Effect of Selenium Deficiency on the Development of Overt Hepatic Encephalopathy in Patients with Chronic Liver Disease
Abstract
1. Introduction
2. Methods
2.1. Study Design and Ethical Considerations
2.2. Study Population
2.3. Data Collection
2.4. Outcome
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Enrolled Patients
3.2. Selenium Deficiency and Liver Function Reserve in Patients with CLD
3.3. Factors Associated with Selenium Deficiency
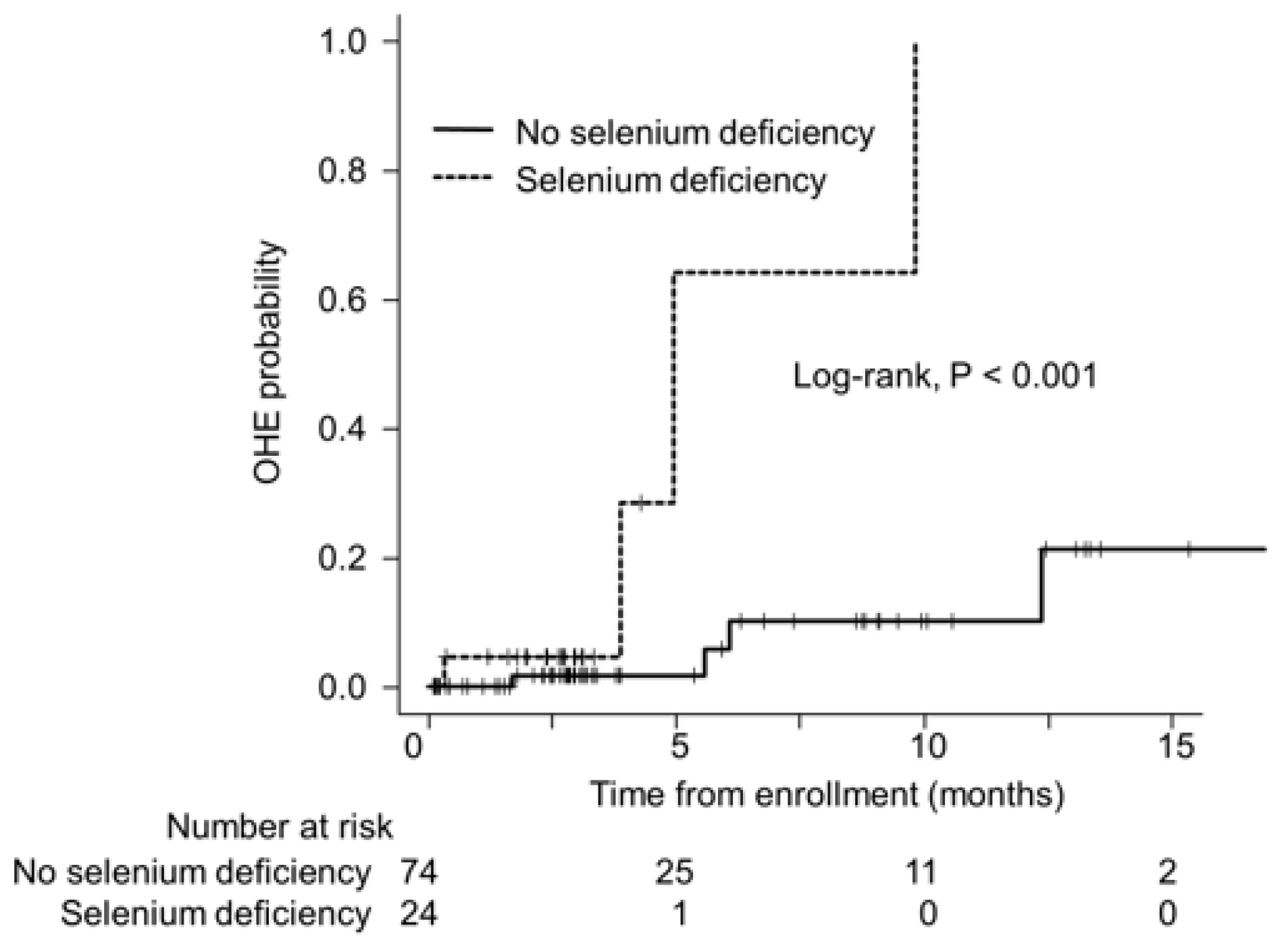
3.4. Selenium Deficiency and OHE
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Y.; He, F.; Lian, S.; Xie, B.; Liu, T.; He, J.; Liu, C. Selenium status in patients with chronic liver disease: A systematic review and meta-analysis. Nutrients 2022, 14, 952. [Google Scholar] [CrossRef] [PubMed]
- Himoto, T.; Masaki, T. Current trends of essential trace elements in patients with chronic liver diseases. Nutrients 2020, 12, 2084. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484s–1491s. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E.; Motley, A.K.; Byrne, D.W.; Norsworthy, B.K. Selenium deficiency occurs in some patients with moderate-to-severe cirrhosis and can be corrected by administration of selenate but not selenomethionine: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F., Jr. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Sies, H. Selenium homeostasis and antioxidant selenoproteins in brain: Implications for disorders in the central nervous system. Arch. Biochem. Biophys. 2013, 536, 152–157. [Google Scholar] [CrossRef]
- Xu, L.; Lu, Y.; Wang, N.; Feng, Y. The role and mechanisms of selenium supplementation on fatty liver-associated disorder. Antioxidants 2022, 11, 922. [Google Scholar] [CrossRef]
- Kodama, H.; Asagiri, K.; Ida, S.; Etani, Y.; Koyama, H.; Soh, H.; Tanaka, Y.; Takayanagi, M.; Funakoshi, M.; Yoshida, M. Diagnosis and treatment of selenium deficiency. J. Jpn. Soc. Clin. Nutr. 2018, 40, 238–283. [Google Scholar]
- Burk, R.F.; Early, D.S.; Hill, K.E.; Palmer, I.S.; Boeglin, M.E. Plasma selenium in patients with cirrhosis. Hepatology 1998, 27, 794–798. [Google Scholar] [CrossRef]
- Nangliya, V.; Sharma, A.; Yadav, D.; Sunder, S.; Nijhawan, S.; Mishra, S. Study of trace elements in liver cirrhosis patients and their role in prognosis of disease. Biol. Trace Elem. Res. 2015, 165, 35–40. [Google Scholar] [CrossRef]
- Taylor, E.W.; Ruzicka, J.A.; Premadasa, L.; Zhao, L. Cellular selenoprotein mrna tethering via antisense interactions with ebola and hiv-1 mrnas may impact host selenium biochemistry. Curr. Top. Med. Chem. 2016, 16, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; López, H.; Ruiz, M.L.; González, S.; Pérez, V.; López, M.C. Determination of selenium in serum by hydride generation atomic absorption spectrometry for calculation of daily dietary intake. Sci. Total Environ. 1995, 175, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Davidson, H.I.; Richardson, R.; Sutherland, D.; Garden, O.J. Macronutrient preference, dietary intake, and substrate oxidation among stable cirrhotic patients. Hepatology 1999, 29, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Sanz Alaejos, M.; Romero, C.D. Urinary selenium concentrations. Clin. Chem. 1993, 39, 2040–2052. [Google Scholar] [CrossRef] [PubMed]
- Thuluvath, P.J.; Triger, D.R. Selenium in chronic liver disease. J. Hepatol. 1992, 14, 176–182. [Google Scholar] [CrossRef]
- Misra, V.; Misra, S.P.; Dwivedi, M.; Gupta, S.C. Histomorphometric study of portal hypertensive enteropathy. Am. J. Clin. Pathol. 1997, 108, 652–657. [Google Scholar] [CrossRef]
- Chen, Y.L.; Yang, K.C.; Chang, H.H.; Lee, L.T.; Lu, C.W.; Huang, K.C. Low serum selenium level is associated with low muscle mass in the community-dwelling elderly. J. Am. Med. Dir. Assoc. 2014, 15, 807–811. [Google Scholar] [CrossRef]
- Hirato, J.; Nakazato, Y.; Koyama, H.; Yamada, A.; Suzuki, N.; Kuroiwa, M.; Takahashi, A.; Matsuyama, S.; Asayama, K. Encephalopathy in megacystis-microcolon-intestinal hypoperistalsis syndrome patients on long-term total parenteral nutrition possibly due to selenium deficiency. Acta Neuropathol. 2003, 106, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, K.; Iida, M.; Matsumoto, T.; Mochizuki, Y.; Doi, K.; Aoyagi, K.; Fujishima, M. Progressive encephalopathy in a crohn’s disease patient on long-term total parenteral nutrition: Possible relationship to selenium deficiency. Postgrad. Med. J. 1994, 70, 215–219. [Google Scholar] [CrossRef]
- Agamy, O.; Zeev, B.B.; Lev, D.; Marcus, B.; Fine, D.; Su, D.; Narkis, G.; Ofir, R.; Hoffmann, C.; Leshinsky-Silver, E.; et al. Mutations disrupting selenocysteine formation cause progressive cerebello-cerebral atrophy. Am. J. Hum. Genet. 2010, 87, 538–544. [Google Scholar] [CrossRef]
- Görg, B.; Bidmon, H.J.; Häussinger, D. Gene expression profiling in the cerebral cortex of patients with cirrhosis with and without hepatic encephalopathy. Hepatology 2013, 57, 2436–2447. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Gluud, L.L.; Nikolova, D.; Bjelakovic, M.; Nagorni, A.; Gluud, C. Antioxidant supplements for liver diseases. Cochrane Database Syst. Rev. 2011, 3, Cd007749. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Total Cohort (n = 98) | No selenium Deficiency (n = 74) | Selenium Deficiency † (n = 24) | p Value * |
|---|---|---|---|---|
| Age (years) | 73 (66–79) | 73 (66–79) | 70 (63–79) | 0.374 |
| Men | 56 (57) | 46 (62) | 10 (42) | 0.098 |
| Body mass index (kg/m2) | 24.4 (22.2–26.9) | 23.6 (21.9–25.3) | 27.8 (24.0–30.1) | 0.004 |
| Etiology (HBV/HCV/Alcohol/Others) | 14/6/23/55 | 14/4/17/39 | 0/2/6/16 | 0.081 |
| Liver cirrhosis | 69 (70) | 50 (68) | 19 (79) | 0.279 |
| Hepatocellular carcinoma | 70 (71) | 59 (80) | 11 (46) | 0.003 |
| M2BPGi | 2.77 (1.25–5.64) | 1.83 (1.06–4.74) | 5.19 (3.34–9.13) | <0.001 |
| FIB-4 index | 4.13 (2.80–6.46) | 3.96 (2.74–5.93) | 5.53 (3.89–8.70) | 0.035 |
| ALBI score | −2.36 (−2.78–−1.81) | −2.52 (−2.82–−2.14) | −1.52 (−2.29–−1.18) | <0.001 |
| ALBI grade (1/2a/2b/3) | 36/22/28/12 | 34/17/19/4 | 2/5/9/8 | <0.001 |
| Child–Pugh score | 5 (5–7) | 5 (5–6) | 7 (6–9) | <0.001 |
| Child–Pugh class (A/B/C) | 71/19/8 | 61/10/3 | 10/9/5 | <0.001 |
| Albumin (g/dL) | 3.7 (3.2–4.2) | 3.9 (3.4–4.3) | 2.9 (2.6–3.6) | <0.001 |
| Creatinine (mg/dL) | 0.75 (0.64–0.91) | 0.74 (0.64–0.85) | 0.82 (0.66–1.12) | 0.100 |
| Total bilirubin (mg/dL) | 1.0 (0.7–1.5) | 0.9 (0.7–1.3) | 1.5 (1.0–1.9) | 0.006 |
| Platelet (10⁹/L) | 139 (91–166) | 145 (107–173) | 102 (77–148) | 0.051 |
| International normalized ratio | 1.00 (0.93–1.11) | 0.98 (0.92–1.05) | 1.15 (1.02–1.31) | <0.001 |
| Zinc (μg/dL) | 67 (57–76) | 70 (61–78) | 55 (42–69) | 0.001 |
| Ammonia (µg/dL) | 62 (48–90) | 60 (44–79) | 90 (57–131) | 0.002 |
| BTR | 4.8 (3.3–6.3) | 5.0 (4.0–6.3) | 2.9 (2.6–5.0) | 0.001 |
| Selenium (µg/dL) | 11.8 (10.1–13.4) | 12.6 (10.9–13.9) | 8.8 (8.2–9.5) | <0.001 |
| Characteristics | OR (95% CI) | p Value * |
|---|---|---|
| Body mass index, kg/m2 | 1.12 (0.99–1.25) | 0.065 |
| ALBI score | 3.23 (1.56–6.67) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakahata, Y.; Hanai, T.; Miwa, T.; Maeda, T.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M. Effect of Selenium Deficiency on the Development of Overt Hepatic Encephalopathy in Patients with Chronic Liver Disease. J. Clin. Med. 2023, 12, 2869. https://doi.org/10.3390/jcm12082869
Nakahata Y, Hanai T, Miwa T, Maeda T, Imai K, Suetsugu A, Takai K, Shimizu M. Effect of Selenium Deficiency on the Development of Overt Hepatic Encephalopathy in Patients with Chronic Liver Disease. Journal of Clinical Medicine. 2023; 12(8):2869. https://doi.org/10.3390/jcm12082869
Chicago/Turabian StyleNakahata, Yuki, Tatsunori Hanai, Takao Miwa, Toshihide Maeda, Kenji Imai, Atsushi Suetsugu, Koji Takai, and Masahito Shimizu. 2023. "Effect of Selenium Deficiency on the Development of Overt Hepatic Encephalopathy in Patients with Chronic Liver Disease" Journal of Clinical Medicine 12, no. 8: 2869. https://doi.org/10.3390/jcm12082869
APA StyleNakahata, Y., Hanai, T., Miwa, T., Maeda, T., Imai, K., Suetsugu, A., Takai, K., & Shimizu, M. (2023). Effect of Selenium Deficiency on the Development of Overt Hepatic Encephalopathy in Patients with Chronic Liver Disease. Journal of Clinical Medicine, 12(8), 2869. https://doi.org/10.3390/jcm12082869







