Soleimani, M.; Shirazi, R. Vitamin D3 affects mitochondrial biogenesis through mitogen-activated protein kinase in polycystic ovary syndrome mouse model. J. Cell Physiol. 2020, 235, 6113–6126. [Google Scholar] [CrossRef] [PubMed]
- Zaree, M.; Shahnazi, V.; Fayezi, S.; Darabi, M.; Mehrzad-Sadaghiani, M.; Darabi, M.; Khani, S.; Nouri, M. Expression Levels of PPARgamma and CYP-19 in Polycystic Ovarian Syndrome Primary Granulosa Cells: Influence of omega-3 Fatty Acid. Int. J. Fertil. Steril. 2015, 9, 197–204. [Google Scholar] [PubMed]
- Nasri, K.; Hantoushzadeh, S.; Aghadavod, E.; Taghizadeh, M.; Asemi, Z. The Effects of Omega-3 Fatty Acids Supplementation on Gene Expression Involved in the Insulin and Lipid Signaling Pathway in Patients with Polycystic Ovary Syndrome. Horm. Metab. Res. 2017, 49, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Shojaei, A.; Samimi, M.; Ebrahimi, F.A.; Aghadavod, E.; Karamali, M.; Taghizadeh, M.; Jamilian, H.; Alaeinasab, S.; Jafarnejad, S.; et al. The effects of omega-3 and vitamin E co-supplementation on parameters of mental health and gene expression related to insulin and inflammation in subjects with polycystic ovary syndrome. J. Affect. Disord. 2018, 229, 41–47. [Google Scholar] [CrossRef]
- Jamilian, M.; Foroozanfard, F.; Kavossian, E.; Aghadavod, E.; Shafabakhsh, R.; Hoseini, A.; Asemi, Z. Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2020, 36, 128–133. [Google Scholar] [CrossRef]
- Heshmati, J.; Moini, A.; Sepidarkish, M.; Morvaridzadeh, M.; Salehi, M.; Palmowski, A.; Mojtahedi, M.F.; Shidfar, F. Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Phytomedicine 2021, 80, 153395. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Foroozanfard, F.; Kavossian, E.; Kia, M.; Aghadavod, E.; Amirani, E.; Asemi, Z. Effects of Chromium and Carnitine Co-supplementation on Body Weight and Metabolic Profiles in Overweight and Obese Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2019, 193, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Amiri Siavashani, M.; Zadeh Modarres, S.; Mirhosseini, N.; Aghadavod, E.; Salehpour, S.; Asemi, Z. The Effects of Chromium Supplementation on Gene Expression of Insulin, Lipid, and Inflammatory Markers in Infertile Women with Polycystic Ovary Syndrome Candidate for in vitro Fertilization: A Randomized, Double-Blinded, Placebo-Controlled Trial. Front. Endocrinol. 2018, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Zadeh Modarres, S.; Heidar, Z.; Foroozanfard, F.; Rahmati, Z.; Aghadavod, E.; Asemi, Z. The Effects of Selenium Supplementation on Gene Expression Related to Insulin and Lipid in Infertile Polycystic Ovary Syndrome Women Candidate for In Vitro Fertilization: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace. Elem. Res. 2018, 183, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, E.; Jamilian, M.; Samimi, M.; Mehrizi, M.Z.; Aghadavod, E.; Akbari, E.; Tamtaji, O.R.; Asemi, Z. The effects of coenzyme Q10 supplementation on gene expression related to insulin, lipid and inflammation in patients with polycystic ovary syndrome. Gynecol. Endocrinol. 2018, 34, 217–222. [Google Scholar] [CrossRef]
- Rahmani, E.; Jamilian, M.; Dadpour, B.; Nezami, Z.; Vahedpoor, Z.; Mahmoodi, S.; Aghadavod, E.; Taghizadeh, M.; Hassan, A.B.; Asemi, Z. The effects of fish oil on gene expression in patients with polycystic ovary syndrome. Eur. J. Clin. Investig. 2018, 48, e12893. [Google Scholar] [CrossRef] [PubMed]
- Shabani, A.; Foroozanfard, F.; Kavossian, E.; Aghadavod, E.; Ostadmohammadi, V.; Reiter, R.J.; Eftekhar, T.; Asemi, Z. Effects of melatonin administration on mental health parameters, metabolic and genetic profiles in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. J. Affect. Disord. 2019, 250, 51–56. [Google Scholar] [CrossRef]
- Shokrpour, M.; Foroozanfard, F.; Ebrahimi, F.A.; Vahedpoor, Z.; Aghadavod, E.; Ghaderi, A.; Asemi, Z. Comparison of myo-inositol and metformin on glycemic control, lipid profiles, and gene expression related to insulin and lipid metabolism in women with polycystic ovary syndrome: A randomized controlled clinical trial. Gynecol. Endocrinol. 2019, 35, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Komar, C.M. Peroxisome proliferator-activated receptors (PPARs) and ovarian function--implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod. Biol. Endocrinol. 2005, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Komar, C.M.; Curry, T.E. Localization and expression of messenger RNAs for the peroxisome proliferator-activated receptors in ovarian tissue from naturally cycling and pseudopregnant rats. Biol. Reprod. 2002, 66, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Froment, P.; Fabre, S.; Dupont, J.; Pisselet, C.; Chesneau, D.; Staels, B.; Monget, P. Expression and functional role of peroxisome proliferator-activated receptor-gamma in ovarian folliculogenesis in the sheep. Biol. Reprod. 2003, 69, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Long, M.J.; Sairam, M.R.; Komar, C.M. Initiation of the expression of peroxisome proliferator—Activated receptor gamma (PPAR gamma) in the rat ovary and the role of FSH. Reprod. Biol. Endocrinol. 2009, 7, 145. [Google Scholar] [CrossRef]
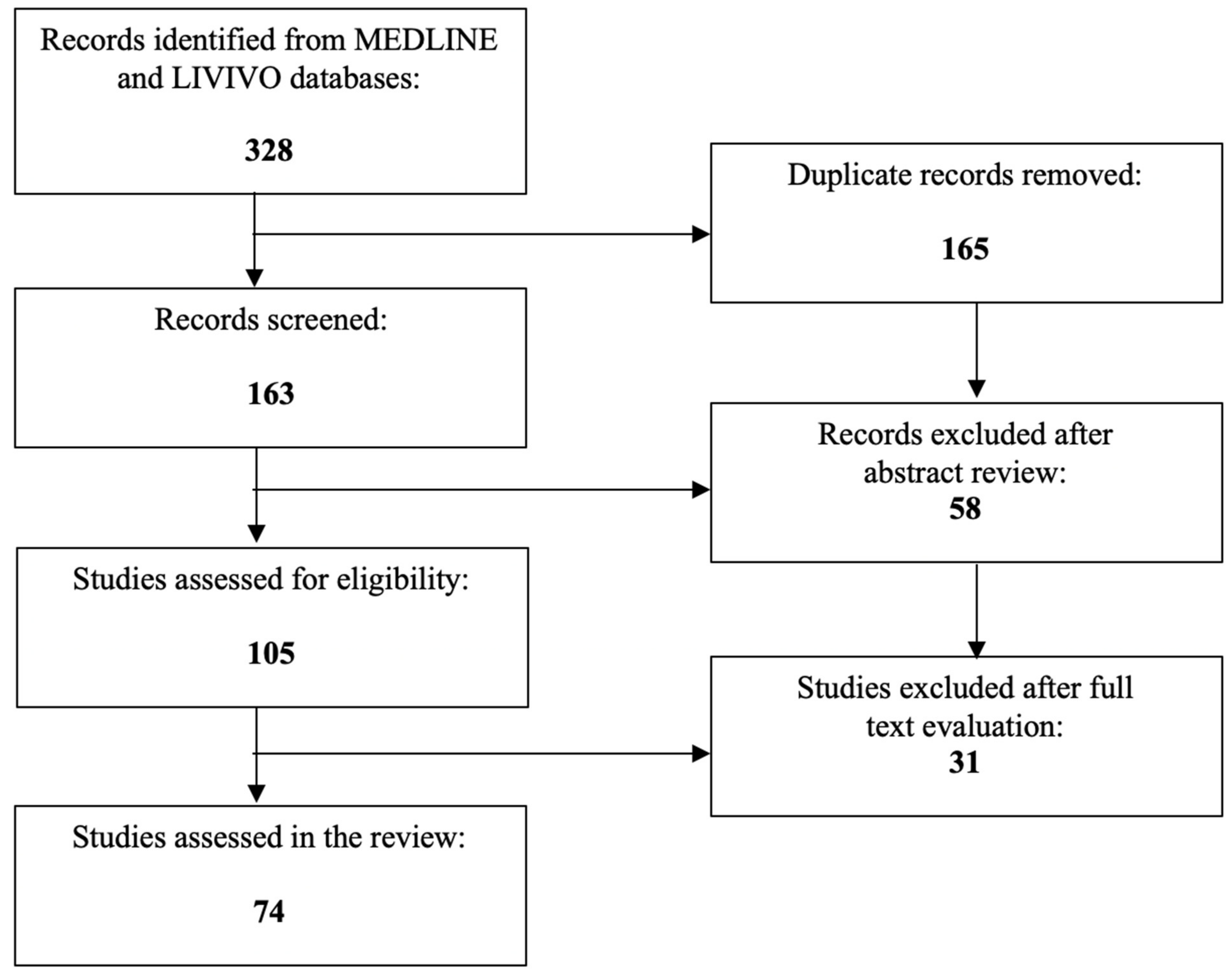
| Study | Ethnic Group (Study Group Size) | PPARγ Polymorphism | Role in PCOS |
|---|---|---|---|
| Baldani et al. [76] | Croatian (330) | Pro12Ala | Positive effect on insulin sensitivity and BMI |
| Chae et al. [77] | Korean (440) | Pro12Ala | Modulation of HDL levels |
| Gu et al. [78] | Korean (238) | Pro12Ala, His447His | Correlation with PCOS |
| Wang et al. [79] | Chinese (348) | Pro12Ala | No significant correlation |
| Yang et al. [80] | Chinese (238) | Pro12Ala | No significant correlation |
| Dasgupta et al. [81] | South Indian (549) | Exon 2 Ala allele, Exon 6 His447His T allele | Reduced frequency of hyperandrogenic and metabolic characteristics |
| Shaikh et al. [82] | Indian (750) | Pro12Ala, His447His | Improved glucose metabolism, fasting insulin, and insulin resistance |
| Thangavelu et al. [83] | Indian (338) | rs3856806 | No significant correlation |
| Study | Study Group Size | PCOS Organ Tissue | PPAR Expression |
|---|---|---|---|
| Tepavčević et al. [84] | 24 rats | Cardiac cells | Enhanced nuclear PPARα and PGC-1 expression |
| Dantas et al. [85] | 8 women | Skeletal myocytes | Significant PPARα and PGC-1α upregulation |
| Keller et al. [86] | 12 monkeys | Subcutaneous adipocytes | No significant differences |
| Wang et al. [87] | 16 rats | Adipocytes | Low PPARγ expression levels |
| Nada et al. [88] | 13 sheep | Hepatocytes, Adipocytes | Low PPARγ levels in the liver, High PPARγ levels in the adipose tissue |
| Siemienowicz et al. [89] | 121 ewes/lamps | Hepatocytes, Subcutaneous adipocytes | Decreased PGC-1α/PPARγ expression |
| Maxel et al. [90] | 59 women | Subcutaneous adipocytes | Downregulation with increasing BMI, Positive correlation with the ZIP14 gene |
| Tao et al. [91] | 34 women | Subcutaneous adipocytes | Low PPARγ expression levels |
| Wang et al. [92] | 24 women | Subcutaneous adipocytes | Low PPARγ expression levels |
| Mlinar et al. [93,94] | 129 women | Subcutaneous adipocytes Visceral adipocytes | Positive correlation of lipin 1β expression in subcutaneous adipose tissue with PPARγ, Positive correlation of HSD11B1 expression in visceral adipose tissue with PPARγ |
| Dumesic et al. [95] | 16 women | Subcutaneous adipocytes | Positive prediction of total body mass, total body fat, and gynoid fat masses by PPARγ gene expression |
| Natural Agent | Study Group Size | PPARα Upregulation | PPARγ Upregulation | Reference |
|---|---|---|---|---|
| Eugenol | 30 rats | X | [96] | |
| Icariin | 36 rats | X | [97] | |
| Labisia pumila standardized water extract | 22 rats | X | [98] | |
| γ-linolenic acid | Rat model | X | [99] | |
| Fresh leaves extracts of Ficus religiosa | 42 rats | X | [100] | |
| Astragaloside IV | 30 rats | X | [101] | |
| Myo-inositol | 45 rats; 53 women | X | [102,115] | |
| Vitamin D3 | Mouse model | X | [103] | |
| Eicosapentaenoic acid | 30 women | X | [104] | |
| ω-3 fatty acids +/− vitamin E | 100 women | X | [105,106] | |
| Curcumin | 132 women | X | [107,108] | |
| Chromium +/− carnitine | 94 women | X | [109,110] | |
| Selenium | 40 women | X | [111] | |
| Coenzyme Q10 | 40 women | X | [112] | |
| Fish oil | 40 women | X | [113] | |
| Melatonin | 58 women | X | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psilopatis, I.; Vrettou, K.; Nousiopoulou, E.; Palamaris, K.; Theocharis, S. The Role of Peroxisome Proliferator-Activated Receptors in Polycystic Ovary Syndrome. J. Clin. Med. 2023, 12, 2912. https://doi.org/10.3390/jcm12082912
Psilopatis I, Vrettou K, Nousiopoulou E, Palamaris K, Theocharis S. The Role of Peroxisome Proliferator-Activated Receptors in Polycystic Ovary Syndrome. Journal of Clinical Medicine. 2023; 12(8):2912. https://doi.org/10.3390/jcm12082912
Chicago/Turabian StylePsilopatis, Iason, Kleio Vrettou, Eleni Nousiopoulou, Kostas Palamaris, and Stamatios Theocharis. 2023. "The Role of Peroxisome Proliferator-Activated Receptors in Polycystic Ovary Syndrome" Journal of Clinical Medicine 12, no. 8: 2912. https://doi.org/10.3390/jcm12082912
APA StylePsilopatis, I., Vrettou, K., Nousiopoulou, E., Palamaris, K., & Theocharis, S. (2023). The Role of Peroxisome Proliferator-Activated Receptors in Polycystic Ovary Syndrome. Journal of Clinical Medicine, 12(8), 2912. https://doi.org/10.3390/jcm12082912








