A Randomized Placebo-Controlled Study of a Transcranial Photobiomodulation Helmet in Parkinson’s Disease: Post-Hoc Analysis of Motor Outcomes
Abstract
1. Introduction
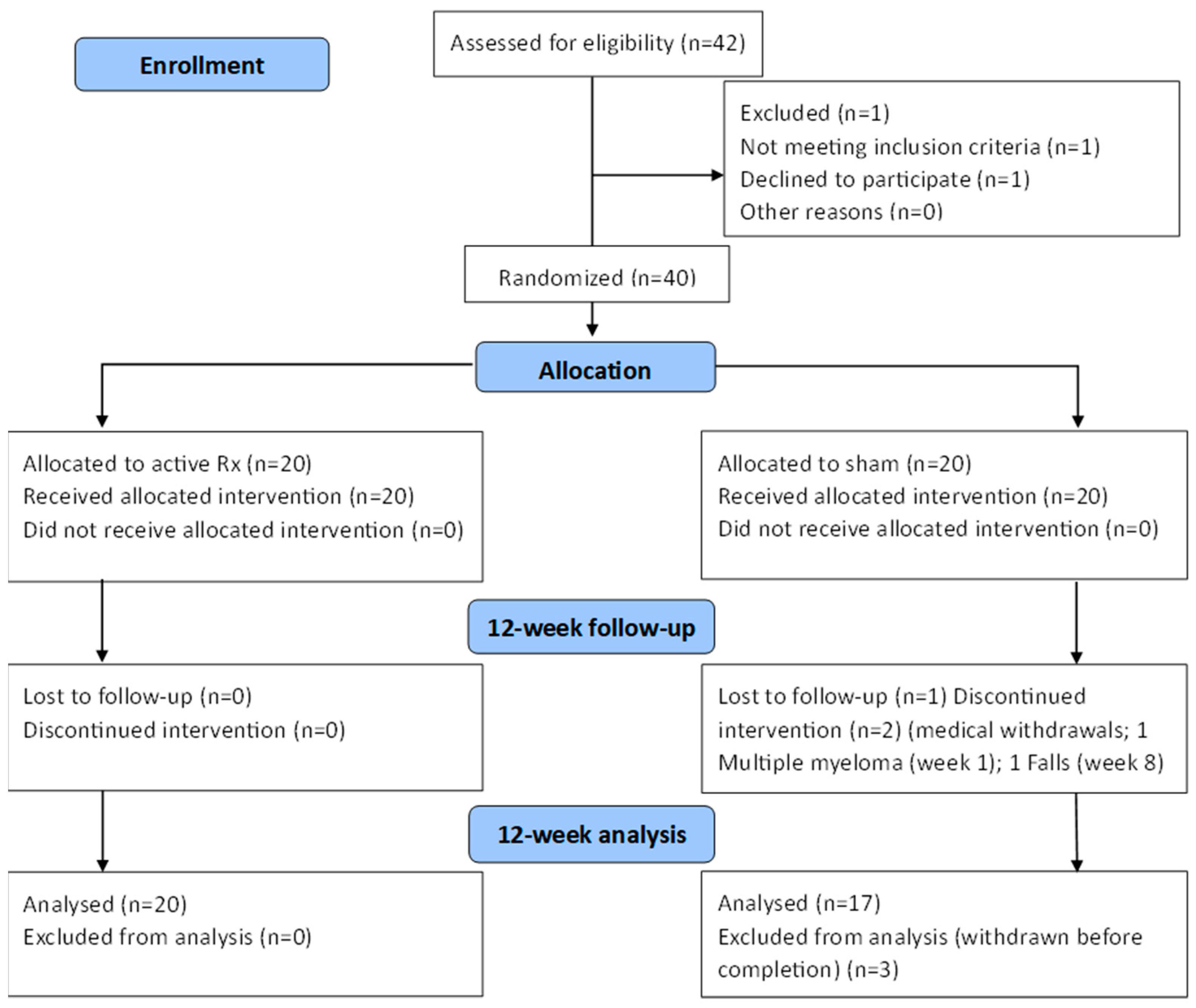
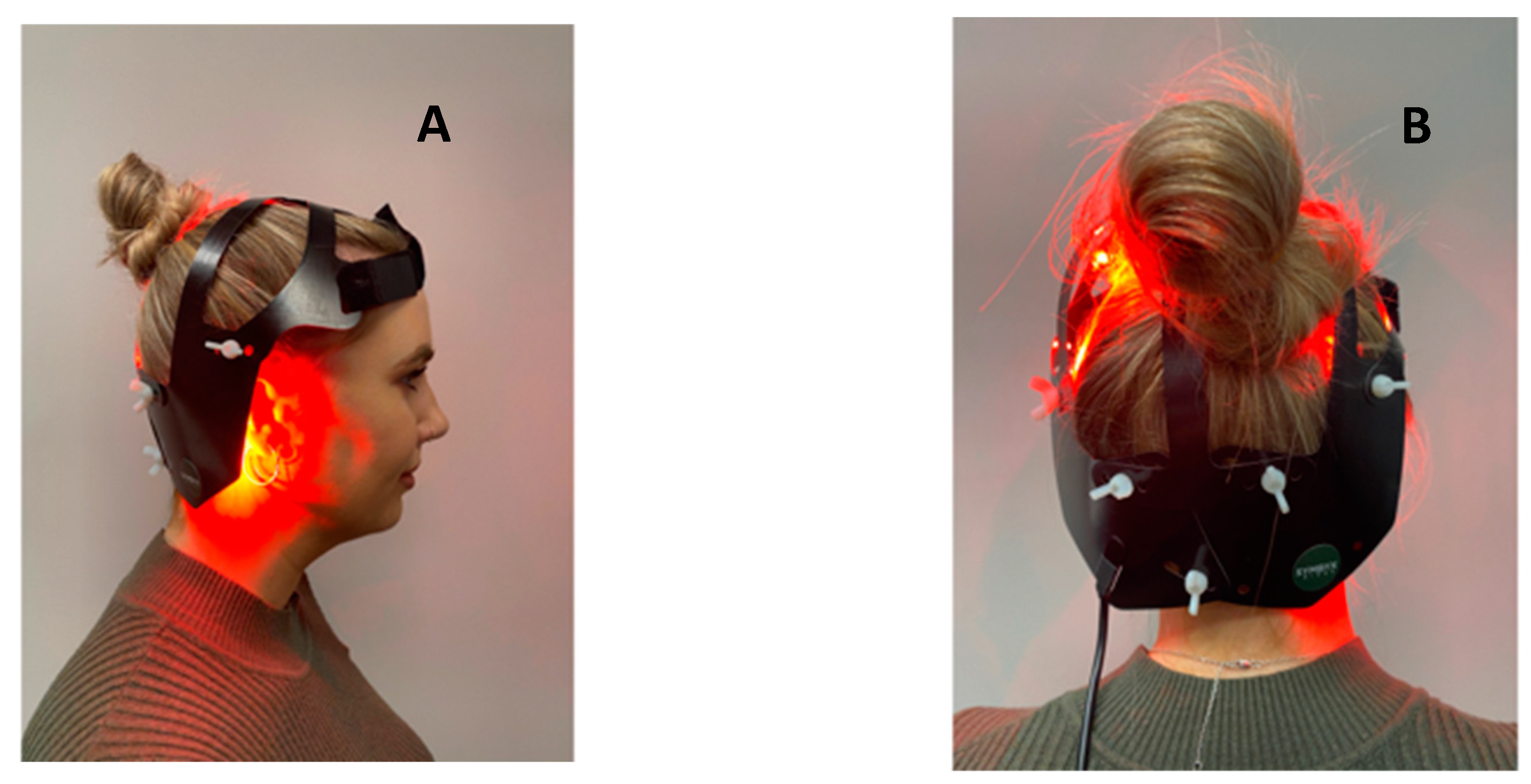
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zeng, X.S.; Geng, W.S.; Jia, J.J.; Chen, L.; Zhang, P.P. Cellular and Molecular Basis of Neurodegeneration in Parkinson Disease. Front. Aging Neurosci. 2018, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Rocca, W.A. The future burden of Parkinson’s disease. Mov. Dis. 2018, 33, 8–9. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef]
- Twelves, D.; Perkins, K.S.M.; Counsell, C. Systematic review of incidence studies of Parkinson’s disease. Mov. Dis. 2003, 18, 19–31. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Lin, F.; Wu, D.; Chen, L.; Weng, H.; Yu, J.; Wang, Y.; Chen, Y.; Chen, X.; Ye, Q.; et al. Comparative Efficacy and Safety of Dopamine Agonists in Advanced Parkinson’s Disease with Motor Fluctuations: A Systematic Review and Network Meta-Analysis of Double-Blind Randomized Controlled Trials. Front. Neurosci. 2021, 15, PMC8586709. [Google Scholar] [CrossRef]
- Limousin, P.; Foltynie, T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat. Rev. Neurol. 2019, 15, 234–242. [Google Scholar] [CrossRef]
- Zahoor, I.; Shafi, A.; Haq, E. Pharmacological Treatment of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Codon Publications: Singapore, 2018; pp. 129–144. [Google Scholar] [CrossRef]
- Hill, E.J.; Mangleburg, C.G.; Alfradique-Dunham, I.; Ripperger, B.; Stillwell, A.; Saade, H.; Rao, S.; Fagbongbe, O.; von Coelln, R.; Tarakad, A.; et al. Quantitative mobility measures complement the MDS-UPDRS for characterization of Parkinson’s disease heterogeneity. Park. Relat. Disord. 2021, 84, 105–111. [Google Scholar] [CrossRef]
- Campbell, M.C.; Myers, P.S.; Weigand, A.J.; Foster, E.R.; Cairns, N.J.; Jackson, J.J.; Lessov-Schlaggar, C.N.; Perlmutter, J.S. Parkinson disease clinical subtypes: Key features & clinical milestones. Ann. Clin. Transl. Neurol. 2020, 7, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Salehpour, F. Photobiomodulation of the Brain: Shining Light on Alzheimer’s and Other Neuropathological Diseases. J. Alzheimer’s Dis. 2021, 83, 1395–1397. [Google Scholar] [CrossRef]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers: Vs. light emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Liebert, A. Photobiomodulation Therapy Mechanisms Beyond Cytochrome c Oxidase. Photobiomodulation Photomed. Laser Surg. 2022, 40, 75–77. [Google Scholar] [CrossRef]
- Salehpour, F.; Hamblin, M. Photobiomodulation for Parkinson’s Disease in Animal Models: A Systematic Review. Biomolecules 2020, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Zomorrodi, R.; Loheswaran, G.; Pushparaj, A.; Lim, L. Pulsed Near Infrared Transcranial and Intranasal Photobiomodulation Significantly Modulates Neural Oscillations: A pilot exploratory study. Sci. Rep. 2019, 9, 6309. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinov, D.; Qi, X.; Berman, M.H.; Dougal, G.; Dayawansa, S.; Wu, E.; Yi, S.S.; Stevens, A.B.; Huang, J.H. Transcranial near infrared light stimulations improve cognition in patients with dementia. Aging Dis. 2021, 12, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Naeser, M.A.; Martin, P.I.; Ho, M.D.; Krengel, M.H.; Bogdanova, Y.; Knight, J.A.; Hamblin, M.R.; Fedoruk, A.E.; Poole, L.G.; Cheng, C.; et al. Transcranial Photobiomodulation Treatment: Significant Improvements in Four Ex-Football Players with Possible Chronic Traumatic Encephalopathy. J. Alzheimer’s Dis. Rep. 2023, 7, 77–105. [Google Scholar] [CrossRef]
- Liebert, A.; Bicknell, B.; Laakso, E.L.; Heller, G.; Jalilitabaei, P.; Tilley, S.; Mitrofanis, J.; Kiat, H. Improvements in clinical signs of Parkinson’s disease using photobiomodulation: A prospective proof-of-concept study. BMC Neurol. 2021, 21, 256. [Google Scholar] [CrossRef] [PubMed]
- McGee, C.; Liebert, A.; Herkes, G.; Bicknell, B.; Pang, V.; McLachlan, C.S.; Kiat, H. Protocol for randomized controlled trial to evaluate the safety and feasibility of a novel helmet to deliver transcranial light emitting diodes photobiomodulation therapy to patients with Parkinson’s disease. Front. Neurosci. 2022, 16. [Google Scholar] [CrossRef] [PubMed Central]
- Karanicolas, P.J.; Farrokhyar, F.; Bhandari, M. Practical tips for surgical research: Blinding: Who, what, when, why, how? Can. J. Surg. 2010, 53, 345–348. [Google Scholar]
- Tarolli, C.G.; Andrzejewski, K.; Zimmerman, G.A.; Bull, M.; Goldenthal, S.; Auinger, P.; O’Brien, M.; Dorsey, E.; Biglan, K.; Simuni, T. Feasibility, Reliability, and Value of Remote Video-Based Trial Visits in Parkinson’s Disease. J. Parkinsons. Dis. 2020, 10, 1779–1786. [Google Scholar] [CrossRef]
- Stillerova, T.; Liddle, J.; Gustafsson, L.; Lamont, R.; Silburn, P. Remotely Assessing Symptoms of Parkinson’s Disease Using Videoconferencing: A Feasibility Study. Neurol. Res. Int. 2016, 2016, 4802570. [Google Scholar] [CrossRef]
- Abdolahi, A.; Scoglio, N.; Killoran, A.; Dorsey, E.R.; Biglan, K.M. Potential reliability and validity of a modified version of the Unified Parkinson’s Disease Rating Scale that could be administered remotely. Park. Relat. Disord. 2013, 19, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Shulman, L.M.; Gruber-Baldini, A.L.; Anderson, K.E.; Fishman, P.S.; Reich, S.G.; Weiner, W.J. The Clinically Important Difference on the Unified Parkinson’s Disease Rating Scale. Arch. Neurol. 2010, 67, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Wuu, J.; McDermott, M.P.; Adler, C.H.; Fahn, S.; Freed, C.R.; Hauser, R.A.; Olanow, W.C.; Shoulson, I.; Tandon, P.K.; et al. Placebo response in Parkinson’s disease: Comparisons among 11 trials covering medical and surgical interventions. Mov. Dis. 2008, 23, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Lidstone, S.C.; Schulzer, M.; Dinelle, K.; Mak, E.; Sossi, V.; Ruth, T.J.; de la Fuente-Fernández, R.; Phillips, A.G.; Stoessl, A.J. Effects of Expectation on Placebo-Induced Dopamine Release in Parkinson Disease. Arch. Gen. Psychiatry 2010, 67, 857. [Google Scholar] [CrossRef]
- Lidstone, S.C. Great Expectations: The Placebo Effect in Parkinson’s Disease. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 139–147. [Google Scholar]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Dis. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Athauda, D.; Maclagan, K.; Budnik, N.; Zampedri, L.; Hibbert, S.; Aviles-Olmos, I.; Chowdhury, K.; Skene, S.S.; Limousin, P.; Foltynie, T. Post hoc analysis of the Exenatide-PD trial-Factors that predict response. Eur. J. Neurosci. 2019, 49, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Bologna, M.; Paparella, G.; Fasano, A.; Hallett, M.; Berardelli, A. Evolving concepts on bradykinesia. Brain 2020, 143, 727–750. [Google Scholar] [CrossRef]
- Askari, A.; Zhu, B.J.; Lyu, X.; Chou, K.L.; Patil, P.G. Characterization and localization of upper and lower extremity motor improvements in STN DBS for Parkinson’s disease. Park. Rela Disord. 2022, 94, 84–88. [Google Scholar] [CrossRef]
- Regnault, A.; Boroojerdi, B.; Meunier, J.; Bani, M.; Morel, T.; Cano, S. Does the MDS-UPDRS provide the precision to assess progression in early Parkinson’s disease? Learnings from the Parkinson’s progression marker initiative cohort. J. Neurol. 2019, 266, 1927–1936. [Google Scholar] [CrossRef]
- Dwivedi, R.; Tiwari, P.; Pahuja, M.; Dada, R.; Tripathi, M. Anti-seizure medications and quality of life in person with epilepsy. Heliyon 2022, 8, e11073. [Google Scholar] [CrossRef] [PubMed]
- Stubendorff, K.; Larsson, V.; Ballard, C.; Minthon, L.; Aarsland, D.; Londos, E. Treatment effect of memantine on survival in dementia with Lewy bodies and Parkinson’s disease with dementia: A prospective study. BMJ Open 2014, 4, e005158. [Google Scholar] [CrossRef] [PubMed]
- Standaert, D.G.; Boyd, J.T.; Odin, P.; Robieson, W.Z.; Zamudio, J.; Chatamra, K. Systematic evaluation of levodopa-carbidopa intestinal gel patient-responder characteristics. NPJ Park. Dis. 2018, 4, 4. [Google Scholar] [CrossRef] [PubMed]
| Group | Baseline Mean (SD) | 12-Week Mean (SD) | Mean Difference | Paired t-Test | |||
|---|---|---|---|---|---|---|---|
| Mean % Improvement | T Score | p Value | |||||
| UPDRS scores for all participants (df: active = 19; sham = 17) | |||||||
| Total score | Active | 21.35 (9.43) | 16.45 (9.48) | −4.90 (7.67) | 23% | 2.84 | 0.010 * |
| Sham | 26.00 (13.81) | 20.47 (12.83) | −5.52 (7.93) | 21% | 2.85 | 0.011 * | |
| Facial | Active | 2.26 (1.44) | 1.73 (1.66) | −0.53 (0.77) | 23% | 2.92 | 0.008 * |
| Sham | 2.24 (1.44) | 1.88 (1.49) | −0.36 (0.93) | 16% | 1.56 | 0.138 | |
| Upper limb | Active | 6.63 (3.53) | 4.84 (3.82) | −1.79 (3.88) | 27% | 1.84 | 0.060 |
| Sham | 7.24 (4.68) | 6.59 (4.87) | −0.64 (3.37) | 9% | 0.79 | 0.440 | |
| Lower limb | Active | 4.26 (2.51) | 2.47 (2.38) | −2.26 (2.62) | 53% | 2.61 | 0.017 * |
| Sham | 6.24 (3.68) | 3.88 (2.29) | −2.36 (3.16) | 38% | 3.04 | 0.007 * | |
| Gait | Active | 3.37 (1.54) | 2.79 (1.87) | −0.58 (1.46) | 17% | 1.87 | 0.102 |
| Sham | 5.00 (2.80) | 3.65 (2.85) | −1.35 (2.57) | 27% | 2.16 | 0.046 * | |
| Tremor | Active | 4.84 (3.48) | 4.11 (2.96) | −0.74 (2.58) | 15% | 0.51 | 0.229 |
| Sham | 5.29 (5.59) | 4.47 (4.45) | −0.82 (3.6) | 16% | 0.93 | 0.361 | |
| UPDRS scores for responders (df: active = 13; sham = 9) | |||||||
| Total | Active | 22.86 (10.39) | 14.57 (8.87) | −8.29 (5.17) | 36% | 6.00 | <0.001 * |
| score | Sham | 29.80 10.39) | 18.80 (14.31) | −11.00 (2.98) | 37% | 11.67 | <0.001 * |
| Facial | Active | 2.07 (1.38) | 1.50 (1.51) | −0.57 (0.76) | 28% | 2.83 | 0.014 * |
| Sham | 2.10 (1.52) | 1.50 (1.43) | −0.60 (0.97) | 29% | 1.97 | 0.081 | |
| Upper Limb | Active | 7.07 (3.73) | 4.29 (3.58) | −2.79 (3.89) | 40% | 2.68 | 0.019 * |
| Sham | 8.30 (5.31) | 6.30 (5.25) | −2.00 (2.91) | 24% | 2.18 | 0.058 | |
| Lower Limb | Active | 4.29 (2.73) | 1.79 (2.12) | −2.50 (2.41) | 58% | 3.88 | 0.002 * |
| Sham | 7.60 (3.57) | 3.70 (2.41) | −3.90 (2.57) | 51% | 4.82 | 0.001 * | |
| Gait | Active | 3.57 (1.40) | 2.57 (1.79) | −1.00 (1.24) | 28% | 3.01 | 0.010 * |
| Sham | 5.60 (2.99) | 3.60 (2.91 | −2.00 (2.98) | 36% | 2.12 | 0.063 | |
| Tremor | Active | 5.86 (3.39) | 4.43 (3.03) | −1.43 (2.34) | 24% | 2.28 | 0.040 * |
| Sham | 6.20 (6.51) | 3.70 (4.53) | −2.50 (3.63) | 40% | 2.18 | 0.057 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGee, C.; Liebert, A.; Bicknell, B.; Pang, V.; Isaac, V.; McLachlan, C.S.; Kiat, H.; Herkes, G. A Randomized Placebo-Controlled Study of a Transcranial Photobiomodulation Helmet in Parkinson’s Disease: Post-Hoc Analysis of Motor Outcomes. J. Clin. Med. 2023, 12, 2846. https://doi.org/10.3390/jcm12082846
McGee C, Liebert A, Bicknell B, Pang V, Isaac V, McLachlan CS, Kiat H, Herkes G. A Randomized Placebo-Controlled Study of a Transcranial Photobiomodulation Helmet in Parkinson’s Disease: Post-Hoc Analysis of Motor Outcomes. Journal of Clinical Medicine. 2023; 12(8):2846. https://doi.org/10.3390/jcm12082846
Chicago/Turabian StyleMcGee, Claire, Ann Liebert, Brian Bicknell, Vincent Pang, Vivian Isaac, Craig S. McLachlan, Hosen Kiat, and Geoffrey Herkes. 2023. "A Randomized Placebo-Controlled Study of a Transcranial Photobiomodulation Helmet in Parkinson’s Disease: Post-Hoc Analysis of Motor Outcomes" Journal of Clinical Medicine 12, no. 8: 2846. https://doi.org/10.3390/jcm12082846
APA StyleMcGee, C., Liebert, A., Bicknell, B., Pang, V., Isaac, V., McLachlan, C. S., Kiat, H., & Herkes, G. (2023). A Randomized Placebo-Controlled Study of a Transcranial Photobiomodulation Helmet in Parkinson’s Disease: Post-Hoc Analysis of Motor Outcomes. Journal of Clinical Medicine, 12(8), 2846. https://doi.org/10.3390/jcm12082846








