Risk of Gastric Cancer among Patients with Newly Diagnosed Ulcerative Colitis: A Nationwide Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
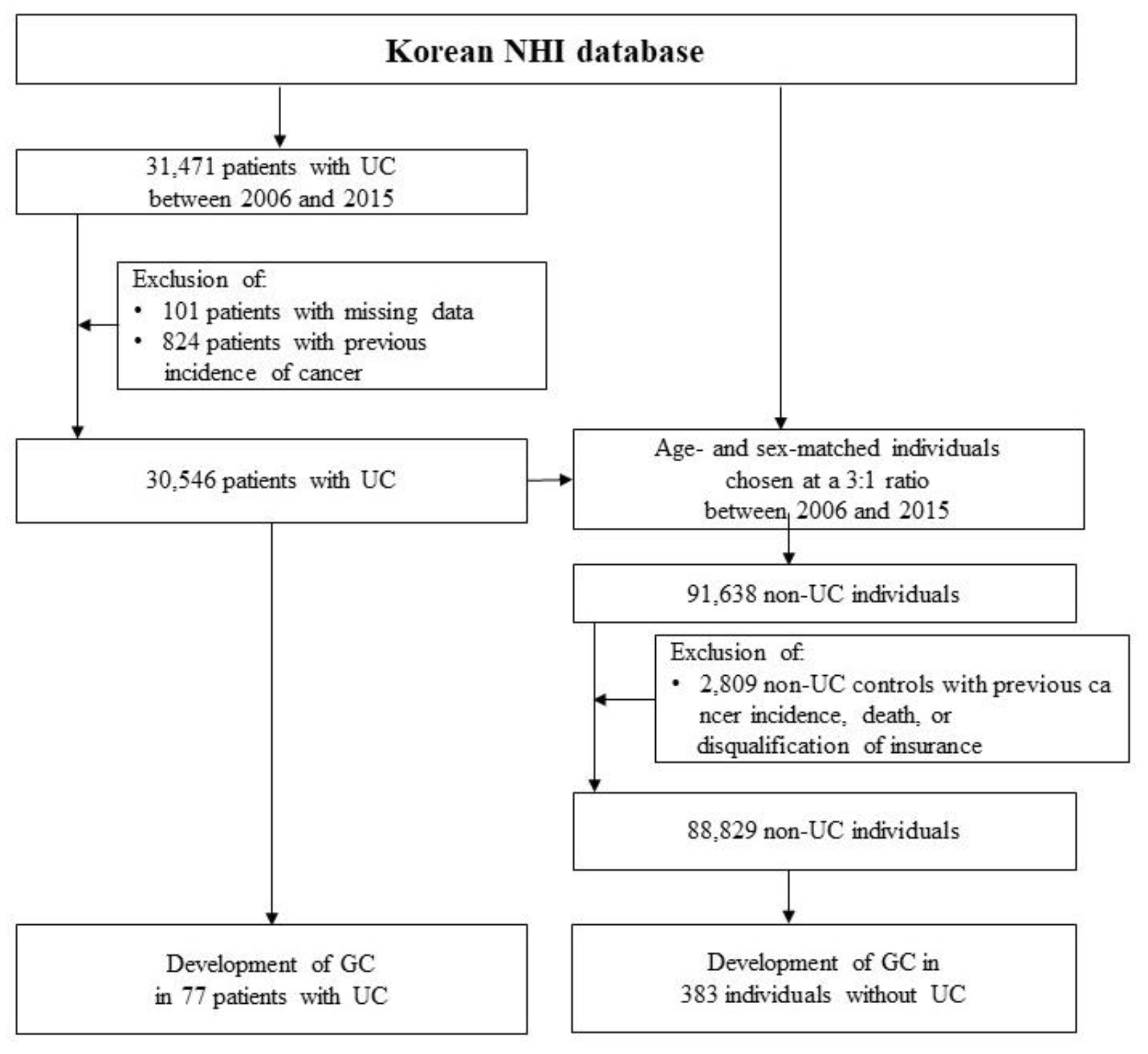
2.1. Study Population
2.2. Ethical Considerations
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Risk of GC in Patients with UC
3.3. Risk Factors for GC within Patients with UC
3.4. The Impact of 5-ASA on the Risk of GC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vavricka, S.R.; Brun, L.; Ballabeni, P.; Pittet, V.; Prinz Vavricka, B.M.; Zeitz, J.; Rogler, G.; Schoepfer, A.M. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am. J. Gastroenterol. 2011, 106, 110–119. [Google Scholar] [CrossRef]
- Jung, Y.S.; Han, M.; Park, S.; Kim, W.H.; Cheon, J.H. Cancer risk in the early stages of inflammatory bowel disease in Korean patients: A nationwide population-based study. J. Crohn’s Colitis 2017, 11, 954–962. [Google Scholar] [CrossRef]
- Pedersen, N.; Duricova, D.; Elkjaer, M.; Gamborg, M.; Munkholm, P.; Jess, T. Risk of extra-intestinal cancer in inflammatory bowel disease: Meta-analysis of population-based cohort studies. Am. J. Gastroenterol. 2010, 105, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.N.; Jess, T. Has the risk of colorectal cancer in inflammatory bowel disease decreased? World J. Gastroenterol. 2013, 19, 7561–7568. [Google Scholar] [CrossRef]
- Lutgens, M.W.; van Oijen, M.G.; van der Heijden, G.J.; Vleggaar, F.P.; Siersema, P.D.; Oldenburg, B. Declining risk of colorectal cancer in inflammatory bowel disease: An updated meta-analysis of population-based cohort studies. Inflamm. Bowel Dis. 2013, 19, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Jess, T.; Rungoe, C.; Peyrin-Biroulet, L. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of population-based cohort studies. Clin. Gastroenterol. Hepatol. 2012, 10, 639–645. [Google Scholar] [CrossRef]
- Jess, T.; Simonsen, J.; Jorgensen, K.T.; Pedersen, B.V.; Nielsen, N.M.; Frisch, M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology 2012, 143, 375–381. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Blanchard, J.F.; Kliewer, E.; Wajda, A. Cancer risk in patients with inflammatory bowel disease: A population-based study. Cancer 2001, 91, 854–862. [Google Scholar] [CrossRef]
- Jussila, A.; Virta, L.J.; Pukkala, E.; Farkkila, M.A. Malignancies in patients with inflammatory bowel disease: A nationwide register study in Finland. Scand. J. Gastroenterol. 2013, 48, 1405–1413. [Google Scholar] [CrossRef]
- So, J.; Tang, W.; Leung, W.K.; Li, M.; Lo, F.H.; Wong, M.T.L.; Sze, A.S.F.; Leung, C.M.; Tsang, S.W.C.; Shan, E.H.S.; et al. Cancer risk in 2621 Chinese patients with inflammatory bowel disease: A population-based cohort study. Inflamm. Bowel Dis. 2017, 23, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Yun, S.; Kim, J.H.; Park, J.Y.; Kim, H.Y.; Kim, Y.H.; Chang, D.K.; Kim, J.S.; Song, I.S.; Park, J.B.; et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986–2005: A KASID study. Inflamm. Bowel Dis. 2008, 14, 542–549. [Google Scholar] [CrossRef]
- Eom, B.W.; Jung, K.W.; Won, Y.J.; Yang, H.; Kim, Y.W. Trends in gastric cancer incidence according to the clinicopathological characteristics in Korea, 1999–2014. Cancer Res. Treat. 2018, 50, 1343–1350. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, J.H.; Lee, J.K.; Kang, D.R.; Kim, H.; Kim, S.Y.; Kim, H.S. Age- and sex-specific risk of colorectal cancer in incident ulcerative colitis during the first 10 years after diagnosis: A nationwide population-based study. Scand. J. Gastroenterol. 2021, 56, 1279–1285. [Google Scholar] [CrossRef]
- Kwak, M.S.; Cha, J.M.; Lee, H.H.; Choi, Y.S.; Seo, S.I.; Ko, K.J.; Park, D.I.; Kim, S.H.; Kim, T.J. Emerging trends of inflammatory bowel disease in South Korea: A nationwide population-based study. J. Gastroenterol. Hepatol. 2019, 34, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Ha, H.J.; Oh, S.J.; Kim, J.W.; Lee, J.K.; Kim, H.S.; Yoon, S.M.; Kang, S.B.; Kim, E.S.; Kim, T.O.; et al. Nationwide validation study of diagnostic algorithms for inflammatory bowel disease in Korean National Health Insurance Service database. J. Gastroenterol. Hepatol. 2020, 35, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J.; Kim, N.; Yun, C.Y.; Yoon, H.; Shin, C.M.; Park, Y.S.; Son, I.T.; Oh, H.K.; Kim, D.W.; Kang, S.B.; et al. Validation of administrative big database for colorectal cancer searched by international classification of disease 10th codes in Korean: A retrospective big-cohort study. J. Cancer Prev. 2018, 23, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Wan, Q.; Zhao, R.; Xia, L.; Wu, Y.; Zhou, Y.; Wang, Y.; Cui, Y.; Shen, X.; Wu, X.T. Inflammatory bowel disease and risk of gastric, small bowel and colorectal cancer: A meta-analysis of 26 observational studies. J. Cancer Res. Clin. Oncol. 2021, 147, 1077–1087. [Google Scholar] [CrossRef]
- Jung, K.-W.; Won, Y.-J.; Kong, H.-J.; Lee, E.S. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2015. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2018, 50, 303–316. [Google Scholar] [CrossRef]
- 2020 Inflammatory Bowel Disease Fact Sheet in Korea. Available online: http://m.kasid.org/file/IBD%20fact%20sheet_1217.pdf (accessed on 28 March 2023).
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar]
- Huang, J.Q.; Sridhar, S.; Chen, Y.; Hunt, R.H. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology 1998, 114, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Takada, K. Epstein-Barr virus and gastric carcinoma. Mol. Pathol. 2000, 53, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Konstantopoulos, P.; Mantzaris, G.J. Helicobacter pylori infection and inflammatory bowel disease: Is there a link? World J. Gastroenterol. 2014, 20, 6374–6385. [Google Scholar] [CrossRef]
- Song, M.J.; Park, D.I.; Hwang, S.J.; Kim, E.R.; Kim, Y.H.; Jang, B.I.; Lee, S.H.; Ji, J.S.; Shin, S.J. The prevalence of Helicobacter pylori infection in Korean patients with inflammatory bowel disease, a multicenter study. Korean J. Gastroenterol. 2009, 53, 341–347. [Google Scholar] [CrossRef]
- Wu, X.W.; Ji, H.Z.; Yang, M.F.; Wu, L.; Wang, F.Y. Helicobacter pylori infection and inflammatory bowel disease in Asians: A meta-analysis. World J. Gastroenterol. 2015, 21, 4750–4756. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.H.; Assendorp, E.L.; van der Post, R.S.; Derikx, L.A.; de Jong, D.J.; Kievit, W.; Pierik, M.; van den Heuvel, T.; Verhoeven, R.; Overbeek, L.I.; et al. Impaired gastric cancer survival in patients with inflammatory bowel disease. J. Gastrointest. Liver Dis. 2016, 25, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Itzkowitz, S.H. Colorectal cancer in inflammatory bowel disease: Mechanisms and management. Gastroenterology 2022, 162, 715–730. [Google Scholar] [CrossRef]
- Hsiao, S.W.; Yen, H.H.; Chen, Y.Y. Chemoprevention of colitis-associated dysplasia or cancer in inflammatory bowel disease. Gut Liver 2022, 16, 840–848. [Google Scholar] [CrossRef]
| Characteristic | UC Patients | Non-UC Individuals | p Value |
|---|---|---|---|
| Total | 30,546 | 88,829 | |
| Person-year | 158,380 | 467,232 | |
| Gastric cancer | 77 (0.25%) | 383 (0.43%) | <0.0001 |
| Person-year | 291 | 1413 | |
| Incidence rate (1/100,000 person-years) | 48.6 | 82.0 | |
| Sex (male) | 17,914 (58.65) | 52,032 (58.58) | 0.8291 |
| Age at diagnosis of UC * | |||
| Mean ± SD | 42.7 ± 16.1 | 42.3 ± 16.0 | <0.0001 |
| 0–19 | 2021 (6.62) | 6055 (6.82) | 0.0010 |
| 20–39 | 11,646 (38.13) | 34,450 (38.78) | |
| 40–59 | 11,848 (38.79) | 34,515 (38.86) | |
| ≥60 | 5031 (16.47) | 13,809 (15.55) | |
| Social economic status | <0.0001 | ||
| Low | 7218 (23.63) | 22,141 (24.93) | |
| Mid | 8934 (29.25) | 32,129 (36.17) | |
| High | 14,394 (47.12) | 34,559 (38.91) | |
| Residential area | <0.0001 | ||
| Urban | 28,099 (91.99) | 78,266 (88.11) | |
| Rural | 2424 (7.94) | 9274 (10.44) | |
| Comorbidities | |||
| HTN | 8548 (27.98) | 24,417 (27.49) | 0.0942 |
| DM | 7694 (25.19) | 19,408 (21.85) | <0.0001 |
| Cerebral vascular disease | 502 (1.64) | 969 (1.09) | <0.0001 |
| Cardiovascular disease | 504 (1.65) | 935 (1.05) | <0.0001 |
| Cholangitis | 652 (2.13) | 846 (0.95) | <0.0001 |
| CCI without tumor factor | <0.0001 | ||
| 0 | 8891 (29.11) | 31,466 (35.42) | |
| 1 | 10,203 (33.40) | 28,827 (32.45) | |
| 2 | 6016 (19.69) | 15,188 (17.10) | |
| ≥3 | 5436 (17.80) | 13,348 (15.03) | |
| IBD medication | |||
| Steroid | 14,170 (46.39) | 5801 (6.53) | <0.0001 |
| 5-ASA | 30,285 (99.15) | 25 (0.03) | <0.0001 |
| Thiopurines | 5181 (16.96) | 84 (0.09) | <0.0001 |
| Biologics | 1579 (5.17) | 30 (0.03) | <0.0001 |
| Follow-up period (years) | 5.11 ± 2.96 | 5.20 ± 2.95 | <0.0001 |
| Age | Age at Diagnosis (Year) | GC Cases | Adjusted HR a | 95% CI | p Value |
|---|---|---|---|---|---|
| Age groups | All | 77 | 0.60 | 0.47–0.77 | <0.0001 |
| 0–19 years | 1 | 5.81 | 0.05–714.65 | 0.473 | |
| 20–39 years | 1 | 0.19 | 0.04–0.98 | 0.047 | |
| 40–59 years | 35 | 0.65 | 0.45–0.94 | 0.021 | |
| ≥60 years | 40 | 0.60 | 0.49–0.80 | 0.004 | |
| Sex | Age at Diagnosis (year) | GC Cases | Adjusted HR a | 95% CI | p Value |
| Male | All | 54 | 0.54 | 0.41–0.73 | <0.001 |
| 0–19 years | 1 | 4.99 | 0.05–1.54 | 0.4893 | |
| 20–39 years | 0 | - | - | - | |
| 40–59 years | 21 | 0.51 | 0.32–0.81 | 0.0045 | |
| ≥60 years | 32 | 0.61 | 0.42–0.89 | 0.0111 | |
| Female | All | 23 | 0.81 | 0.51–1.27 | 0.3559 |
| 0–19 years | 0 | - | - | - | |
| 20–39 years | 1 | 0.49 | 0.08–2.89 | 0.4277 | |
| 40–59 years | 14 | 1.12 | 0.61–2.06 | 0.7232 | |
| ≥60 years | 8 | 0.62 | 0.29–1.31 | 0.2089 |
| Characteristic | UC Patients | ||
|---|---|---|---|
| Gastric Cancer | Non-Gastric Cancer | p Value | |
| Total number | 77 | 30,469 | |
| Person-years | 291 | 258,089 | |
| Sex (male) | 54 (70.13) | 17,860 (58.62) | 0.0405 |
| Age at diagnosis of UC | |||
| Mean ± SD | 59.73 ± 12.04 | 42.66 ± 16.11 | <0.0001 |
| 0–19 years | 1 (1.30) | 2020 (6.63) | <0.0001 |
| 20–39 years | 1 (1.30) | 11,645 (38.22) | |
| 40–59 years | 35 (45.45) | 11,813 (38.77) | |
| ≥60 years | 40 (51.95) | 4991 (16.38) | |
| Social economic status | 0.7381 | ||
| Low | 19 (24.68) | 7199 (23.63) | |
| Mid | 25 (32.47) | 8909 (29.24) | |
| High | 33 (42.86) | 14,361 (47.13) | |
| Residential area | 0.7237 | ||
| Urban | 35 (45.45) | 14,421 (47.37) | |
| Suburban | 34 (44.16) | 13,609 (44.70) | |
| Rural | 8 (10.39) | 2416 (7.94) | |
| Comorbidities | |||
| Hypertension | 40 (51.95) | 8508 (27.92) | <0.0001 |
| Diabetes Mellitus | 32 (41.56) | 7662 (25.15) | 0.0009 |
| Cerebral vascular disease | 2 (2.60) | 500 (1.64) | 0.3618 |
| Cardiovascular disease | 4 (5.19) | 500 (1.64) | 0.0386 |
| Cholangitis | 3 (3.90) | 649 (2.13) | 0.2268 |
| Modified CCI score 1 | <0.0001 | ||
| 0 | 9 (11.69) | 8882 (29.15) | |
| 1 | 14 (18.18) | 10,189 (33.44) | |
| 2 | 28 (36.36) | 5988 (19.65) | |
| ≥3 | 26 (33.77) | 5410 (17.76) | |
| IBD medication | |||
| Steroid | 39 (50.65) | 14,131 (46.38) | 0.4931 |
| 5-ASA | 75 (97.40) | 30,210 (99.15) | 0.1407 |
| Thiopurines | 6 (7.79) | 5175 (16.98) | 0.0321 |
| Biologics | 1 (1.30) | 1578 (5.18) | 0.1898 |
| Follow-up period (years) | 3.78 ± 2.83 | 5.19 ± 2.95 | <0.0001 |
| UC | |||
|---|---|---|---|
| Adjusted HR 1 | 95% CI | p Value | |
| Sex | |||
| Male | 1 | ||
| Female | 0.51 | 0.32–0.84 | 0.0077 |
| Age at diagnosis | |||
| 0–19 years | 1 | ||
| 20–39 years | 0.15 | 0.02–1.56 | 0.1125 |
| 40–59 years | 3.90 | 0.72–21.04 | 0.1133 |
| ≥60 years | 12.34 | 2.23–68.16 | 0.0040 |
| Social economic status | |||
| Low | 1 | ||
| Mid | 1.18 | 0.65–2.14 | 0.5959 |
| High | 0.78 | 0.44–1.37 | 0.3779 |
| Residential area | |||
| Urban | 1 | ||
| Suburban | 0.99 | 0.62–1.59 | 0.9688 |
| Rural | 0.93 | 0.43–1.98 | 0.8450 |
| Comorbidities | |||
| Hypertension | 0.90 | 0.54–1.49 | 0.6667 |
| Diabetes Mellitus | 0.92 | 0.57–1.29 | 0.7292 |
| Cerebral vascular disease | 0.75 | 0.21–2.71 | 0.660 |
| Cardiovascular disease | 1.58 | 0.59–4.19 | 0.3625 |
| Cholangitis | 1.79 | 0.61–5.25 | 0.2920 |
| Duration of Medication Use | UC | |||
|---|---|---|---|---|
| GC Cases | Adjusted HR a | 95% CI | p Value | |
| 5-ASA | ||||
| 1st quartile | 30 | 1 | ||
| 2nd quartile | 15 | 0.74 | 0.39–1.38 | 0.3373 |
| 3rd quartile | 12 | 0.44 | 0.22–0.87 | 0.0186 |
| 4th quartile | 20 | 0.42 | 0.24–0.75 | 0.0033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.M.; Kim, J.; Kim, H.; Park, S.C.; Lee, J.K.; Kang, D.R.; Kim, S.Y.; Kim, H.-S. Risk of Gastric Cancer among Patients with Newly Diagnosed Ulcerative Colitis: A Nationwide Population-Based Study. J. Clin. Med. 2023, 12, 2843. https://doi.org/10.3390/jcm12082843
Kim HM, Kim J, Kim H, Park SC, Lee JK, Kang DR, Kim SY, Kim H-S. Risk of Gastric Cancer among Patients with Newly Diagnosed Ulcerative Colitis: A Nationwide Population-Based Study. Journal of Clinical Medicine. 2023; 12(8):2843. https://doi.org/10.3390/jcm12082843
Chicago/Turabian StyleKim, Hee Man, Jihoon Kim, Hyunil Kim, Soon Chang Park, Jung Kuk Lee, Dae Ryong Kang, Su Young Kim, and Hyun-Soo Kim. 2023. "Risk of Gastric Cancer among Patients with Newly Diagnosed Ulcerative Colitis: A Nationwide Population-Based Study" Journal of Clinical Medicine 12, no. 8: 2843. https://doi.org/10.3390/jcm12082843
APA StyleKim, H. M., Kim, J., Kim, H., Park, S. C., Lee, J. K., Kang, D. R., Kim, S. Y., & Kim, H.-S. (2023). Risk of Gastric Cancer among Patients with Newly Diagnosed Ulcerative Colitis: A Nationwide Population-Based Study. Journal of Clinical Medicine, 12(8), 2843. https://doi.org/10.3390/jcm12082843






