Abstract
Neonatal hemochromatosis (NH) is an uncommon, severe disorder that results in fetal loss or neonatal death due to liver failure. NH is currently regarded as the phenotypic expression of gestational alloimmune liver disease (GALD). The diagnosis of NH-GALD is rarely prenatally established. In addition to providing a systematic review of the prenatal features that are identifiable using ultrasound (US) and MRI, we suggest a prenatal diagnosis algorithm for use in suspected NH during the first affected pregnancy. From a total of 586 database entries identified in PubMed, Google Scholar, and ResearchGate, we selected 18 studies published from 1993 to 2021 that reported maternal medical and obstetric history, prenatal ultrasound findings, and postpartum outcomes. We investigated the ultrasound and MRI features of these studies, along with the outcome due to this condition. A total of 74 cases were identified. The main reported prenatal US finding was fetal growth restriction (FGR) (33%), followed by oligohydramnios (13%) and hydrops fetalis (13%), with 13% cases described as uneventful. Other rare prenatal findings were fetal anemia, ascites, and abnormal fetal liver and spleen. Most pregnancies ended with fetal/perinatal death or therapeutic interruption of pregnancy. Favorable evolution with treatment (ensanguine transfusion and intravenous immunoglobulin (IVIG)) was reported for only 7% of fetuses. Using T2-weighted MRI, fetal extrahepatic siderosis confirmed prenatally in two cases and postnatally in 11 cases. IVIG treatment throughout subsequent pregnancies was found to significantly improve fetal prognosis. MRI should be indicated in selected cases of oligohydramnios, fetal hydrops, fetal hepatomegaly, ascites, or unexplained FGR or anemia after ruling out all other more frequently encountered conditions. MRI can be used to detect iron overload in the liver and extrahepatic siderosis.
1. Introduction
Neonatal hemochromatosis (NH) (OMIM 231100) is defined as a severe liver illness accompanied by extrahepatic siderosis in newborns [1]. Evidence points to gestational disease, mainly manifested in utero by fetal liver injury, receiving the name of congenital hemochromatosis [2]. While causality was originally thought to lie in iron metabolism defects, it is now known to be an effect of severe fetal injury, with more than 95% of cases being linked to gestational alloimmune liver disease (GALD) [3]. In rare cases, NH may be caused by other conditions, such as certain perinatal infections (parvovirus B19 or cytomegalovirus), GRACILE (growth retardation, amino-aciduria, cholestasis, iron overload, lactic acidosis, and early death) syndrome, mitochondrial deoxyribonucleic acid depletion from deoxyguanosine kinase deficiency gene mutations, trichohepatoenteric syndrome, deficiency of delta 4-3-oxosteroid 5 beta-reductase due to defects in the synthesis of this bile acid, or 21 trisomy [1,4,5].
While the actual incidence of NH is uncertain (a 2018 study estimated the incidence of GALD in the United States to be 4 per 10,000 live births), relevant findings have resulted in reclassification of NH as both congenital and familial [1,6]. Even if preceding pregnancies have resulted in healthy live births, once the alloimmune reaction occurs, up to 90% of the affected women’s further pregnancies will be impacted by fetal liver disease, regardless of changes in the father [3]. Moreover, the affliction does not occur in pregnancies of other maternal siblings [7].
Information regarding fetal hemochromatosis remains scarce. Since its initial description, evidence has largely been gathered from case reports or case series [8].
NH is often only diagnosed at autopsy, and failure to acquire a correct diagnosis in stillbirths or neonatal deaths with liver failure leads to dire consequences for the mother’s future pregnancies [7]. Left untreated, GALD leads to fetal/neonatal death, making it necessary to consider GALD as a possible cause in all cases of liver disease and unexplained stillbirth, neonatal demise, or early infant death [1]. Because of these findings, the course of diagnosis and treatment of NH has shifted from neonatal to in utero [7].
From 18 weeks of gestation, intrauterine signs that could suggest NH are fetal growth restriction (FGR), oligohydramnios, fetal hydrops, fetal hepatomegaly, ascites, prematurity, unexplained anemia, or fetal demise in the late-second and third trimesters [1,3,9]. These signs are observed early in known affected mothers and lead to a clear diagnosis. However, in the absence of positive maternal history, the diagnosis of NH is easily overlooked, as these ultrasound (US) findings are often encountered in more common pathologies. One method that could lead to prenatal diagnosis of NH in the first affected pregnancy is fetal MRI. MRI is an acceptable, non-invasive method that does not involve significant risks and can be used to confirm hepatic and extrahepatic siderosis in utero or in the first postnatal hours [4].
The current review aims to gather the available evidence regarding the antenatal features related to NH-GALD and create an algorithm for potential assessment when there is prenatal suspicion of this rare pathology characterized by a severe prognosis, wherein selected fetuses will be referred for MRI and the initiation of specific treatment where appropriate.
2. Materials and Methods
We conducted a literature review on the subject, focusing on reports of patients diagnosed with NH and with confirmed extrahepatic siderosis that reported maternal medical and obstetric history, prenatal ultrasound findings, and postpartum outcome.
Despite the strong impact of this condition on present and future pregnancies, the prenatal findings that might suggest fetal NH-GALD are often non-specific, leading to an infrequent prenatal diagnosis; therefore, we used strict inclusion criteria.
The protocol for systematic review was written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. The review was not registered. The literature review was conducted through systematic searches of the PubMed, Google Scholar, and ResearchGate medical databases, independently conducted by A.S. and R.PS, for studies reported from January 1993 to December 2021, with no restrictions on study design. The keyword term used in searching was “neonatal hemochromatosis”. Additional articles were identified from the reference lists cited in the included articles. We used bibliography software (Zotero version 6.0.23) to collect all query entries and to remove duplicates based on the title, author(s), and journal names.
The studies were screened for relevance (abstracts and then full-text articles) based on the PICOS criteria and comprised case reports, case series, and observational studies (study design) of patients diagnosed with NH. These studies reported maternal medical and obstetric history and prenatal ultrasound findings (population), confirmed extrahepatic siderosis (intervention), and the postpartum outcome (outcome).
Papers with no description of prenatal evolution of the pregnancy or cases where extrahepatic siderosis was not confirmed (the absence of NH diagnosis is debatable) were excluded. We considered papers in English, French, and Spanish.
Using the search method, 586 articles were identified from the databases. A total of 128 duplicate articles were removed from the screening, and five were written in languages other than those being considered. Of the 453 articles remaining after screening by title and abstract, 398 were excluded as they referred to another subject. Of the 50 articles assessed for eligibility, 18 met the inclusion criteria (Figure 1).
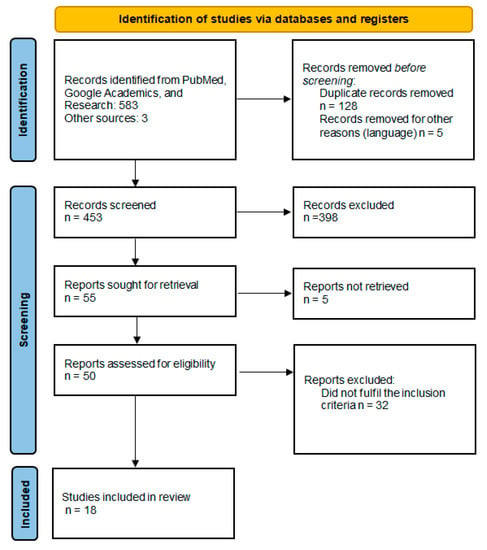
Figure 1.
Details of the literature review—PRISMA diagram.
3. Results
The 74 cases of postnatally diagnosed NH with confirmed extrahepatic siderosis found in the current literature review are listed in Table 1 (Table 1). For each case, all the available data are presented, including maternal medical and obstetric history, prenatal findings, postpartum outcome, and subsequent pregnancy outcome, if available. We identified fourteen case reports and three retrospective observational studies.

Table 1.
Literature review of the cases diagnosed with neonatal hemochromatosis, with confirmed extrahepatic siderosis (1993 to 2021) and maternal medical and obstetric history, prenatal findings, postpartum outcome, and subsequent pregnancy outcome.
NH was reported to affect mainly multiparous, healthy gravidas at a mean of 33 weeks of gestational age (WGA) (range 21 to 40). A small percentage of the gravidas presented autoimmune pathologies, such as systemic erythematosus lupus (4%), autoimmune thyroiditis (4%), or were part of a consanguineous couple (4%). A significant percentage of the patients (28%) had experienced recurrent fetal/neonatal death or neonatal liver failure. The main prenatal ultrasound finding reported in the 74 cases was FGR (33% cases), followed by oligohydramnios (13% cases) and hydrops fetalis (13% cases). In 13% of the cases, the course of the pregnancy was described as uneventful. Other rare prenatal findings were fetal anemia, ascites, or abnormal fetal liver and spleen (Figure 2). MRI had been used as a prenatal diagnostic tool in only two cases (1.48%), at 32 WGA in both cases, and depicted an atrophic liver. In one case, the parents opted for ensanguine transfusion and intravenous immunoglobulin, with good results. In the other case, they opted for therapeutic interruption of pregnancy (TOP) [17]. MRI was performed in 11 cases (8.14%) to confirm extrahepatic siderosis in neonates, which was described mainly in the pancreas in nine cases (6.66%).
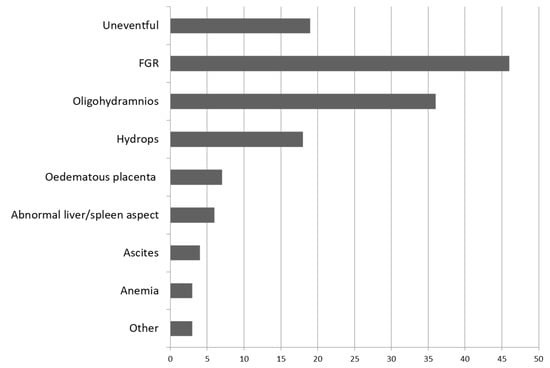
Figure 2.
Prenatal findings in 74 cases evaluated in the literature review diagnosed with neonatal hemochromatosis and with confirmed extrahepatic siderosis, reported from 1993 to 2021.
Most fetuses had an inauspicious prognosis, with the pregnancies ending in fetal death, perinatal death, or therapeutic interruption of pregnancy. Favorable evolution with treatment was reported for only 7% of the fetuses. Autopsy was the primary method used to confirm diagnosis. The postnatal treatment varied from chelation/antioxidant therapy, with reserve results, to ensanguine transfusion and intravenous immunoglobulin, with a favorable prognosis. When reported, the subsequent pregnancy had favorable evolution in cases of prenatal intravenous immunoglobulin (IVIG) treatment.
Following these results, in Figure 3, we propose a prenatal diagnosis algorithm for suspected NH in the first affected pregnancy.
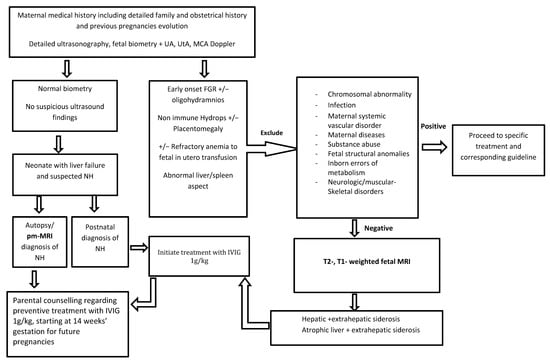
Figure 3.
Prenatal diagnosis algorithm proposal for suspected neonatal hemochromatosis in the first affected pregnancy.
4. Discussion
The objective of this paper was to systematically review the current literature regarding the main prenatal features of patients diagnosed with NH-GALD in order to create an algorithm for potential prenatal suspicion of this rare pathology characterized by a severe prognosis, in which selected fetuses will be referred for fetal MRI and the initiation of specific treatment where appropriate. While neonatal diagnosis has become more reliable, prenatal suspicion of NH in first affected pregnancies is exceptional. We reviewed 18 studies involving 74 fetuses diagnosed with NH and with confirmed extrahepatic siderosis, following which we could draw some conclusions regarding the prenatal features of fetuses with NH.
4.1. Etiology and Pathogenesis
Although the nature of the triggering antigen is still unknown, the central hypothesis regarding the pathogenesis of NH-GALD is focused on liver disease.
Fetal hepatocytes suffer injuries through the attack of membrane complexes resulting from transplacental transfer of maternal IgG antibodies [7], followed by impairment of iron homeostasis and siderosis in extrahepatic tissues [3]. Hemochromatosis often begins with iron buildup in hepatocytes, with the exception of Kupffer cells. The liver tissue of newborns with GALD is severely damaged, with hepatocyte loss leading to cirrhosis [1].
Iron deposition in diverse places is the ultimate phase in a multi-step process in which liver damage mediated by the complement may be the initial step [28].
Usually, extrahepatic siderosis is encountered in the pancreas, myocardium, thyroid, and salivary glands. Whittington et al. suggested that the possibility of alloimmune gestational should be considered since genetic causes for transmission do not align with known patterns of inheritance [7]. Even if NH is both congenital and familial, it is not hereditable [1].
During gestation, fetal iron levels are controlled by placental function, with trophoblastic tissue functioning as duodenal mucosa in tuning iron absorption and balancing the substantial maternal iron pool, thereby carefully regulating the fetal iron pool [2].
Fetal iron stores are regulated via the fetal hepcidin pathway [1]. The damage to fetal hepatocytes caused by maternal antibodies results in a decreased level of hepcidin, producing iron overload in the fetal liver [2]. However, recent data indicate that additional pathways, such as BMP/SMAD and JAK/STAT, can influence hepcidin expression [29].
Two main patterns of iron accumulation have been described: parenchymal iron overload and Kupffer cell hemosiderosis [30]. Parenchymal iron accumulation refers to accumulation in hepatocytes and bile duct epithelium, and in severe cases, portal fibrosis and eventual cirrhosis can be encountered [30].
Kupffer cell hemosiderosis refers to a pattern accumulation of hemosiderin in Kupffer cells and, in some cases, in the portal macrophages and endothelial cells, and secondary iron overload can be encountered. Moreover, mixed patterns of iron accumulation create even more issues, resulting in further difficulties in assessing these cases [30].
More recent data suggest that hepatic C5b-9 deposition could represent an important marker for GALD in an appropriate clinical situation, but the literature data are still contradictory [31].
An excellent study was conducted by Bonilla et al. in which liver tissues and extrahepatic tissues from GALD infants were investigated and compared with normal age-matched tissues. GALD-related liver injury may reduce hepcidin synthesis such that a cascade of reactions may follow, such as impaired placental iron flux feedback control. The authors pointed out that hepatic HAMP (hepatic antimicrobial protein) expression was reduced, and the pattern of extrahepatic siderosis is apparently determined by the capacity of various tissues to import non-transferrin-bound iron and not export cellular iron. Their findings suggest that NH is not primarily an iron overload disease [32].
An essential step is distinguishing the NH-GALD–related phenotype from other non-GALD disorders with different clinical presentations and prognoses. In order to accurately diagnose GALD, a comprehensive evaluation must be performed. Shanmugam et al. summarized the investigations that must be conducted to rule out other potential causes of a comparable phenotype [33]. For the other illnesses, it is postulated that fetal liver injury leading to poor regulation of placental iron flux causes NH. According to some authors, these causes account for approximately 2% of all NH cases [1].
The most frequent causes that should be taken into consideration for the differential diagnosis of NH in neonatal liver failure, other than gestational alloimmune, are presented in Table 2 [34,35,36,37].
Some writers emphasize the importance of the histologic findings and the function of autopsy, arguing that biological and molecular tests may be required to rule out alternative diagnoses, such as mitochondrial illnesses [28]. One particular finding in the histologic report is a grade of renal tubular dysgenesis with paucity or an absence of proximal tubules, explained as a developmental abnormality due to impaired hepatic angiotensinogen secretion [1,38], placing the onset of liver injury prior to the 24th week of gestation [2].
Even in suspected cases determined in utero, the detection of liver siderosis is insufficient for diagnosis due to the physiological presence of stainable iron in the neonatal liver, necessitating extrahepatic siderosis for confirmation or the detection of other specific markers that point to gestational alloimmune liver disease [7].
There have been reported cases of children with phosphatidylinositol N-acetylglucosaminyltransferase subunit A (PIGA) germ-line mutations presenting NH features, but their clinical course differs significantly. Therefore, PIGA gene testing is another option to be considered when evaluating newborns who present with NH [39].
4.2. Ultrasonography—The Key to Early Prenatal Diagnosis of Neonatal Hemochromatosis
FGR is one of the first manifestations visible in ultrasound evaluations that can be suggestive of NH, starting as early as the 18th week [17]. Since FGR has various causes, from utero-placental to genetic and infection, finding the right approach to correctly diagnose FGR-NH is a challenge.
Frequently encountered oligohydramnios is explained by renal tubular dysgenesis due to hepatocyte injury and secondary reduced angiotensinogen production as early as 20 to 25 weeks of gestation [1,38].
However, the FGR–oligohydramnios sequence related to NH often functions in diagnosis of exclusion during the first affected pregnancy but is also the most likely to be omitted since this clinical picture can be explained on the basis of more common causes. The process exclusion may be sufficiently long to result in an accurate verdict. Therefore, birth or fetal demise can occur, leaving the final diagnosis for neonatal or autopsy evaluation. Most of the time, these children are not born in tertiary centers. Until the transfer is successful and the treatment with immunoglobulin is initiated, the child’s prognosis may be compromised.
MRI is the only investigation technique that can guide the prenatal diagnosis algorithm to NH in a first affected pregnancy presenting FGR and oligohydramnios, since liver biopsy is not a viable option. Although expensive at first and not yet a part of the diagnosis algorithm of FGR, prenatal MRI can be used to elucidate a diagnosis of NH and detect placental dysfunction where Doppler flows are still normal [40], justifying the initiation of specific treatment.
Non-immune hydrops fetalis (NIHF) occurs in the end-stage state of 14 various diseases, according to a meta-analysis conducted by Bellini and colleagues in 2015 [41], with no identifiable primary cause in approximately 20% of cases [42]. The diagnostic algorithm used to determine the underlying cause of NIHF is a time trial challenge to assess whether there is a therapeutic solution to reverse the consequences. NIHF is a frequent prenatal finding in NH, which can most likely be explained by secondary hypoproteinemia [43]. After excluding immune hydrops fetalis, a hydropic and anemic fetus with associated hepatomegaly may raise suspicion of NH and justify a fetal MRI for diagnosis of exclusion.
As the fetus grows, unexplained hepatomegaly, sometimes accompanied by ascites, could be encountered during prenatal US surveillance.
In 1996, Achiron et al. examined a group of fetuses with various grades of hepatic hyperechogenicity. The authors identified several causes responsible for this particular imaging aspect, ranging from abnormal karyotype through to fetal infection to in utero vascular accidents [44]. They concluded that hepatic hyperechogenicity alone, with a lack of other markers, poses little clinical significance. Further investigation, such as by MRI, is required to diagnose NH accompanied by extrahepatic siderosis.
An aspect worth mentioning in terms of the difficulties of prenatal diagnosis of NH was described regarding a 33-week fetus presenting on US evaluation with hydrops, oligohydramnios, and placentomegaly. The re-evaluation of fetal ultrasound images resulted in identification of anemia and shrunken liver with multiple regenerative nodules, confirmed by MRI and autopsy [45]. Sometimes, re-evaluating the initial images and assessment may provide vital information for patient care.

Table 2.
Differential diagnosis of neonatal hemochromatosis in neonatal liver failure.
Table 2.
Differential diagnosis of neonatal hemochromatosis in neonatal liver failure.
| Causes of Neonatal Hemochromatosis Phenotype Other Than Gestational Alloimmune Liver Disease | Prenatal Ultrasound Features | Investigations | Available Treatments | Perinatal Findings | Prognosis of Affected/Subsequent Pregnancies |
|---|---|---|---|---|---|
| 1. Intrauterine infections Viral infections (e.g., viruses of the herpes family, cytomegalovirus, parvovirus, enterovirus, adenovirus) [33,46,47] | Fetal hydrops, cerebral ventriculomegaly, ± microcephaly, hyperechogenic fetal bowel, hepatosplenomegaly, cerebral periventricular echogenicity/intracranial calcifications, fetal cardiac arrhythmias, anemia, FGR, abnormal amniotic fluid volume, placental enlargement | Maternal serology AF PCR | Ganciclovir, valacyclovir, foscarnet, CMV hyperimmune globulin (not approved for routine use) Fetal intrauterine transfusion in case of an anemic and hydropic fetus | Fever, lethargy, abdominal distension, seizures | Poor/good |
| 2. Metabolic disorders * Transaldolase deficiency [33,48,49] | FGR, hydrops fetalis, oligohydramnios, fetal distress, hepatosplenomegaly, hyperechogenic bowel mostly uneventful | DNA analysis (variants in the TALDO1 gene) | No established treatment in humans Benefit to supplementation with the glutathione precursor N-acetylcysteine in knockout mouse model | Jaundice, hypoglycemia | Poor/AR |
| Tyrosinemia type I [33,50,51] | Mostly uneventful | Blood succinylacetone level (screening marker) Molecular testing of FAH, encoding FAH | 2-[2-Nitro-4-trifluoromethylbenzoyl]-1,3cyclohexanedione | Jaundice, hypoglycemia | Good with early treatment/AR |
| Galactosemia/hereditary fructose intolerance [33,52] | Mostly uneventful | Measurement of GALT enzyme activity in red blood cells (absent or significantly decreased GALT gene analysis) | Galactose-restricted diet | Jaundice, hypoglycemia | Good with early diagnosis and diet/AR |
| Niemann Pick type C [53,54,55] | FGR, oligohydramnios, hepatomegaly, splenomegaly, hydrops fetalis, hygroma | Genetic testing (NPC2 gene) | No curative treatment Iminosugar N-butyldeoxynojirimycin Management of the neurological disease | NLF without a free interval after birth | Poor/AR |
| Urea cycle disorders [56] | Mostly uneventful, premature birth | Mutation analysis using DNA from CVS or AFC Citrulline and ASA determinations in AF for prenatal diagnosis of ASSD and ASLD | Nitrogen scavengers benzoate and phenylacetate arginine and/or citrulline N-Carbamylglutamate | Neurological impairment, seizures, hyperammonemia, lethargy, anorexia, hyper-/hypoventilation | Outcomes are generally poor without early treatment/good with strict supervision |
| Disorders of glycosylation [57] | FGR, skeletal anomalies, pericardial effusion | Multiple, DNA analysis | No curative treatment Organ transplantation to relieve selected symptoms Promising new monosaccharides (particularly mannose and galactose) and chaperone therapies | Axial hypotonia, hyperreflexia, esotropia, development delay. A non-fatal neurological form and a multivisceral neurological form. | Poor/most forms of CDG are inherited in AR |
| Mitochondrial cytopathies [58] | Usually uneventful | Gene sequencing Mutation in the mitochondrial DNA or nuclear DNA | No curative treatment | Hypotonia, seizures, abnormal liver function, impaired feeding | Depends on how many organ systems and tissues are affected/AR, AD Mitochondrial Random mutations |
| 4. Primary bile acid disorders [59] | Usually uneventful | HSD3B7 gene for 3β-HSD deficiency; AKR1D1 gene (formerly SRD5B1) for Δ4-3-oxoR deficiency. | Cholic and chenodeoxycholic acid | Neonatal hepatitis, cholestasis, diarrhea, steatorrhea, malabsorption, progressive neurological disease, cirrhosis | 50% mortality without treatment/AR |
| 5. Genetic—syndromic Trisomy 21, trisomy 18, cerebrohepatorenal syndrome, renal tubular dysgenesis syndrome, Donohue syndrome, trichohepatoenteric syndrome [3] | FGR, diverse structural anomalies | Karyotype/gene testing | No curative treatment | Clinical presentation may vary widely from single anomaly to multiple malformations | Poor/depends on parental genetic status |
| 6. Rhesus incompatibility, AB0-incompatibility, congenital hemolysis [60] | Hydramnios, FGR, hydrops fetalis, hepato-splenomegaly, ascites | Clinical and laboratory assessment (Coombs test, qPCR to detect RHD gene in cfDNA) | The main principle is the prevention of maternal sensitization anti-D immune globulin | Anemia, hyperbilirubinemia, thrombocytopenia, neutropenia, kernicterus, hypomagnesemia, neurological complications | Generally good/good with early administration of Rh IgG if the fetus is Rh positive |
| 7. Hematological disorders Hemophagocytic lymphohistiocytosis [61] | No data found regarding prenatal ultrasound findings of HLH | Hematological and imagistic tests Evaluations for infections and malignancies Rapid screening tests for genetic HLH | Immunosuppressive and chemotherapeutic treatment | Fever, splenomegaly, cytopenias, hypofibrinogenemia, ±hypertriglyceridemia, hyperferritinemia, hemophagocytosis, low NK activity, high concentration of soluble interleukin 2receptor (sCD25/sIL-2R) | High mortality rate/various genetic causes of predisposition |
| 8. Exposure to toxic agents Pyrrolizidine [62,63] | No data found regarding prenatal ultrasound findings of pyrrolizidine exposure | Classic methods (urine colorimetric screening test), chromatography, mass spectrometry, immunoassays | Stopping the exposure, symptomatic treatment, anticoagulant therapy, transjugular intrahepatic portosystemic shunt, liver transplant, defibrotide | Abdominal pain, jaundice, hepatomegaly, ascites, weight gain | Chronic exposure causes hepatic sinusoidal obstruction syndrome/good |
| 11. Exogenous iron overload [64] | No data found regarding prenatal ultrasound findings of exogenous iron overload | Mutations in the genes for transferrin receptor 2 (TfR2) or ferroportin (SLC40A1) Fasting serum testing for ferritin, total iron-binding capacity, transferrin saturation, iron levels and metabolic panel, including hepatic enzymes MRI | Phlebotomy Iron chelators: deferoxamine and deferasirox Orthotopic liver transplantation Diet modification | Fetal growth retardation, aminoaciduria, cholestasis, liver hemosiderosis, hyperferritinemia, hypotransferrinemia, increased transferrin iron saturation, and free plasma iron, lactic acidosis | Good if no end-organ disfunction/AD |
* Some of the metabolic disorders are also genetic metabolic disorders. AF = amniotic fluid; CDG = congenital disorders of glycosylation; CVS = chorionic villous sample; AFC = amniotic fluid cells; ASA = argininosuccinate; ASSD = argininosuccinate synthetase deficiency, citrullinemia type I; ASLD = argininosuccinate lyase deficiency; UCD = urea cycle disorders; HLH = hemophagocytic lymphohistiocytosis; AR = autosomal recessive disorder; AD = autosomal dominant; FGR = fetal growth restriction; MRI = magnetic resonance imaging; NLF = neonatal liver failure; NK = natural killer.
This particular aspect of the liver, similar to that in adult cirrhosis, is challenging to evaluate in fetuses. Therefore, additional investigations such as fetal MRI are needed to confirm any suspicions.
Unexplained fetal anemia, refractory to fetal in utero transfusion, is another US finding that can be prenatally detected without difficulty after introducing middle cerebral artery systolic peak measurement in routine practice. This could raise suspicion of NH [9].
Encountering NH during the first pregnancy in consanguine couples is an aspect for which clinical significance is yet to be revealed [12]. Another observation that does not fit the NH prenatal pattern is the different liver damage grades observed in some twin pregnancies [1]. In unexplained cases of fetal death, NH should be considered as one of the differential diagnoses, although cautiously, considering that US signs of NH are insufficient in the first affected pregnancy [3].
4.3. Magnetic Resonance Imaging—New Horizons for Prenatal Diagnosis and Follow-Up of Neonatal Hemochromatosis
Although there is evidence of its effectiveness [65], MRI is scarcely used in prenatal NH diagnosis. It is mainly utilized as a research tool rather than a diagnostic tool in neonates [45]. Quantitative assessment of organ iron deposition for the adult population is available using T1 or T2* relaxometry, and this can be applied in pediatrics. However, there is still no available normative data on iron quantity in various neonate tissues [4,66].
Fetal MRI in GALD could help assess clinical responses to in utero treatment [67]. The methods used encompass a qualitative assessment of the signal intensity of the respective organs on T2, T2*, T1, and GRE (gradient recalled echo) sequences.
However, depicting the liver overcharged with iron is insufficient for diagnosis, even if other immune manifestations, such as hydrops fetalis, are present. Extrahepatic siderosis is considered a fundamental criterion for diagnosis, but a GALD hypothesis should be raised, even in its absence, when considering confirmed affliction in previous pregnancies. Recent studies emphasize this, recommending that prenatal treatment should not be postponed when there is no finding of extrahepatic non-reticuloendothelial siderosis but there are other specific markers pointing to gestational alloimmune liver disease [31].
Even if fetal organ iron deposition can be demonstrated by MRI, it may not always be present at the moment of examination, as hypothesized as far back as 1990 by Hoogstraten [67]. Cases have been reported with an atrophic liver both with and without observable extrahepatic iron impregnation. On the other hand, liver siderosis could be absent, as the targeted hepatocytes could have already been destroyed [17]. In these cases, the presence of extrahepatic iron impregnation could help with diagnosis [7].
There have been reports of several MRI sequences that can show organ iron overload in the fetus. In the first case of intrauterine hemochromatosis diagnosed with MRI in 1993, Marti-Bonmati et al. used a T2* sequence. Extremely low hepatic parenchymal signal intensity (SI) was shown compared with that of fetal and maternal fat tissue [68].
Presently, fast, single-shot T2-weighted sequences are the primary tool in fetal MRI and the first to raise suspicion of liver iron deposition. The affected fetal liver shows an SI drop that is even more conspicuous when compared with the fetal spleen or maternal liver SI.
The same pattern of signal intensity alteration seen in neonates with NH has been described in fetuses with liver siderosis in a few case presentations—lower median SI of the liver on in-phase sequences compared with the opposed-phase SI.
The in-phase/opposed-phase SI ratio (SIR) in fetal NH has been reported as approximately 0.5 in an untreated fetus and 0.78 in a fetus with prior IVIG treatment. In one case of prior clinically and histologically proven GALD, Sasaki et al. also calculated the SIR of the fetal liver, spleen, and pancreas to the fetal iliopsoas muscle. They obtained values of 0.7, 1.36, and 1.74, respectively, suggesting that iron deposition in this treated case was limited to the liver [66,69].
Organ iron load quantification could be achieved with MRI T2* relaxometry techniques, as described in 2013 by Schoennagel et al. in a fetal sheep model and a human neonate with hemochromatosis [70]. Sethi et al. achieved further progress in a future quantitative assessment of fetal organ iron load by setting the baseline for quantifying the T1 and T2* relaxation times of fetal tissues in uncomplicated pregnancies at the gestational age of 28–38 weeks [71].
A French retrospective multicentric study of the importance of autopsy and auto-immune maternal manifestations in neonatal hemochromatosis observed that, in fetuses, iron storage was more frequent in the thyroid than in the pancreas [28]. As far as we know, there has been no reported MRI finding (or search) of fetal thyroid iron load in hemochromatosis. The thyroid signal intensity in 3D GRE T1 was evaluated in seven fetuses with hypothyroidism and 17 fetuses with normal thyroid function (GA of 28–41 weeks). The mean and maximum SIRs in fetuses with a normal thyroid was 1.85 ± 0.20 and 2.61 ± 0.39 [72]. This finding could be a reference for normal fetal thyroid SI on GRE T1 sequences when assessing fetuses with hemochromatosis.
Extrahepatic siderosis can be detected non-invasively by a multi-echo gradient recalled echo T2*-weighted MRI sequence within hours of birth and graded as mild, moderate, or severe based on the echo in which organs start showing darkening/a signal drop [4,66].
Another essential step in confirming NH where MRI may play an important role is postmortem evaluation. In selected cases where parents’ religious or moral views prevent a classical autopsy evaluation, a virtual evaluation by postmortem MRI might be a valuable alternative with a high grade of acceptability from the genitors. Postmortem MRI can easily depict liver siderosis [73] or extrahepatic siderosis and can therefore be the necessary justification for further investigations through a guided liver biopsy ±C5b-9 immunostaining.
4.4. In Utero Treatment and the Prevention of Neonatal Hemochromatosis Sequelae
In 2004, Whittington proposed the antenatal administration of a high dose of intravenous immunoglobulin (IVIG), starting in the second trimester, for women with a previous pregnancy with NH [7]. Today, treatment with IVIG has practically changed the prognosis of affected women, allowing them to bear a healthy child after a pregnancy with NH [74,75]. The current guidelines recommend administering IVIG to secondary pregnancies at a dose of 1 g/kg at 14 and 16 weeks and then weekly from 18 weeks of gestation. The treatment significantly improved disease evolution with absent US manifestations and a better life expectancy than in untreated pregnancies [67]. However, IVIG treatment is expensive; availability can be limited in some countries, and severe adverse effects may present [75].
The mechanism by which IVIG administration improves fetal evolution remains unclear, but some theories have been proposed. One considers the dilution of maternal antibodies directed against the fetal liver antigens as a possible mechanism [3]. Another proposes that IVIG may block placenta receptors and reduce the placental transmission of maternal antibodies to the fetus, or that IVIG may even block Fc receptors on the macrophages in the fetal circulation, limiting the destruction of fetal hepatocytes sensitized by maternal antibodies [3].
4.5. Future Diagnostic Perspective
An in utero diagnosis of NH would be a remarkable achievement, as early diagnosis would prove useful for fetal treatment therapy. Based on the wide range of risks posed by a fetal liver biopsy and its relatively poor specificity, a more effective method is required [75].
We consider that a prenatal MRI should be considered earlier in cases presenting FGR with normal Doppler parameters or unexplained non-immune hydrops fetalis, refractory anemia to fetal in utero transfusion, or liver abnormalities in the diagnosis algorithm. Furthermore, for parents that refuse to allow a classical autopsy of the fetus/neonate, postmortem MRI is a non-invasive solution that can easily identify liver siderosis and guide biopsy. To illustrate this idea, a figure of a fetus with confirmed NH investigated using both in vivo and postmortem MRI is rendered in Figure 4.
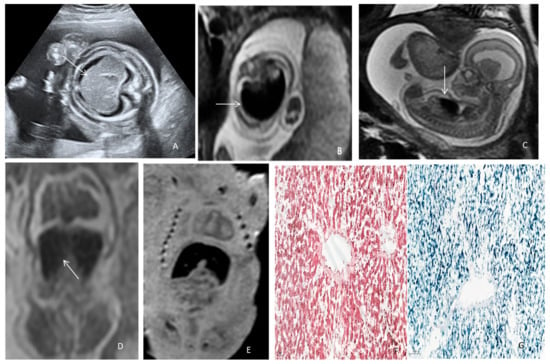
Figure 4.
Neonatal hemochromatosis in a hypotrophic fetus with idiopathic non-immune hydrops. (A) Ultrasound axial abdominal section depicting hepatosplenomegaly (arrow), ascites, and subcutaneous edema at 17 WGA. (B) Prenatal MRI 3T, T2 WI depicting fetoplacental anasarca and the liver with axial T2 hypersignal and (C) sagittal section (arrow) at 18 WGA. (D,E) Virtual autopsy using postmortem MRI, T2WI, and T1WI in the coronal plane depicting fetal liver with low signal (arrow), indicating the presence of iron at 21 WGA. (F) Hematoxylin–eosin staining (7×) showing two centrilobular veins and cords of hepatocytes. (G) Perls staining showing hemosiderin deposits (blue) in liver parenchyma (8×).
4.6. Limitations
NH-GALD is a rare, severe disorder that results in fetal loss or neonatal death due to liver failure; therefore, the present study is limited by the small number of included studies and the retrospective nature of the reported cases.
5. Conclusions
Despite the strong impact of this condition on present and future pregnancies, the prenatal findings that might suggest fetal NH are often nonspecific. MRI should be indicated in selected cases of unexplained FGR, oligohydramnios, fetal hydrops, fetal hepatomegaly, and ascites or anemia, usually after all the other more frequently encountered conditions have been ruled out. MRI can detect iron overload in the liver and extrahepatic siderosis, confirming NH. For the parents that refuse a fetal autopsy, postmortem MRI should be available for all cases of unexplained fetal and newborn deaths with liver failure, as it can easily detect liver siderosis and guide future investigations. Once the diagnosis is made, the administration of specific IVIG should be initiated immediately after diagnosis, allowing for a much better prognosis. C5b-9 staining of the tissues can confirm the diagnosis from the liver biopsy or autopsy specimen.
In subsequent pregnancies, women with a previously NH-affected fetus should be carefully evaluated, and high-dose IVIG treatment should be started at the end of the first trimester, as evidence shows significant improvement in the disease evolution compared with untreated pregnancies. In neonates, exchange transfusion and a high dose of IVIG is the preferred treatment to remove the maternal NH-associated antibody and block its action, including complement activation.
Author Contributions
Conceptualization, A.S., R.C., C.A. and R.P.-S.; methodology, investigation, resources, writing—original draft preparation, A.S., R.C., R.P.-S., D.B.-B., A.C., I.C.R. and M.S.; writing—review and editing, A.S., R.C., R.P.-S., A.C. and M.S.; supervision, G.C. and D.M.; funding acquisition, A.S., M.S. and D.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank “Iuliu Hațieganu” University of Medicine and Pharmacy Research for grant no. 35.166/17.12.2022 received by A.S. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors received no specific funding for this work.
Institutional Review Board Statement
The “Iuliu Hațieganu” University of Medicine and Pharmacy Ethics Committee approved the research No. DEP50/22.02.2022, approved no. DEP50/22.02.2022.
Informed Consent Statement
Informed consent was waived because the review used anonymous clinical data and the patients were not identifiable in the images.
Data Availability Statement
The data presented in this study are available on request from A.S.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Feldman, A.G.; Whitington, P.F. Neonatal hemochromatosis. J. Clin. Exp. Hepatol. 2013, 3, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Whitington, P.F. Fetal and infantile hemochromatosis. Hepatology 2006, 43, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Lopriore, E.; Mearin, M.L.; Oepkes, D.; Devlieger, R.; Whitington, P.F. Neonatal hemochromatosis: Management, outcome, and prevention. Prenat. Diagn. 2013, 33, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Chavhan, G.B.; Kamath, B.M.; Siddiqui, I.; Tomlinson, C. Magnetic resonance imaging of neonatal hemochromatosis. Pediatr. Radiol. 2022, 52, 334–349. [Google Scholar] [CrossRef]
- Neil, E.; Cortez, J.; Joshi, A.; Bawle, E.V.; Poulik, J.; Zilberman, M.; El-Baba, M.F.; Sood, B.G. Hepatic failure, neonatal hemochromatosis and porto-pulmonary hypertension in a newborn with trisomy 21—A case report. Ital. J. Pediatr. 2010, 36, 38. [Google Scholar] [CrossRef]
- Kasko, O.; Klose, E.; Rama, G.; Newberry, D.; Jnah, A. Gestational Alloimmune Liver Disease: A Case Study. Neonatal. Netw. 2018, 37, 271–280. [Google Scholar] [CrossRef]
- Whitington, P.F. Gestational Alloimmune Liver Disease and Neonatal Hemochromatosis. Semin. Liver Dis. 2012, 32, 325–332. [Google Scholar]
- Cottier, H. A hemochromatosis similar disease in newborn. Schweiz. Med. Wochenschr. 1957, 87, 39–43. [Google Scholar]
- Amann, C.; Geipel, A.; Müller, A.; Heep, A.; Ritgen, J.; Stressig, R.; Kozlowski, P.; Gembruch, U.; Berg, C. Fetal Anemia of Unknown Cause—A Diagnostic Challenge. Ultraschall Der Med. Stuttg. Ger. 1980, 32, E134–E140. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Mugarab-Samedi, V.; Ryan, M.D.; Awad, E.H.A.; Elsharkawy, A. The Effect of Prenatal and Postnatal Treatment with Intravenous Immunoglobulin on Severity of Neonatal Hemochromatosis: The Tale of Two Brothers (Case Report). AJP Rep. 2021, 11, e102–e104. [Google Scholar] [CrossRef] [PubMed]
- Mude, S.; Miraje, B.; Gavade, V.; Patil, U.S.; Patil, R.S. Neonatal hemochromatosis: A rare case report with consanguinity. Int. J. Med. Biomed. Stud. 2020, 4, 125–127. [Google Scholar] [CrossRef]
- Maisonneuve, E.; Ben M’Barek, I.; Leblanc, T.; Da Costa, L.; Friszer, S.; Pernot, F.; Thomas, P.; Castaigne, V.; Toly N’Dour, C.; Mailloux, A.; et al. Managing the Unusual Causes of Fetal Anemia. Fetal Diagn. Ther. 2020, 47, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Zaharie, G.; Muresan, D.; Andreica, S.; Hosu, A.; Boitor, D.; Staicu, A.; Hasmasanu, M.; Ighian, I.; Botan, E.C.; Trif, A.R.; et al. A rare case of gastrointestinal bleeding: Neonatal hemochromatosis. Obstetrica. Si Ginecologia. 2018, 66, 51–56. [Google Scholar]
- Darouich, S.; Boujelbène, N.; Amraoui, J.; Amraoui, N.; Masmoudi, A. Hemochromatosis associated with cholelithiasis as a cause of hydrops fetalis and stillbirth: Prenatal diagnosis. J. Clin. Ultrasound JCU 2019, 47, 47–50. [Google Scholar] [CrossRef]
- Babor, F.; Hadzik, B.; Stannigel, H.; Mayatepek, E.; Hoehn, T. Successful management of neonatal hemochromatosis by exchange transfusion and immunoglobulin: A case report. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2013, 33, 83–85. [Google Scholar] [CrossRef]
- Sciard, C.; Collardeau-Frachon, S.; Atallah, A.; Combourieu, D.; Massardier, J.; Heissat, S.; Gaucherand, P.; Guibaud, L.; Massoud, M. Prenatal imaging features suggestive of liver gestational allo immune disease. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 61–64. [Google Scholar] [CrossRef]
- Roos Mariano da Rocha, C.; Rostirola Guedes, R.; Kieling, C.O.; Rossato Adami, M.; Cerski, C.T.S.; Gonçalves Vieira, S.M. Neonatal Liver Failure and Congenital Cirrhosis due to Gestational Alloimmune Liver Disease: A Case Report and Literature Review. Case Rep. Pediatr. 2017, 2017, 7432859. [Google Scholar] [CrossRef]
- Lopes, H.I.; Montes, A.L.; Novais, M.V.; Brandão, O.; Valente, F. Diagnóstico Prénatal de Hemocromatose Neonatal: Será possível? Case Rep. 2015, 3, 88–90. [Google Scholar]
- Rodrigues, F.; Kallas, M.; Nash, R.; Cheeseman, P.; D’Antiga, L.; Rela, M.; Heaton, N.D.; Mieli-Vergani, G. Neonatal hemochromatosis—Medical treatment vs. transplantation: The king’s experience. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2005, 11, 1417–1424. [Google Scholar] [CrossRef]
- Heissat, S.; Collardeau-Frachon, S.; Baruteau, J.; Dubruc, E.; Bouvier, R.; Fabre, M.; Cordier, M.P.; Broué, P.; Guigonis, V.; Debray, D. Neonatal Hemochromatosis: Diagnostic Work-Up Based on a Series of 56 Cases of Fetal Death and Neonatal Liver Failure. J. Pediatr. 2015, 166, 66–73. [Google Scholar] [CrossRef]
- Baruteau, J.; Heissat, S.; Broué, P.; Collardeau-Frachon, S.; Bouvier, R.; Fabre, M.; Debiec, H.; Ronco, P.; Uzan, M.; Narcy, P. Transient neonatal liver disease after maternal antenatal intravenous Ig infusions in gestational alloimmune liver disease associated with neo-natal haemochromatosis. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 629–635. [Google Scholar] [CrossRef]
- Tanaka, H.; Haba, R.; Itoh, S.; Sakamoto, H.; Hata, T. Prenatal high-dose immunoglobulin treatment for neonatal hemochromatosis: A case report and review of the literature. J. Obstet. Gynaecol. Res. 2011, 37, 1891–1894. [Google Scholar] [CrossRef]
- Cakir, M.; Mutlu, M.; Aydin-Mungan, S.; Cansu, A.; Aslan, Y.; Erduran, E. Neonatal hemochromatosis: A case report with unique presentation. Turk. J. Pediatr. 2011, 53, 455–459. [Google Scholar]
- Kassem, E.; Dolfin, T.; Litmanowitz, I.; Regev, R.; Arnon, S.; Kidron, D. Familial perinatal hemochromatosis: A disease that causes recurrent non-immune hydrops. J. Perinat. Med. 1999, 27, 122–127. [Google Scholar] [CrossRef]
- Verloes, A.; Lombet, J.; Lambert, Y.; Hubert, A.-F.; Deprez, M.; Fridman, V.; Gosseye, S.; Rigo, J.; Sokal, E. Tricho-hepato-enteric syndrome: Further delineation of a distinct syndrome with neonatal hemochromatosis phenotype, intractable diarrhea, and hair anomalies. Am. J. Med. Genet. 1997, 68, 391–395. [Google Scholar] [CrossRef]
- Wisser, J.; Schreiner, M.; Diem, H.; Roithmeier, A. Neonatal hemochromatosis: A rare cause of nonimmune hydrops fetalis and fetal anemia. Fetal Diagn. Ther. 1993, 8, 273–278. [Google Scholar] [CrossRef]
- Collardeau-Frachon, S.; Heissat, S.; Bouvier, R.; Fabre, M.; Baruteau, J.; Broue, P.; Cordier, M.P.; Debray, D.; Debiec, H.; Ronco, P.; et al. French retrospective multicentric study of neonatal hemochromatosis: Importance of autopsy and autoimmune maternal manifestations. Pediatr. Dev. Pathol. Off. J. Soc. Pediatr. Pathol. Paediatr. Pathol. Soc. 2012, 15, 450–470. [Google Scholar] [CrossRef]
- Silvestri, L.; Nai, A.; Dulja, A.; Pagani, A. Hepcidin and the BMP-SMAD pathway: An unexpected liaison. Vitam. Horm. 2019, 110, 71–99. [Google Scholar]
- Salomao, M.A. Pathology of Hepatic Iron Overload. Clin. Liver Dis. 2021, 17, 232–237. [Google Scholar] [CrossRef]
- Wu, H.; Ferguson, W.; Castro, E.; Kearney, D.; Finegold, M.; Patel, K. Extrahepatic Nonreticuloendothelial Siderosis Is Not Specific to Gestational Alloimmune Liver Disease. Pediatr. Dev. Pathol. Off. J. Soc. Pediatr. Pathol. Paediatr. Pathol. 2019, 22, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, S.; Prozialeck, J.D.; Malladi, P.; Pan, X.; Yu, S.; Melin-Aldana, H.; Whitington, P.F. Neonatal iron overload and tissue siderosis due to gestational alloimmune liver disease. J. Hepatol. 2012, 56, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, N.P.; Bansal, S.; Greenough, A.; Verma, A.; Dhawan, A. Neonatal liver failure: Aetiologies and management—State of the art. Eur. J. Pediatr. 2011, 170, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Clayton, P. Diagnosis of inherited disorders of liver metabolism. J. Inherit. Metab. Dis. 2003, 26, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Broué, P.; Baruteau, J. Diagnosis and management of metabolic liver failure in children. Arch. Pediatr. Organe Off. La Soc. Fr. Pediatr. 2009, 16, 640–642. [Google Scholar] [CrossRef]
- Pietrangelo, A.; Caleffi, A.; Corradini, E. Non-HFE hepatic iron overload. Semin. Liver Dis. 2011, 31, 302–318. [Google Scholar] [CrossRef]
- Sergi, C.; Himbert, U.; Weinhardt, F.; Heilmann, W.; Meyer, P.; Beedgen, B.; Zilow, E.; Hofmann, W.J.; Linderkamp, O.; Otto, H.F. Hepatic failure with neonatal tissue siderosis of hemochromatotic type in an infant presenting with meconium ileus. Case report and differential diagnosis of the perinatal iron storage disorders. Pathol. Res. Pract. 2001, 197, 699–709, discussion 711–713. [Google Scholar]
- Bonilla, S.F.; Melin-Aldana, H.; Whitington, P.F. Relationship of Proximal Renal Tubular Dysgenesis and Fetal Liver Injury in Neonatal Hemochromatosis. Pediatr. Res. 2010, 67, 188–193. [Google Scholar] [CrossRef]
- Flores-Torres, J.; Carver, J.D.; Sanchez-Valle, A. PIGA Mutations Can Mimic Neonatal Hemochromatosis. Pediatrics 2021, 147, e20200918. [Google Scholar] [CrossRef]
- Sørensen, A.; Sinding, M. Placental Magnetic Resonance Imaging: A Method to Evaluate Placental Function in Vivo. Obstet. Gynecol. Clin. N. Am. 2020, 47, 197–213. [Google Scholar] [CrossRef]
- Bellini, C.; Donarini, G.; Paladini, D.; Calevo, M.G.; Bellini, T.; Ramenghi, L.A.; Hennekam, R.C. Etiology of non-immune hydrops fetalis: An update. Am. J. Med. Genet. Part A 2015, 167, 1082–1088. [Google Scholar] [CrossRef]
- Swearingen, C.; Colvin, Z.A.; Leuthner, S.R. Nonimmune Hydrops Fetalis. Clin. Perinatol. 2020, 47, 105–121. [Google Scholar] [CrossRef]
- Society for Maternal-Fetal Medicine (SMFM); Norton, M.E.; Chauhan, S.P.; Dashe, J.S. Society for maternal-fetal medicine (SMFM) clinical guideline #7: Nonimmune hydrops fetalis. Am. J. Obstet. Gynecol. 2015, 212, 127–139. [Google Scholar]
- Achiron, R.; Seidman, D.S.; Afek, A.; Malinger, G.; Lipitz, S.; Mashiach, S.; Goldman, B.; Yagel, S. Prenatal ultrasonographic diagnosis of fetal hepatic hyperechogenicities: Clinical significance and implications for management. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 1996, 7, 251–255. [Google Scholar] [CrossRef]
- Skaife, T.; Callen, P.W.; Coakley, F. Prenatal sonographic findings in fetal cirrhosis secondary to hemochromatosis. J. Ultrasound Med. 2000, 19, 285–288. [Google Scholar] [CrossRef]
- Rawlinson, W.D.; Boppana, S.B.; Fowler, K.B.; Kimberlin, D.W.; Lazzarotto, T.; Alain, S.; Daly, K.; Doutré, S.; Gibson, L.; Giles, M.L.; et al. Congenital cytomegalo-virus infection in pregnancy and the neonate: Consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect. Dis. 2017, 17, e177–e188. [Google Scholar] [CrossRef]
- Ornoy, A.; Ergaz, Z. Parvovirus B19 infection during pregnancy and risks to the fetus. Birth Defects Res. 2017, 109, 311–323. [Google Scholar] [CrossRef]
- Xue, J.; Han, J.; Zhao, X.; Zhen, L.; Mei, S.; Hu, Z.; Li, X. Prenatal Diagnosis of Fetus with Transaldolase Deficiency Identifies Compound Heterozygous Variants: A Case Report. Front. Genet. 2021, 12, 752272. [Google Scholar] [CrossRef] [PubMed]
- Rodan, L.H.; Berry, G.T. N-Acetylcysteine Therapy in an Infant with Transaldolase Deficiency Is Well Tolerated and Associated with Normalization of Alpha Fetoprotein Levels. JIMD Rep. 2017, 31, 73–77. [Google Scholar] [PubMed]
- Rafati, M.; Mohamadhashem, F.; Hoseini, A.; Ramandi, S.D.; Ghaffari, S.R. Prenatal Diagnosis of Tyrosinemia Type 1 Using Next Generation Sequencing. Fetal Pediatr. Pathol. 2016, 35, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Chinsky, J.M.; Singh, R.; Ficicioglu, C.; van Karnebeek, C.D.M.; Grompe, M.; Mitchell, G.; Waisbren, S.E.; Gucsavas-Calikoglu, M.; Wasserstein, M.P.; Coakley, K.; et al. Diagnosis and treatment of tyrosinemia type I: A US and Canadian consensus group review and recommendations. Genet. Med. Off. J. Am. Coll. Med. Genet. 2017, 19, 1380–1395. [Google Scholar] [CrossRef]
- Welling, L.; Bernstein, L.E.; Berry, G.T.; Burlina, A.B.; Eyskens, F.; Gautschi, M.; Grünewald, S.; Gubbels, C.S.; Knerr, I.; Labrune, P.; et al. International clinical guideline for the management of classical galactosemia: Diagnosis, treatment, and follow-up. J. Inherit. Metab. Dis. 2017, 40, 171–176. [Google Scholar] [CrossRef]
- Ples, L.; Sima, R.-M.; Nedelea, F.; Moga, M. First Prenatal Diagnosis of a Niemann-Pick Disease Type C2 Revealed by a Cystic Hygroma: A Case Report and Review of the Literature. Front. Endocrinol. 2018, 9, 292. [Google Scholar] [CrossRef]
- Surmeli-Onay, O.; Yakarisik, S.; Korkmaz, A.; Akcoren, Z.; Yuce, A.; Runz, H.; Stampfer, M.; Yurdakok, M. Prenatal-Onset Niemann-Pick Type C Disease with Nonimmune Hydrops Fetalis. Pediatr. Neonatol. 2013, 54, 344–347. [Google Scholar] [CrossRef]
- Ribas, G.S.; Pires, R.; Coelho, J.C.; Rodrigues, D.; Mescka, C.P.; Vanzin, C.S.; Biancini, G.B.; Negretto, G.; Wayhs, C.A.; Wajner, M.; et al. Oxidative stress in Niemann-Pick type C patients: A protective role of N-butyl-deoxynojirimycin therapy. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2012, 30, 439–444. [Google Scholar] [CrossRef]
- Häberle, J.; Burlina, A.; Chakrapani, A.; Dixon, M.; Karall, D.; Lindner, M.; Mandel, H.; Martinelli, D.; Pintos-Morell, G.; Santer, R.; et al. Suggested guidelines for the diagnosis and management of urea cycle disorders: First revision. J. Inherit. Metab. Dis. 2019, 42, 1192–1230. [Google Scholar] [CrossRef]
- Ondruskova, N.; Cechova, A.; Hansikova, H.; Honzik, T.; Jaeken, J. Congenital disorders of glycosylation: Still ‘hot’ in 2020. Biochim. Et Biophys. Acta Gen. Subj. 2021, 1865, 129751. [Google Scholar] [CrossRef]
- Nesbitt, V.; Alston, C.L.; Blakely, E.L.; Fratter, C.; Feeney, C.; Poulton, J.; Brown, G.K.; Turnbull, D.M.; Taylor, R.W.; McFarland, R. A national perspective on prenatal testing for mitochondrial disease. Eur. J. Hum. Genet. 2014, 22, 1255–1259. [Google Scholar] [CrossRef]
- Sundaram, S.S.; Bove, K.E.; Lovell, M.A.; Sokol, R.J. Mechanisms of Disease: Inborn errors of bile acid synthesis. Nat. Clin. Pr. Gastroenterol. Hepatol. 2008, 5, 456–468. [Google Scholar] [CrossRef]
- Ayenew, A.A. Prevalence of rhesus D-negative blood type and the challenges of rhesus D immunoprophylaxis among obstetric population in Ethiopia: A systematic review and meta-analysis. Matern. Health Neonatol. Perinatol. 2021, 7, 8. [Google Scholar] [CrossRef]
- Canna, S.W.; Marsh, R.A. Pediatric hemophagocytic lymphohistiocytosis. Blood 2020, 135, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, M.; Steenkamp, V.; Stewart, M.J. Hepatic veno-occlusive disease as a result of a traditional remedy: Confirmation of toxic pyrrolizidine alkaloids as the cause, using an in vitro technique. J. Clin. Pathol. 2002, 55, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Q.; Ye, J.; Li, X.; Li, Q.; Song, Y.-H. Pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome: Pathogenesis, clinical manifestations, diagnosis, treatment, and outcomes. World J. Gastroenterol. 2019, 25, 3753–3763. [Google Scholar] [CrossRef] [PubMed]
- Palmer, W.C.; Vishnu, P.; Sanchez, W.; Aqel, B.; Riegert-Johnson, D.; Seaman, L.A.K.; Bowman, A.W.; Rivera, C.E. Diagnosis and Management of Genetic Iron Overload Disorders. J. Gen. Intern. Med. 2018, 33, 2230–2236. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.M.; Jaramillo, D.; Levy, H.L.; Knisely, A.S. Neonatal hemochromatosis: Diagnosis with MR imaging. Am. J. Roentgenol. 1992, 159, 623–625. [Google Scholar] [CrossRef]
- Alenezi, K.; Kamath, B.M.; Siddiqui, I.; Tomlinson, C.; Chavhan, G.B. Magnetic Resonance Imaging Findings in Neo-Natal Hemochromatosis. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 581–587. [Google Scholar] [CrossRef]
- Hoogstraten, J.; de Sa, D.J.; Knisely, A.S. Fetal liver disease may precede extrahepatic siderosis in neonatal hemochromatosis. Gastroenterology 1990, 98, 1699–1701. [Google Scholar] [CrossRef]
- Martí-Bonmatí, L.; Baamonde, A.; Poyatos, C.R.; Monteagudo, E. Prenatal diagnosis of idiopathic neonatal hemochromatosis with MRI. Abdom. Imaging 1994, 19, 55–56. [Google Scholar] [CrossRef]
- Concejo Iglesias, P.; Liébana de Rojas, C.; Bartolomé Mateos, A.; Risco Montemayor, B.; Rasero Ponferrada, M.; Galindo, A. Usefulness of dual gradient-echo MR imaging for the prenatal diagnosis of gestational alloimmune disease. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2021, 41, 981–983. [Google Scholar] [CrossRef]
- Schoennagel, B.P.; Remus, C.C.; Wedegaertner, U.; Salzmann, I.; Grabhorn, E.; Adam, G.; Fischer, R.; Harmatz, P.; Kooijman, H.; Yamamura, J. Quantification of prenatal liver and spleen iron in a sheep model and assessment of iron stores in a human neonate with neonatal hemochromatosis using R2* mapping. Magn. Reson. Med. Sci. MRMS Off. J. Jpn. Soc. Magn. Reson. Med. 2014, 13, 167–173. [Google Scholar] [CrossRef]
- Sethi, S.; Giza, S.A.; Goldberg, E.; Empey, M.E.E.T.; de Ribaupierre, S.; Eastabrook, G.D.M.; de Vrijer, B.; McKenzie, C.A. Quantification of 1.5 T T1 and T2 * Relaxation Times of Fetal Tissues in Uncomplicated Pregnancies. J. Magn. Reson. Imaging JMRI 2021, 54, 113–121. [Google Scholar] [CrossRef]
- Fujii, S.; Nagaishi, J.; Mukuda, N.; Kaneda, S.; Inoue, C.; Fukunaga, T.; Ogawa, T. Evaluation of Fetal Thyroid with 3D Gradient Echo T1-weighted MR Imaging. Magn. Reson. Med. Sci. MRMS Off. J. Jpn. Soc. Magn. Reson. Med. 2017, 16, 203–208. [Google Scholar]
- Staicu, A.; Albu, C.; Popa-Stanila, R.; Bondor, C.I.; Rotar, I.C.; Stamatian, F.; Muresan, D. Diagnostic value of virtual autopsy using pm-MRI at 3T on malformed second trimester fetuses vs. classic autopsy. PLoS ONE 2021, 16, e0260357. [Google Scholar] [CrossRef]
- Gissen, P.; Kelly, D. New hope for treatment of neonatal haemochromatosis. Lancet 2004, 364, 1644–1645. [Google Scholar] [CrossRef]
- Shneider, B.L. Genetic counseling in neonatal hemochromatosis. J. Pediatr. Gastroenterol. Nutr. 2002, 34, 328. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).