Application of Artificial Intelligence in the Diagnosis, Treatment, and Prognostic Evaluation of Mediastinal Malignant Tumors
Abstract
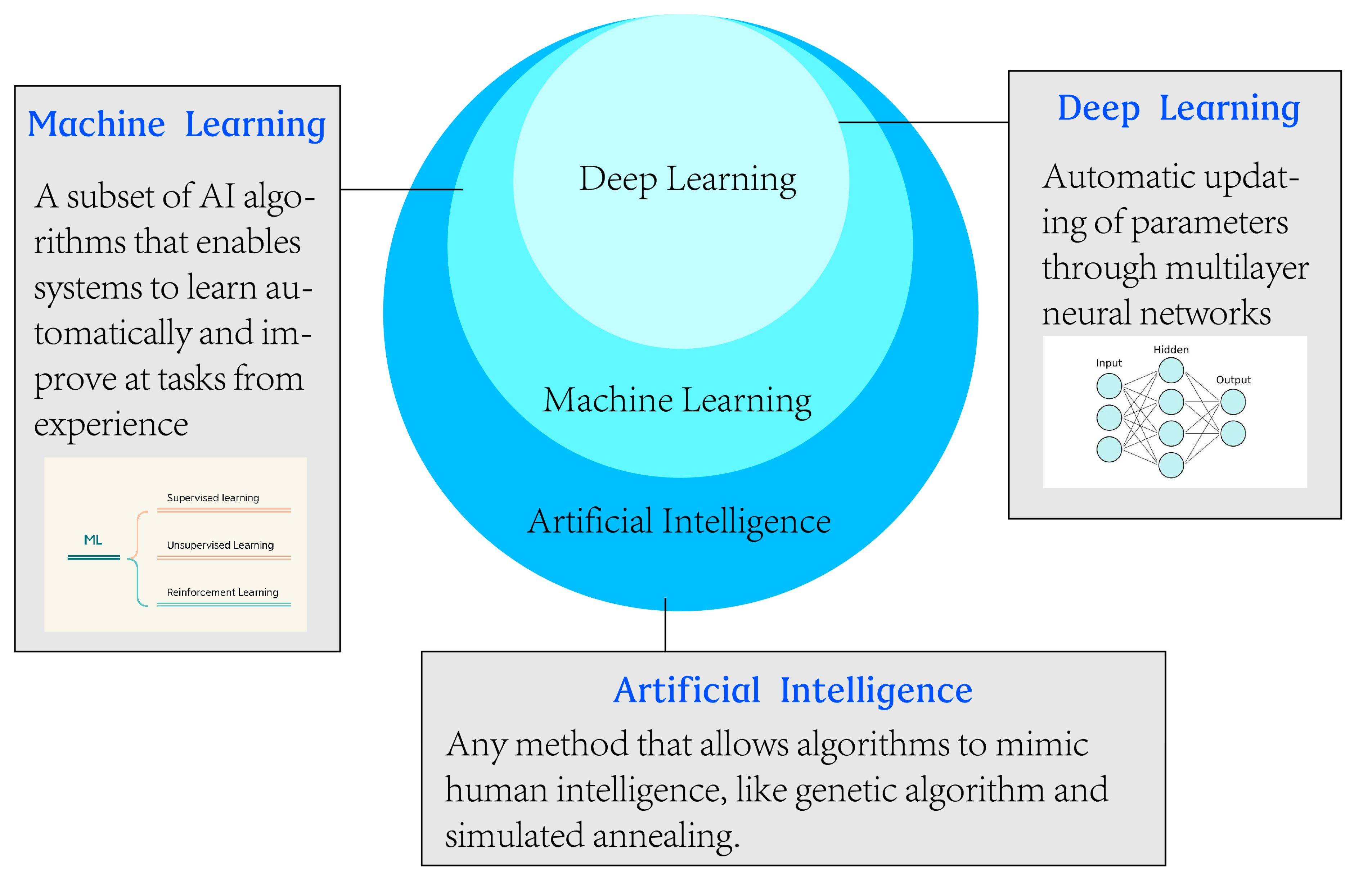
1. Introduction
2. Diagnosing Mediastinal Malignant Tumors
2.1. Diagnosis Using Imaging
2.2. Diagnosis by Pathological Examinations
3. Treatment of Mediastinal Malignancies
3.1. Surgical Resection
3.2. Chemotherapy
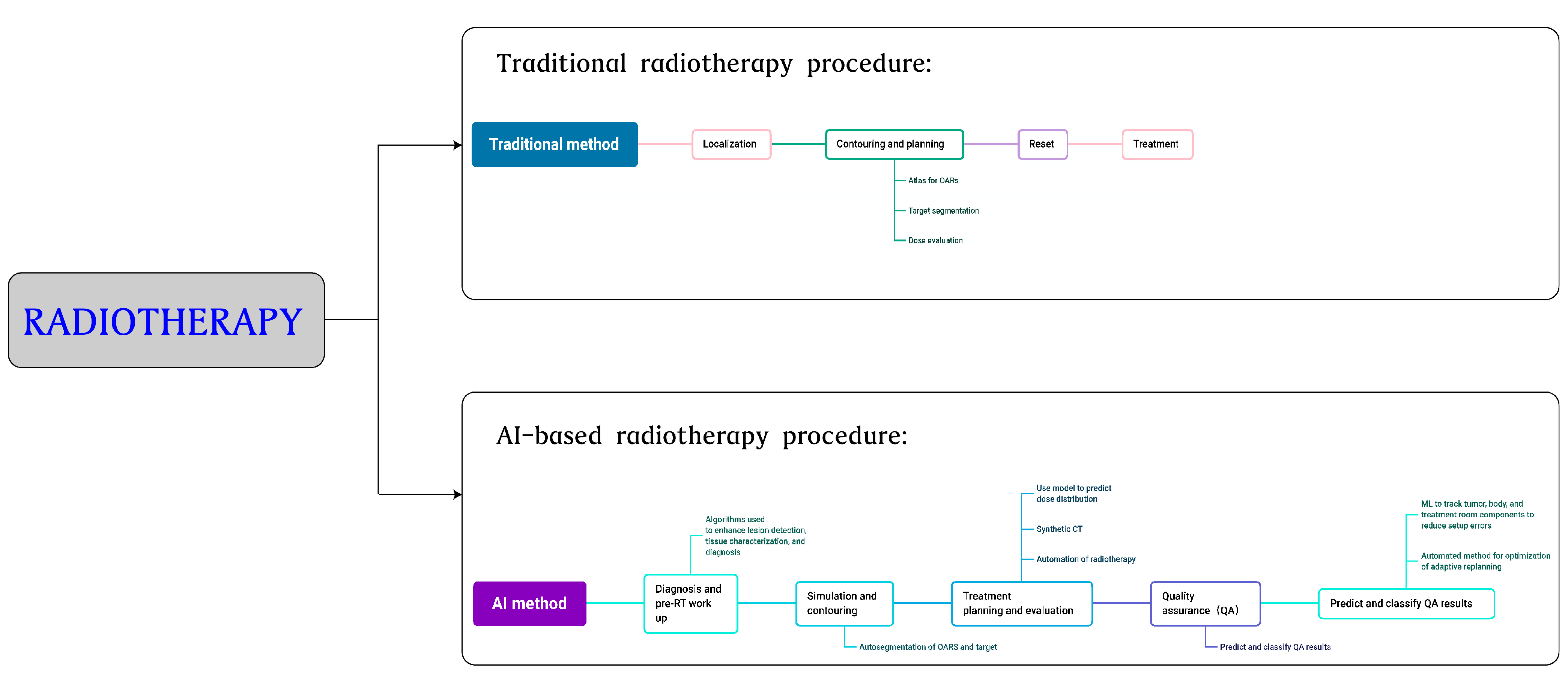
3.3. Radiotherapy
4. Prognostic Analysis
5. Prospects of Using Artificial Intelligence
6. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramesh, A.N.; Kambhampati, C.; Monson, J.R.; Drew, P.J. Artificial intelligence in medicine. Ann. R. Coll. Surg. Engl. 2004, 86, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Jean, A. A brief history of artificial intelligence. Med. Sci. 2020, 36, 1059–1067. [Google Scholar]
- Choi, R.Y.; Coyner, A.S.; Kalpathy-Cramer, J.; Chiang, M.F.; Campbell, J.P. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl. Vis. Sci. Technol. 2020, 9, 14. [Google Scholar] [PubMed]
- Hirschberg, J.; Manning, C.D. Advances in natural language processing. Science 2015, 349, 261–266. [Google Scholar] [CrossRef]
- Moravčík, M.; Schmid, M.; Burch, N.; Lisý, V.; Morrill, D.; Bard, N.; Davis, T.; Waugh, K.; Johanson, M.; Bowling, M. DeepStack: Expert-level artificial intelligence in heads-up no-limit poker. Science 2017, 356, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Milne-Ives, M.; de Cock, C.; Lim, E.; Shehadeh, M.H.; de Pennington, N.; Mole, G.; Normando, E.; Meinert, E. The Effectiveness of Artificial Intelligence Conversational Agents in Health Care: Systematic Review. J. Med. Internet Res. 2020, 22, e20346. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.A.D.A.; Hovenburg, A.R.; de de Lima, L.N.; Rodin, C.D.; Johansen, T.A.; Storvold, R.; Correia, C.A.M.; Haddad, D.B. Autonomous Unmanned Aerial Vehicles in Search and Rescue Missions Using Real-Time Cooperative Model Predictive Control. Sensors 2019, 19, 4067. [Google Scholar] [CrossRef] [PubMed]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, E.; Oliaro, A.; Novero, D.; Campisi, P.; Filosso, P.L. Neuroendocrine tumors of the thymus. Thorac. Surg. Clin. 2011, 21, 13–23. [Google Scholar] [CrossRef]
- Marx, A.; Chan, J.K.C.; Chalabreysse, L.; Dacic, S.; Detterbeck, F.; French, C.A.; Hornick, J.L.; Inagaki, H.; Jain, D.; Lazar, A.J.; et al. The 2021 WHO Classification of Tumors of the Thymus and Mediastinum: What Is New in Thymic Epithelial, Germ Cell, and Mesenchymal Tumors? J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2022, 17, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Arrossi, A.V.; Dermawan, J.K.; Bolen, M.; Raymond, D. Thymomas with Intravascular and Intracardiac Growth. Front. Oncol. 2022, 12, 881553. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Matsuno, Y.; Noguchi, M.; Mukai, K.; Asamura, H.; Goya, T.; Shimosato, Y. A review of 79 thymomas: Modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol. Int. 1994, 44, 359–367. [Google Scholar] [CrossRef]
- Vobugari, N.; Raja, V.; Sethi, U.; Gandhi, K.; Raja, K.; Surani, S.R. Advancements in Oncology with Artificial Intelligence—A Review Article. Cancers 2022, 14, 1349. [Google Scholar] [CrossRef] [PubMed]
- Aamir, S.; Rahim, A.; Aamir, Z.; Abbasi, S.F.; Khan, M.S.; Alhaisoni, M.; Khan, K.; Ahmad, J. Predicting Breast Cancer Leveraging Supervised Machine Learning Techniques. Comput. Math. Methods Med. 2022, 2022, 5869529. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; Schwartz, K.M.; Eckel, L.J.; Diehn, F.E.; Hunt, C.H.; Bartholmai, B.J.; Erickson, B.J.; Kallmes, D.F. The effects of changes in utilization and technological advancements of cross-sectional imaging on radiologist workload. Acad. Radiol. 2015, 22, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H. Artificial intelligence in radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef]
- Castellino, R.A. Computer aided detection (CAD): An overview. Cancer Imaging 2005, 5, 17–19. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, E.; Orhan, K.; Soydal, C.; Kahya, Y.; Tunc, S.S.; Celik, O.; Sak, S.D.; Cangir, A.K. Combined clinical and specific positron emission tomography/computed tomography-based radiomic features and machine-learning model in prediction of thymoma risk groups. Nucl. Med. Commun. 2022, 43, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Huang, Y.; Xiao, G.; Lan, B.; Jiang, G.; Tian, J. Predictive Features of Thymic Carcinoma and High-Risk Thymomas Using Random Forest Analysis. J. Comput. Assist. Tomogr. 2020, 44, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Yen, Y.-T.; Huang, L.-T.; Chen, T.-Y.; Liu, Y.-S.; Tang, S.-Y.; Huang, W.-L.; Chen, Y.-Y.; Lai, C.-H.; Fang, Y.-H.D.; et al. An MRI-Based Clinical-Perfusion Model Predicts Pathological Subtypes of Prevascular Mediastinal Tumors. Diagnostics 2022, 12, 889. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, R.; Chufal, K.; Ahmad, I.; Chhabra, A.; Jwala, M.; Pahuja, A.; Sharma, M.; Gairola, M. Artificial Intelligence Enabled Prognostic Modelling for Thymomas. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, e787. [Google Scholar] [CrossRef]
- Shen, Y.-T.; Chen, L.; Yue, W.-W.; Xu, H.-X. Artificial intelligence in ultrasound. Eur. J. Radiol. 2021, 139, 109717. [Google Scholar] [CrossRef] [PubMed]
- Seyyed-Kalantari, L.; Zhang, H.; McDermott, M.B.A.; Chen, I.Y.; Ghassemi, M. Underdiagnosis bias of artificial intelligence algorithms applied to chest radiographs in under-served patient populations. Nat. Med. 2021, 27, 2176–2182. [Google Scholar] [CrossRef] [PubMed]
- Bondeven, P.; Laurberg, S.; Hagemann-Madsen, R.H.; Pedersen, B.G. Suboptimal surgery and omission of neoadjuvant therapy for upper rectal cancer is associated with a high risk of local recurrence. Color. Dis. 2015, 17, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.A.; Kondrashova, O.; Bradley, A.; Williams, E.D.; Pearson, J.V.; Waddell, N. Deep learning in cancer diagnosis, prognosis and treatment selection. Genome Med. 2021, 13, 152. [Google Scholar] [CrossRef]
- Vu, T.H.; Mousavi, H.S.; Monga, V.; Rao, G.; Rao, U.K.A. Histopathological Image Classification Using Discriminative Feature-Oriented Dictionary Learning. IEEE Trans. Med. Imaging 2016, 35, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Tizhoosh, H.R.; Shah, S.; Choi, C.; Damaskinos, S.; Safarpoor, A.; Shafiei, S.; Babaie, M.; Diamandis, P.; Campbell, C.J.V.; et al. Pan-cancer diagnostic consensus through searching archival histopathology images using artificial intelligence. NPJ Digit. Med. 2020, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Mellors, R.C.; Silver, R. A micro-fluorometric scanner for the differential detection of cells; application of exfoliative cytology. Science 1951, 114, 356–360. [Google Scholar]
- Tolles, W.E.; Bostrom, R.C. Automatic screening of cytological smears for cancer: The instrumentation. Ann. N. Y. Acad. Sci. 1956, 63, 1211–1218. [Google Scholar] [CrossRef]
- Qiu, H.; Ding, S.; Liu, J.; Wang, L.; Wang, X. Applications of Artificial Intelligence in Screening, Diagnosis, Treatment, and Prognosis of Colorectal Cancer. Curr. Oncol. 2022, 29, 146. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, R.; Madesta, F.; Nielsen, M.; Krause, J.; Steurer, S.; Werner, R.; Rösch, T. Multi-scale fully convolutional neural networks for histopathology image segmentation: From nuclear aberrations to the global tissue architecture. Med. Image Anal. 2021, 70, 101996. [Google Scholar] [CrossRef] [PubMed]
- DiPalma, J.; Suriawinata, A.A.; Tafe, L.J.; Torresani, L.; Hassanpour, S. Resolution-based distillation for efficient histology image classification. Artif. Intell. Med. 2021, 119, 102136. [Google Scholar] [CrossRef]
- Ryu, H.S.; Jin, M.-S.; Park, J.H.; Lee, S.; Cho, J.; Oh, S.; Kwak, T.-Y.; Woo, J.I.; Mun, Y.; Kim, S.W.; et al. Automated Gleason Scoring and Tumor Quantification in Prostate Core Needle Biopsy Images Using Deep Neural Networks and Its Comparison with Pathologist-Based Assessment. Cancers 2019, 11, 1860. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Okuda, S.; Watanabe, Y.; Tajima, Y.; Nagahashi, M.; Ichikawa, H.; Nakano, M.; Sakata, J.; Takii, Y.; Kawasaki, T.; et al. Histopathological characteristics and artificial intelligence for predicting tumor mutational burden-high colorectal cancer. J. Gastroenterol. 2021, 56, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jia, Z.; Wang, L.-B.; Ai, Y.; Zhang, F.; Lai, M.; Chang, E.I.-C. Large scale tissue histopathology image classification, segmentation, and visualization via deep convolutional activation features. BMC Bioinform. 2017, 18, 281. [Google Scholar] [CrossRef] [PubMed]
- Kather, J.N.; Pearson, A.T.; Halama, N.; Jäger, D.; Krause, J.; Loosen, S.H.; Marx, A.; Boor, P.; Tacke, F.; Neumann, U.P.; et al. Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat. Med. 2019, 25, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- El Achi, H.; Khoury, J.D. Artificial Intelligence and Digital Microscopy Applications in Diagnostic Hematopathology. Cancers 2020, 12, 797. [Google Scholar] [CrossRef]
- Bejnordi, B.E.; Mullooly, M.; Pfeiffer, R.M.; Fan, S.; Vacek, P.M.; Weaver, D.L.; Herschorn, S.; Brinton, L.A.; van Ginneken, B.; Karssemeijer, N.; et al. Using deep convolutional neural networks to identify and classify tumor-associated stroma in diagnostic breast biopsies. Mod. Pathol. 2018, 31, 1502–1512. [Google Scholar]
- Coudray, N.; Ocampo, P.S.; Sakellaropoulos, T.; Narula, N.; Snuderl, M.; Fenyö, D.; Moreira, A.L.; Razavian, N.; Tsirigos, A. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat. Med. 2018, 24, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Leiro-Fernández, V.; Fernández-Villar, A. Mediastinal staging for non-small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Saito, Y.; Imaoka, H.; Saiko, M.; Yamada, S.; Kondo, H.; Takamaru, H.; Sakamoto, T.; Sese, J.; Kuchiba, A.; et al. Development of a real-time endoscopic image diagnosis support system using deep learning technology in colonoscopy. Sci. Rep. 2019, 9, 14465. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mittal, B.R.; Bhattacharya, A.; Singh, H.; Bal, A.; Prakash, G.; Singh, N. 18F-FDG PET/CT-Guided Real-Time Automated Robotic Arm-Assisted Needle Navigation for Percutaneous Biopsy of Hypermetabolic Bone Lesions: Diagnostic Performance and Clinical Impact. Am. J. Roentgenol. 2019, 212, W10–W18. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, K.E.; DeCamp, M.M.; Yang, A.D.; Bilimoria, K.Y.; Odell, D.D. Treatment Approaches and Outcomes for Primary Mediastinal Sarcoma: Analysis of 976 Patients. Ann. Thorac. Surg. 2018, 106, 333–339. [Google Scholar] [CrossRef]
- Luo, D.X.; Huang, M.J.; Xiong, B.; Li, T.; Xie, K.; Chen, F.R.; Che, G.W.; Wang, J.; Xu, Y.; Zhou, X.J.; et al. Primary mediastinal sarcoma: Surgical outcomes of 21 cases. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Zehani, A.; Ayadi-Kaddour, A.; Daghfous, H.; Ridene, I.; Marghli, A.; Kilani, T.; El Mezni, F. Primary mediastinal sarcomas. Rev. Des Mal. Respir. 2011, 28, 14–24. [Google Scholar]
- Abdel-Rahman, O. An analysis of clinical characteristics and patient outcomes in primary mediastinal sarcomas. Expert Rev. Anticancer. Ther. 2017, 17, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Suster, D.I. The role of molecular pathology in mediastinal sarcomas. Mediastinum 2020, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Capper, D.; Jones, D.T.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef]
- Wightman, S.C.; Shrager, J.B. Non-Myasthenia Gravis Immune Syndromes and the Thymus: Is There a Role for Thymectomy? Thorac. Surg. Clin. 2019, 29, 215–225. [Google Scholar]
- Issoufou, I.; Lakranbi, M.; Sani, R.; Belliraj, L.; Ammor, F.Z.; Ghalimi, J.; Ouadnouni, Y.; Smahi, M. Neurogenic mediastinal tumors in adults. Rev. Pneumol. Clin. 2016, 72, 310–315. [Google Scholar] [PubMed]
- Friedant, A.J.; Handorf, E.A.; Su, S.; Scott, W.J. Minimally Invasive versus Open Thymectomy for Thymic Malignancies: Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2016, 11, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Mehta, C.; Raparia, K.; Bharat, A. Anterior Mediastinal Myelolipoma. Ann. Thorac. Surg. 2017, 103, e81. [Google Scholar] [CrossRef] [PubMed]
- Nagahiro, I.; Andou, A.; Aoe, M.; Sano, Y.; Date, H.; Shimizu, N. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: A comparison of VATS and conventional procedure. Ann. Thorac. Surg. 2001, 72, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Dieter, R.A., Jr.; Kuzyçz, G.B. Complications and contraindications of thoracoscopy. Int. Surg. 1997, 82, 232–239. [Google Scholar]
- Raffort, J.; Adam, C.; Carrier, M.; Ballaith, A.; Coscas, R.; Jean-Baptiste, E.; Hassen-Khodja, R.; Chakfé, N.; Lareyre, F. Artificial intelligence in abdominal aortic aneurysm. J. Vasc. Surg. 2020, 72, 321–333.e1. [Google Scholar] [CrossRef]
- Huo, J.; Huang, G.; Han, D.; Wang, X.; Bu, Y.; Chen, Y.; Cai, D.; Zhao, C. Value of 3D preoperative planning for primary total hip arthroplasty based on artificial intelligence technology. J. Orthop. Surg. Res. 2021, 16, 156. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, P.M.; Kuijpers, M.V.; Khayyat, A.; Iftikhar, A.; De Sa, M.D. Artificial Intelligence: A New Paradigm in Obstetrics and Gynecology Research and Clinical Practice. Cureus 2020, 12, e7124. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Matsuura, Y.; Kondo, Y.; Ichinose, J.; Nakao, M.; Okumura, S.; Mun, M. The predictive power of artificial intelligence on mediastinal lymphnode metastasis. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- De Perrot, M.; McRae, K. Evidence for thymectomy in myasthenia gravis: Getting stronger? J. Thorac. Cardiovasc. Surg. 2017, 154, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Amore, D.; Cicalese, M.; Scaramuzzi, R.; Di Natale, D.; Casazza, D.; Curcio, C. Hybrid robotic thoracic surgery for excision of large mediastinal masses. J. Vis. Surg. 2018, 4, 105. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, Q.; Wang, S.; Zhang, H.; Huang, D. Surgical treatment of posterior mediastinal neurogenic tumors. J. Surg. Oncol. 2019, 119, 807–813. [Google Scholar] [CrossRef]
- Weksler, B.; Tavares, J.; Newhook, T.E.; Greenleaf, C.E.; Diehl, J.T. Robot-assisted thymectomy is superior to transsternal thymectomy. Surg. Endosc. 2012, 26, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Seong, Y.W.; Kang, C.H.; Choi, J.-W.; Kim, H.-S.; Jeon, J.H.; Park, I.K.; Kim, Y.T. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: Its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur. J. Cardiothorac. Surg. 2014, 45, e68–e73; discussion e73. [Google Scholar] [CrossRef]
- Loftus, T.J.; Tighe, P.J.; Filiberto, A.C.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Rashidi, P.; Upchurch, G.R.; Bihorac, A. Artificial Intelligence and Surgical Decision-making. JAMA Surg. 2020, 155, 148–158. [Google Scholar] [CrossRef]
- Cakar, F.; Werner, P.; Augustin, F.; Schmid, T.; Wolf-Magele, A.; Sieb, M.; Bodner, J. A comparison of outcomes after robotic open extended thymectomy for myasthenia gravis. Eur. J. Cardio-Thorac. Surg. 2007, 31, 501–504; discussion 504–505. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, S.; Zirafa, C.C.; Davini, F.; Melfi, F. How to get the best from robotic thoracic surgery. J. Thorac. Dis. 2018, 10 (Suppl. S8), S947–S950. [Google Scholar] [CrossRef]
- Gumbs, A.A.; Frigerio, I.; Spolverato, G.; Croner, R.; Illanes, A.; Chouillard, E.; Elyan, E. Artificial Intelligence Surgery: How Do We Get to Autonomous Actions in Surgery? Sensors 2021, 21, 5526. [Google Scholar] [CrossRef]
- Berishvili, V.; Voronkov, A.E.; Radchenko, E.V.; Palyulin, V.A. Palyulin, Machine Learning Classification Models to Improve the Docking-based Screening: A Case of PI3K-Tankyrase Inhibitors. Mol. Inform. 2018, 37, e1800030. [Google Scholar] [PubMed]
- Sakellaropoulos, T.; Vougas, K.; Narang, S.; Koinis, F.; Kotsinas, A.; Polyzos, A.; Moss, T.J.; Piha-Paul, S.; Zhou, H.; Kardala, E.; et al. A Deep Learning Framework for Predicting Response to Therapy in Cancer. Cell Rep. 2019, 29, 3367–3373.e4. [Google Scholar] [CrossRef]
- Janssen, B.V.; Theijse, R.; van Roessel, S.; de Ruiter, R.; Berkel, A.; Huiskens, J.; Busch, O.R.; Wilmink, J.W.; Kazemier, G.; Valkema, P.; et al. Artificial Intelligence-Based Segmentation of Residual Tumor in Histopathology of Pancreatic Cancer after Neoadjuvant Treatment. Cancers 2021, 13, 5089. [Google Scholar] [PubMed]
- Moussa, H.G.; Husseini, G.A.; Abel-Jabbar, N.; Ahmad, S.E. Use of Model Predictive Control and Artificial Neural Networks to Optimize the Ultrasonic Release of a Model Drug from Liposomes. IEEE Trans. Nanobioscience 2017, 16, 149–156. [Google Scholar] [CrossRef]
- Mak, K.-K.; Pichika, M.R. Artificial intelligence in drug development: Present status and future prospects. Drug Discov. Today 2019, 24, 773–780. [Google Scholar] [CrossRef]
- Patel, V.; Shah, M. Artificial intelligence and machine learning in drug discovery and development. Intell. Med. 2022, 2, 134–140. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Sumner, J.; Ling, L.H.; Quek, R.H.C.; Tan, A.T.H.; Teng, G.G.; Seetharaman, S.K.; Gollamudi, S.P.K.; Ho, D.; Motani, M. Personalised Dosing Using the CURATE.AI Algorithm: Protocol for a Feasibility Study in Patients with Hypertension and Type II Diabetes Mellitus. Int. J. Environ. Res. Public Health 2022, 19, 8979. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Malik, A.; Halim, M.U.; Ali, A.M. The use of robotics in surgery: A review. Int. J. Clin. Pract. 2014, 68, 1376–1382. [Google Scholar] [CrossRef]
- Zhao, J.; Bhatnagar, V.; Ding, L.; Atay, S.M.; David, E.A.; McFadden, P.M.; Stamnes, S.; Lechtholz-Zey, E.; Wightman, S.C.; Detterbeck, F.C.; et al. A systematic review of paraneoplastic syndromes associated with thymoma: Treatment modalities, recurrence, and outcomes in resected cases. J. Thorac. Cardiovasc. Surg. 2020, 160, 306–314.e14. [Google Scholar] [CrossRef]
- Thummerer, A.; Seller Oria, C.; Zaffino, P.; Visser, S.; Meijers, A.; Guterres Marmitt, G.; Wijsman, R.; Seco, J.; Langendijk, J.A.; Knopf, A.C.; et al. Deep learning-based 4D-synthetic CTs from sparse-view CBCTs for dose calculations in adaptive proton therapy. Med. Phys. 2022, 49, 6824–6839. [Google Scholar] [CrossRef]
- Yuzhen, N.W.; Barrett, S. A review of automatic lung tumour segmentation in the era of 4DCT. Rep. Pract. Oncol. Radiother. 2019, 24, 208–220. [Google Scholar]
- Lustberg, T.; van Soest, J.; Gooding, M.; Peressutti, D.; Aljabar, P.; van der Stoep, J.; van Elmpt, W.; Dekker, A. Clinical evaluation of atlas and deep learning based automatic contouring for lung cancer. Radiother. Oncol. 2018, 126, 312–317. [Google Scholar] [CrossRef]
- Nguyen, D.; Long, T.; Jia, X.; Lu, W.; Gu, X.; Iqbal, Z.; Jiang, S. A feasibility study for predicting optimal radiation therapy dose distributions of prostate cancer patients from patient anatomy using deep learning. Sci. Rep. 2019, 9, 1076. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, X.; Hong, J.C.; Zheng, D. Artificial Intelligence in Radiotherapy Treatment Planning: Present and Future. Technol. Cancer Res. Treat. 2019, 18, 1533033819873922. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fan, J.; Li, M.; Yan, H.; Hu, Z.; Huang, P.; Tian, Y.; Miao, J.; Dai, J. A deep learning method for prediction of three-dimensional dose distribution of helical tomotherapy. Med. Phys. 2019, 46, 1972–1983. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Li, T.; Wu, Q.; Yin, F.F. Adaptive radiation therapy: Technical components and clinical applications. Cancer J. 2011, 17, 182–189. [Google Scholar] [CrossRef]
- Schwaab, J.; Prall, M.; Sarti, C.; Kaderka, R.; Bert, C.; Kurz, C.; Parodi, K.; Günther, M.; Jenne, J. Ultrasound tracking for intra-fractional motion compensation in radiation therapy. Phys. Med. 2014, 30, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Chen, Y.; Zhang, Y.; Ge, Y.; Yin, F.-F.; Ren, L. Augmentation of CBCT Reconstructed from Under-Sampled Projections Using Deep Learning. IEEE Trans. Med. Imaging 2019, 38, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Madesta, F.; Sentker, T.; Gauer, T.; Werner, R. Self-contained deep learning-based boosting of 4D cone-beam CT reconstruction. Med. Phys. 2020, 47, 5619–5631. [Google Scholar] [CrossRef] [PubMed]
- Maspero, M.; Savenije, M.H.F.; Dinkla, A.M.; Seevinck, P.R.; Intven, M.P.W.; Juergenliemk-Schulz, I.M.; Kerkmeijer, L.G.W.; Berg, C.A.T.V.D. Dose evaluation of fast synthetic-CT generation using a generative adversarial network for general pelvis MR-only radiotherapy. Phys. Med. Biol. 2018, 63, 185001. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Fu, Y.; Wang, T.; Liu, Y.; Patel, P.; Curran, W.J.; Liu, T.; Yang, X. 4D-CT deformable image registration using multiscale unsupervised deep learning. Phys. Med. Biol. 2020, 65, 085003. [Google Scholar] [CrossRef]
- Pei, Q.; Luo, Y.; Chen, Y.; Li, J.; Xie, D.; Ye, T. Artificial intelligence in clinical applications for lung cancer: Diagnosis, treatment and prognosis. Clin. Chem. Lab. Med. 2022, 60, 1974–1983. [Google Scholar] [CrossRef]
- Bychkov, D.; Linder, N.; Turkki, R.; Nordling, S.; Kovanen, P.E.; Verrill, C.; Walliander, M.; Lundin, M.; Haglund, C.; Lundin, J. Deep learning based tissue analysis predicts outcome in colorectal cancer. Sci. Rep. 2018, 8, 3395. [Google Scholar] [CrossRef]
- Courtiol, P.; Maussion, C.; Moarii, M.; Pronier, E.; Pilcer, S.; Sefta, M.; Manceron, P.; Toldo, S.; Zaslavskiy, M.; Le Stang, N.; et al. Deep learning-based classification of mesothelioma improves prediction of patient outcome. Nat. Med. 2019, 25, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; She, Y.; Deng, J.; Chen, S.; Wang, T.; Yang, M.; Ma, M.; Song, Y.; Qi, H.; Wang, Y.; et al. Deep Learning for Prediction of N2 Metastasis and Survival for Clinical Stage I Non-Small Cell Lung Cancer. Radiology 2022, 302, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Purushotham, S.; Jiang, B.; Mandelbaum, R.S.; Takiuchi, T.; Liu, Y.; Roman, L.D. Survival outcome prediction in cervical cancer: Cox models vs deep-learning model. Am. J. Obstet. Gynecol. 2019, 220, 381.e1–381.e14. [Google Scholar] [CrossRef] [PubMed]
- Sailer, F.; Pobiruchin, M.; Bochum, S.; Martens, U.M.; Schramm, W. Prediction of 5-Year Survival with Data Mining Algorithms. Stud. Health Technol. Inform. 2015, 213, 75–78. [Google Scholar]
- Skrede, O.J.; De Raedt, S.; Kleppe, A.; Hveem, T.S.; Liestøl, K.; Maddison, J.; Askautrud, H.A.; Pradhan, M.; Nesheim, J.A.; Albregtsen, F.; et al. Deep learning for prediction of colorectal cancer outcome: A discovery and validation study. Lancet 2020, 395, 350–360. [Google Scholar] [CrossRef]
- Blasiak, A.; Khong, J.; Kee, T. CURATE.AI: Optimizing Personalized Medicine with Artificial Intelligence. SLAS Technol. 2020, 25, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-C.; Chen, S.-W.; Wu, K.-C.; Hsieh, T.-C.; Liang, J.-A.; Hung, Y.-C.; Yeh, L.-S.; Chang, W.-C.; Lin, W.-C.; Yen, K.-Y.; et al. Prediction of local relapse and distant metastasis in patients with definitive chemoradiotherapy-treated cervical cancer by deep learning from [(18)F]-fluorodeoxyglucose positron emission tomography/computed tomography. Eur. Radiol. 2019, 29, 6741–6749. [Google Scholar] [PubMed]
- Aramendía-Vidaurreta, V.; Cabeza, R.; Villanueva, A.; Navallas, J.; Alcázar, J.L. Ultrasound Image Discrimination between Benign and Malignant Adnexal Masses Based on a Neural Network Approach. Ultrasound Med. Biol. 2016, 42, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, J.; Liu, L. Improved Deep Learning Network Based in combination with Cost-sensitive Learning for Early Detection of Ovarian Cancer in Color Ultrasound Detecting System. J. Med. Syst. 2019, 43, 251. [Google Scholar] [PubMed]
- Pergialiotis, V.; Pouliakis, A.; Parthenis, C.; Damaskou, V.; Chrelias, C.; Papantoniou, N.; Panayiotides, I. The utility of artificial neural networks and classification and regression trees for the prediction of endometrial cancer in postmenopausal women. Public Health 2018, 164, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.B.; Vitzthum, L.K.; Mell, L.K. Challenge of Directly Comparing Imaging-Based Diagnoses Made by Machine Learning Algorithms with Those Made by Human Clinicians. J. Clin. Oncol. 2020, 38, 1868–1869. [Google Scholar] [CrossRef]
- Goecks, J.; Jalili, V.; Heiser, L.M.; Gray, J.W. How Machine Learning Will Transform Biomedicine. Cell 2020, 181, 92–101. [Google Scholar] [CrossRef]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.C.A.; Prudêncio, R.B.C.; Costa, I.G. A Drug-Target Network-Based Supervised Machine Learning Repurposing Method Allowing the Use of Multiple Heterogeneous Information Sources. Methods Mol. Biol. 2019, 1903, 281–289. [Google Scholar]
- Baskin, I.I. The power of deep learning to ligand-based novel drug discovery. Expert Opin. Drug Discov. 2020, 15, 755–764. [Google Scholar] [CrossRef]
- Ballester, P.J. Machine Learning for Molecular Modelling in Drug Design. Biomolecules 2019, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Bhinder, B.; Gilvary, C.; Madhukar, N.S.; Elemento, O. Artificial Intelligence in Cancer Research and Precision Medicine. Cancer Discov. 2021, 11, 900–915. [Google Scholar] [CrossRef]
- Gayvert, K.M.; Madhukar, N.S.; Elemento, O. A Data-Driven Approach to Predicting Successes and Failures of Clinical Trials. Cell Chem. Biol. 2016, 23, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.F.; Teixeira, A.L.; Pinheiro, L.; Falcao, A.O. A Bayesian approach to in silico blood-brain barrier penetration modeling. J. Chem. Inf. Model. 2012, 52, 1686–1697. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Cheng, F.; Xu, Y.; Li, W.; Tang, Y. Estimation of ADME properties with substructure pattern recognition. J. Chem. Inf. Model. 2010, 50, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K.; et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 2017, 171, 1437–1452.e17. [Google Scholar] [CrossRef] [PubMed]
- Kadurin, A.; Aliper, A.; Kazennov, A.; Mamoshina, P.; Vanhaelen, Q.; Khrabrov, K.; Zhavoronkov, A. The cornucopia of meaningful leads: Applying deep adversarial autoencoders for new molecule development in oncology. Oncotarget 2017, 8, 10883–10890. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J.; Gönen, M.; Margineantu, D.H.; Handeli, S.; Swanger, J.; Hoellerbauer, P.; Paddison, P.J.; Gu, H.; Raftery, D.; Grim, J.E.; et al. Pan-cancer transcriptional signatures predictive of oncogenic mutations reveal that Fbw7 regulates cancer cell oxidative metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 5462–5467. [Google Scholar] [CrossRef] [PubMed]
- Poplin, R.; Chang, P.-C.; Alexander, D.; Schwartz, S.; Colthurst, T.; Ku, A.; Newburger, D.; Dijamco, J.; Nguyen, N.; Afshar, P.T.; et al. A universal SNP and small-indel variant caller using deep neural networks. Nat. Biotechnol. 2018, 36, 983–987. [Google Scholar] [CrossRef]
- Cirillo, D.; Núñez-Carpintero, I.; Valencia, A. Artificial intelligence in cancer research: Learning at different levels of data granularity. Mol. Oncol. 2021, 15, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Troyanskaya, O.; Trajanoski, Z.; Carpenter, A.; Thrun, S.; Razavian, N.; Oliver, N. Artificial intelligence and cancer. Nat. Cancer 2020, 1, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Shen, P.-C.; Chen, C.-Y.; Hsu, A.-N.; Cho, Y.-C.; Lai, Y.-L.; Chen, F.-H.; Li, C.-Y.; Wang, S.-C.; Chen, M.; et al. DriverDBv3: A multi-omics database for cancer driver gene research. Nucleic Acids Res. 2020, 48, D863–D870. [Google Scholar] [CrossRef]
- Chaudhary, K.; Poirion, O.B.; Lu, L.; Garmire, L.X. Deep Learning-Based Multi-Omics Integration Robustly Predicts Survival in Liver Cancer. Clin. Cancer Res. 2018, 24, 1248–1259. [Google Scholar] [CrossRef]
- Malik, V.; Kalakoti, Y.; Sundar, D. Deep learning assisted multi-omics integration for survival and drug-response prediction in breast cancer. BMC Genom. 2021, 22, 214. [Google Scholar] [CrossRef] [PubMed]
- Poirion, O.B.; Jing, Z.; Chaudhary, K.; Huang, S.; Garmire, L.X. DeepProg: An ensemble of deep-learning and machine-learning models for prognosis prediction using multi-omics data. Genome Med. 2021, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, J.; Xiu, W.; Ma, X. Application of Artificial Intelligence in the Diagnosis, Treatment, and Prognostic Evaluation of Mediastinal Malignant Tumors. J. Clin. Med. 2023, 12, 2818. https://doi.org/10.3390/jcm12082818
Pang J, Xiu W, Ma X. Application of Artificial Intelligence in the Diagnosis, Treatment, and Prognostic Evaluation of Mediastinal Malignant Tumors. Journal of Clinical Medicine. 2023; 12(8):2818. https://doi.org/10.3390/jcm12082818
Chicago/Turabian StylePang, Jiyun, Weigang Xiu, and Xuelei Ma. 2023. "Application of Artificial Intelligence in the Diagnosis, Treatment, and Prognostic Evaluation of Mediastinal Malignant Tumors" Journal of Clinical Medicine 12, no. 8: 2818. https://doi.org/10.3390/jcm12082818
APA StylePang, J., Xiu, W., & Ma, X. (2023). Application of Artificial Intelligence in the Diagnosis, Treatment, and Prognostic Evaluation of Mediastinal Malignant Tumors. Journal of Clinical Medicine, 12(8), 2818. https://doi.org/10.3390/jcm12082818






