Women Have More Recurrences of Atrial Fibrillation than Men after Thoracoscopic Ablation and Suffer More from Established Risk Factors
Abstract
1. Introduction
2. Methods
3. Clinical Follow-Up
4. Histological Analysis of Atrial Interstitial Collagen
5. Statistical Analysis
6. Results
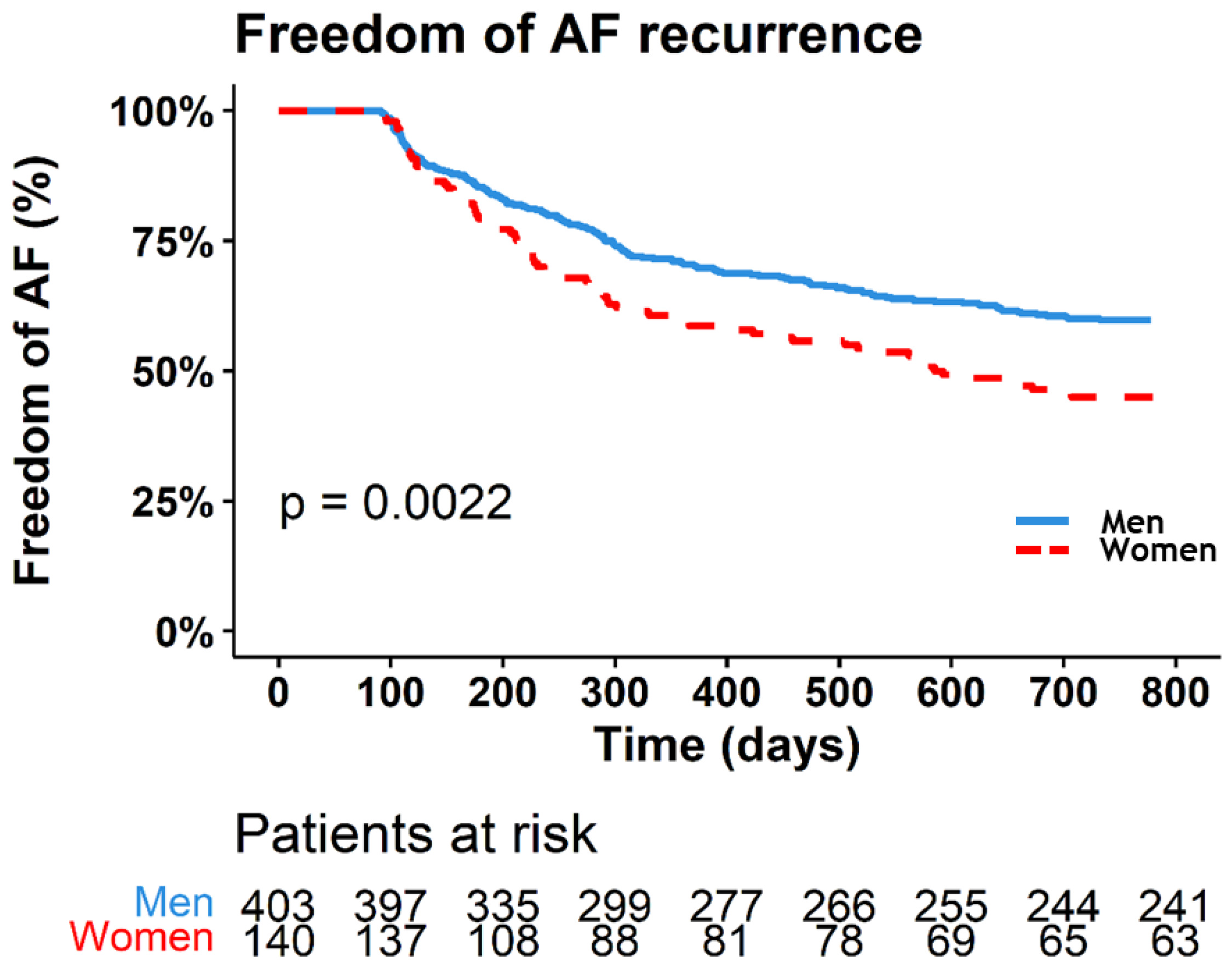
7. Freedom of AF Recurrence
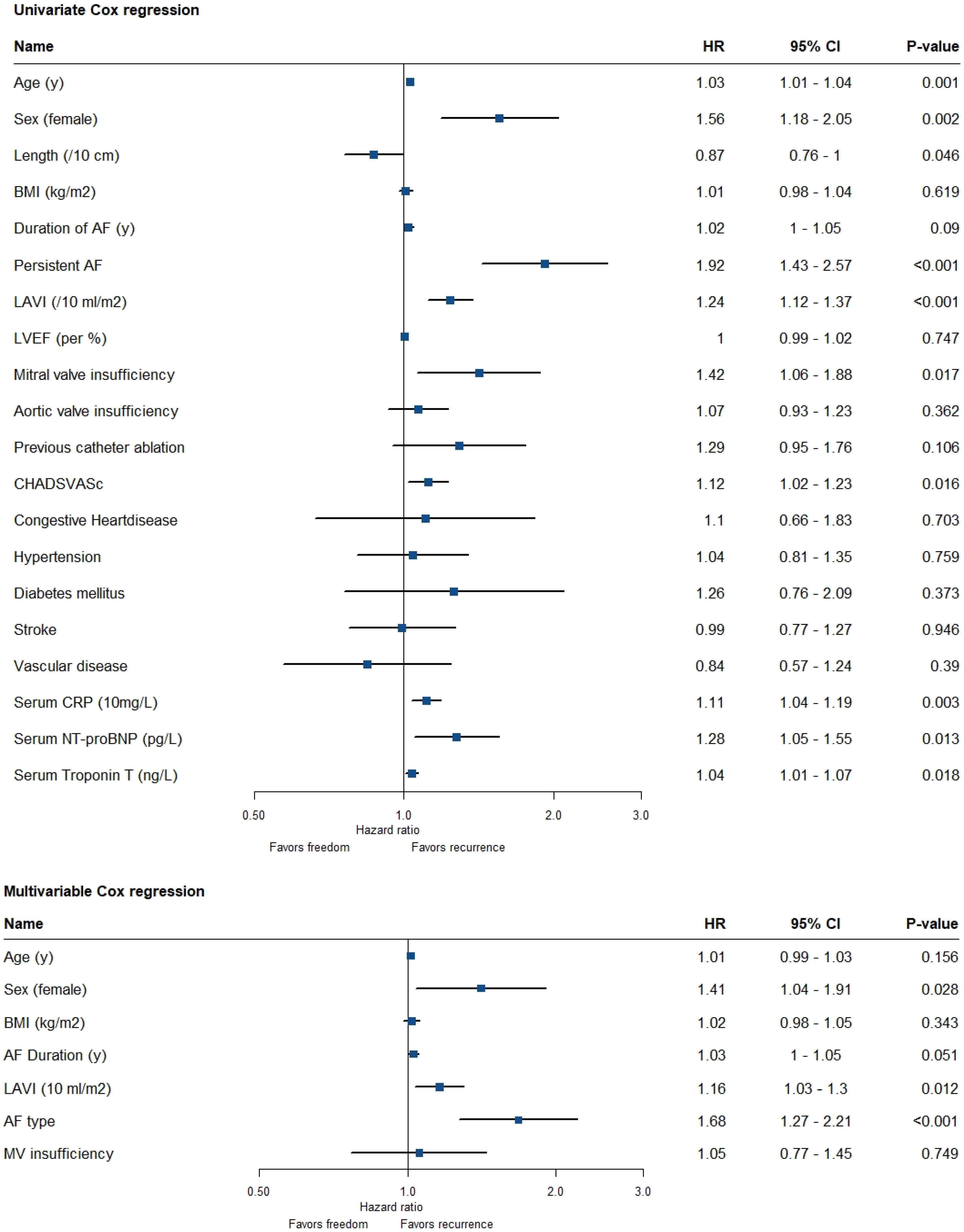
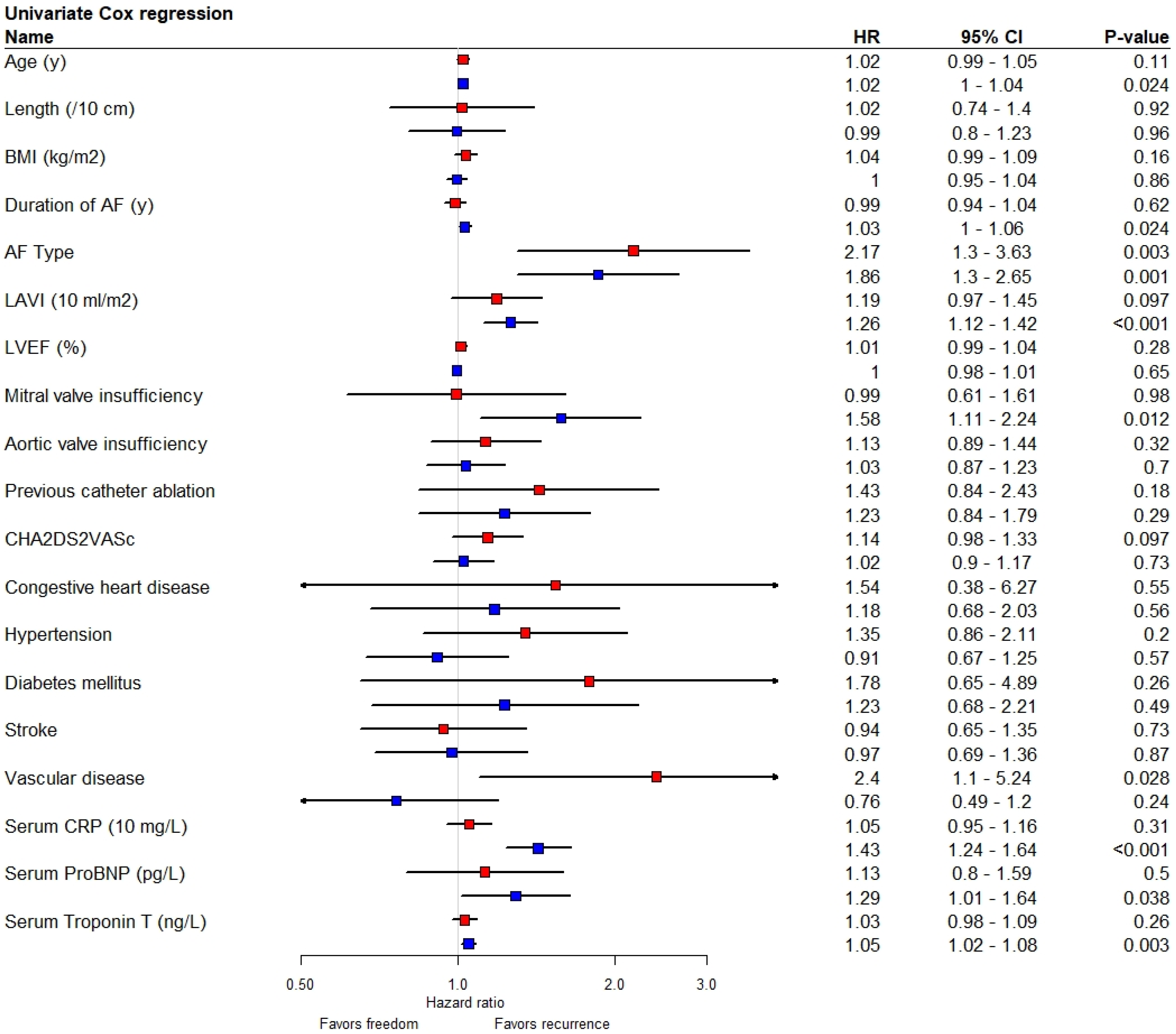
8. Association of Variables with Recurrence of AF
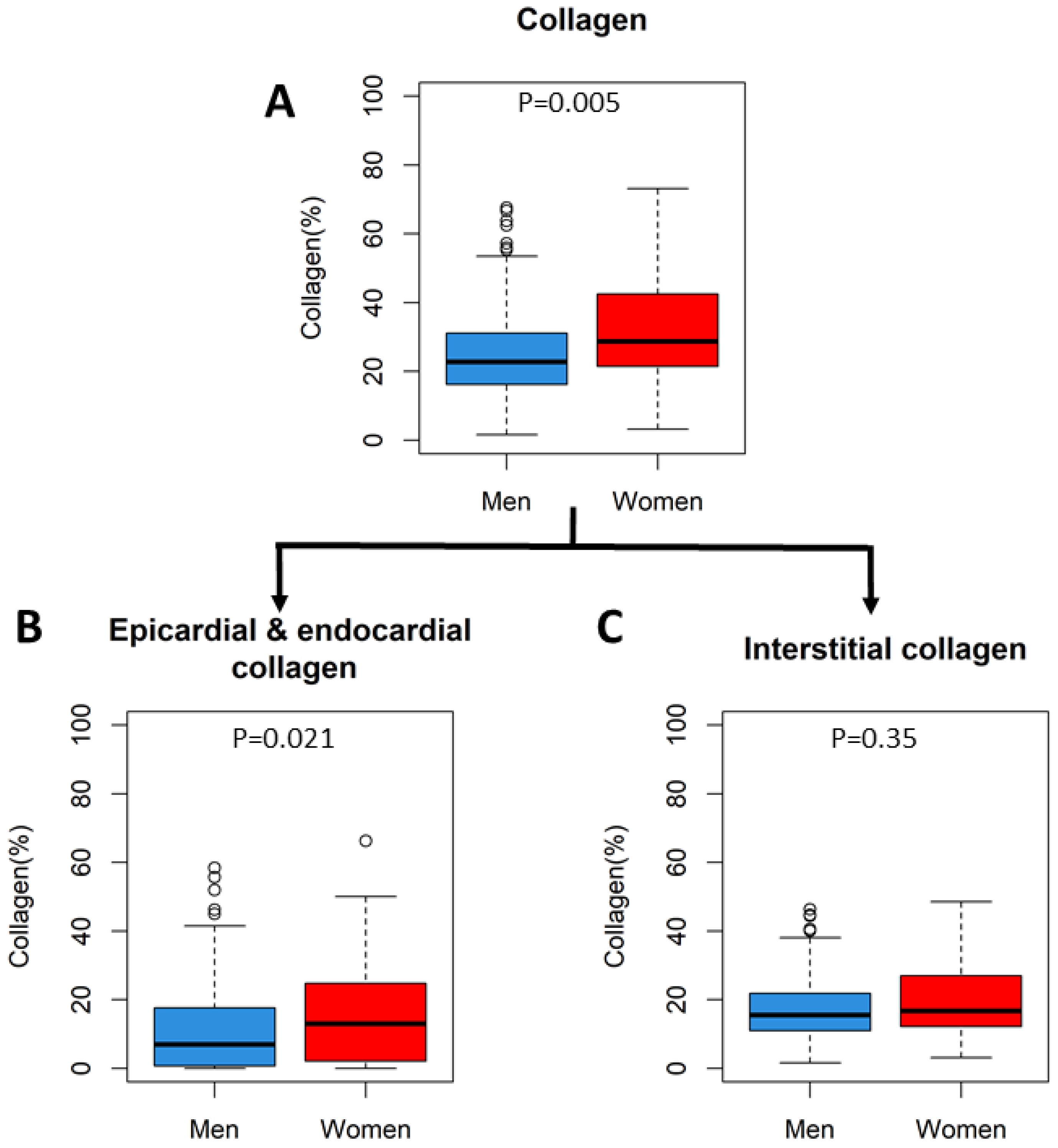
9. Histology
10. Discussion
11. Baseline Differences
12. Strengths and Limitations
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Krijthe, B.P.; Kunst, A.; Benjamin, E.J.; Lip, G.Y.; Franco, O.H.; Hofman, A.; Witteman, J.C.; Stricker, B.H.; Heeringa, J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur. Heart J. 2013, 34, 2746–2751. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.S.; McManus, D.D.; Newton-Cheh, C.; Lubitz, S.A.; Magnani, J.W.; Ellinor, P.T.; et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015, 386, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, C.; Niiranen, T.J.; Ojeda, F.M.; Gianfagna, F.; Blankenberg, S.; Njolstad, I.; Vartiainen, E.; Sans, S.; Pasterkamp, G.; Hughes, M.; et al. Sex Differences and Similarities in Atrial Fibrillation Epidemiology, Risk Factors, and Mortality in Community Cohorts: Results from the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation 2017, 136, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Zylla, M.M.; Brachmann, J.; Lewalter, T.; Hoffmann, E.; Kuck, K.H.; Andresen, D.; Willems, S.; Eckardt, L.; Tebbenjohanns, J.; Spitzer, S.G.; et al. Sex-related outcome of atrial fibrillation ablation: Insights from the German Ablation Registry. Heart Rhythm 2016, 13, 1837–1844. [Google Scholar] [CrossRef]
- Wong, J.A.; Rexrode, K.M.; Sandhu, R.K.; Conen, D.; Albert, C.M. Number of Pregnancies and Atrial Fibrillation Risk: The Women’s Health Study. Circulation 2017, 135, 622–624. [Google Scholar] [CrossRef]
- Ko, D.; Rahman, F.; Schnabel, R.B.; Yin, X.; Benjamin, E.J.; Christophersen, I.E. Atrial fibrillation in women: Epidemiology, pathophysiology, presentation, and prognosis. Nat. Rev. Cardiol. 2016, 13, 321–332. [Google Scholar] [CrossRef]
- Perez, M.V.; Wang, P.J.; Larson, J.C.; Virnig, B.A.; Cochrane, B.; Curb, J.D.; Klein, L.; Manson, J.E.; Martin, L.W.; Robinson, J.; et al. Effects of postmenopausal hormone therapy on incident atrial fibrillation: The Women’s Health Initiative randomized controlled trials. Circ. Arrhythmia Electrophysiol. 2012, 5, 1108–1116. [Google Scholar] [CrossRef]
- Magnani, J.W.; Moser, C.B.; Murabito, J.M.; Sullivan, L.M.; Wang, N.; Ellinor, P.T.; Vasan, R.S.; Benjamin, E.J.; Coviello, A.D. Association of sex hormones, aging, and atrial fibrillation in men: The Framingham Heart Study. Circ. Arrhythmia Electrophysiol. 2014, 7, 307–312. [Google Scholar] [CrossRef]
- Piccini, J.P.; Simon, D.N.; Steinberg, B.A.; Thomas, L.; Allen, L.A.; Fonarow, G.C.; Gersh, B.; Hylek, E.; Kowey, P.R.; Reiffel, J.A.; et al. Patients, Differences in Clinical and Functional Outcomes of Atrial Fibrillation in Women and Men: Two-Year Results from the ORBIT-AF Registry. JAMA Cardiol. 2016, 1, 282–291. [Google Scholar] [CrossRef]
- Andrade, J.G.; Deyell, M.W.; Lee, A.Y.K.; Macle, L. Sex Differences in Atrial Fibrillation. Can. J. Cardiol. 2018, 34, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Rienstra, M.; Van Veldhuisen, D.J.; Hagens, V.E.; Ranchor, A.V.; Veeger, N.J.; Crijns, H.J.; Van Gelder, I.C.; Investigators, R. Gender-related differences in rhythm control treatment in persistent atrial fibrillation: Data of the Rate Control Versus Electrical Cardioversion (RACE) study. J. Am. Coll. Cardiol. 2005, 46, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Hu, Q.; Gao, L.; Liu, J.; Qin, S.; Zhang, D. Sex-related differences in catheter ablation of atrial fibrillation: A systematic review and meta-analysis. Europace 2019, 21, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Wong, C.X.; Hsiao, A.J.; Altman, D.G.; Peters, S.A.; Woodward, M.; Odutayo, A.A. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: Systematic review and meta-analysis of cohort studies. BMJ 2016, 532, h7013. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2020, 42, 373–498. [Google Scholar]
- Mol, D.; Houterman, S.; Balt, J.C.; Bhagwandien, R.E.; Blaauw, Y.; Delnoy, P.-P.H.; van Driel, V.J.; Driessen, A.H.; Folkeringa, R.J.; Hassink, R.J.; et al. Complications in pulmonary vein isolation in the Netherlands Heart Registration differ with sex and ablation technique. EP Eur. 2020, 23, 216–225. [Google Scholar] [CrossRef]
- Dagres, N.; Nieuwlaat, R.; Vardas, P.E.; Andresen, D.; Levy, S.; Cobbe, S.; Kremastinos, D.T.; Breithardt, G.; Cokkinos, D.V.; Crijns, H.J. Gender-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: A report from the Euro Heart Survey on Atrial Fibrillation. J. Am. Coll. Cardiol. 2007, 49, 572–577. [Google Scholar] [CrossRef]
- Kaiser, D.W.; Fan, J.; Schmitt, S.; Than, C.T.; Ullal, A.J.; Piccini, J.P.; Heidenreich, P.A.; Turakhia, M.P. Gender Differences in Clinical Outcomes after Catheter Ablation of Atrial Fibrillation. JACC Clin. Electrophysiol. 2016, 2, 703–710. [Google Scholar] [CrossRef]
- Driessen, A.H.; Berger, W.R.; Krul, S.P.; van den Berg, N.W.; Neefs, J.; Piersma, F.R.; Chan Pin Yin, D.R.; de Jong, J.S.; van Boven, W.P.; de Groot, J.R. Ganglion Plexus Ablation in Advanced Atrial Fibrillation: The AFACT Study. J. Am. Coll. Cardiol. 2016, 68, 1155–1165. [Google Scholar] [CrossRef]
- Krul, S.P.; Driessen, A.H.; van Boven, W.J.; Linnenbank, A.C.; Geuzebroek, G.S.; Jackman, W.M.; Wilde, A.A.; de Bakker, J.M.; de Groot, J.R. Thoracoscopic video-assisted pulmonary vein antrum isolation, ganglionated plexus ablation, and periprocedural confirmation of ablation lesions: First results of a hybrid surgical-electrophysiological approach for atrial fibrillation. Circ. Arrhythmia Electrophysiol. 2011, 4, 262–270. [Google Scholar] [CrossRef]
- Edgerton, J.R.; Jackman, W.M.; Mack, M.J. A new epicardial lesion set for minimal access left atrial maze: The Dallas lesion set. Ann. Thorac. Surg. 2009, 88, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- de Groot, J.R.; Driessen, A.H.; Van Boven, W.J.; Krul, S.P.; Linnenbank, A.C.; Jackman, W.M.; De Bakker, J.M. Epicardial confirmation of conduction block during thoracoscopic surgery for atrial fibrillation--a hybrid surgical-electrophysiological approach. Minim. Invasive Ther. Allied Technol. 2012, 21, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018, 20, e1–e160. [Google Scholar] [CrossRef]
- Gharaviri, A.; Bidar, E.; Potse, M.; Zeemering, S.; Verheule, S.; Pezzuto, S.; Krause, R.; Maessen, J.G.; Auricchio, A.; Schotten, U. Epicardial Fibrosis Explains Increased Endo-Epicardial Dissociation and Epicardial Breakthroughs in Human Atrial Fibrillation. Front. Physiol. 2020, 11, 68. [Google Scholar] [CrossRef]
- Yunus, F.N.; Perino, A.C.; Holmes, D.N.; Matsouaka, R.A.; Curtis, A.B.; Ellenbogen, K.A.; Frankel, D.S.; Knight, B.P.; Russo, A.M.; Lewis, W.R.; et al. Sex Differences in Ablation Strategy, Lesion Sets, and Complications of Catheter Ablation for Atrial Fibrillation: An Analysis From the GWTG-AFIB Registry. Circ. Arrhythmia Electrophysiol. 2021, 14, e009790. [Google Scholar] [CrossRef] [PubMed]
- Kougioumtzoglou, A.M.; Neefs, J.; Wesselink, R.; Terpstra, M.M.; Van den Berg, N.W.E.; Berger, W.R.; Meulendijks, E.R.; Krul, S.P.J.; Piersma, F.R.; De Jong, J.S.S.G.; et al. HFpEF reverses in more than a quarter of patients after thoracoscopic AF ablation. Eur. Heart J. 2019, 40, 1132. [Google Scholar] [CrossRef]
- Al Alawi, A.M.; Al Busaidi, I.; Al Shibli, E.; Al-Senaidi, A.R.; Al Manwari, S.; Al Busaidi, I.; Muhanna, F.; Al Qassabi, A. Health outcomes after acute ischemic stroke:retrospective and survival analysis from Oman. Ann. Saudi Med. 2022, 42, 269–275. [Google Scholar] [CrossRef]
- Wankowicz, P.; Nowacki, P.; Golab-Janowska, M. Atrial fibrillation risk factors in patients with ischemic stroke. Arch. Med. Sci. 2021, 17, 19–24. [Google Scholar] [CrossRef]
- Wong, G.R.; Nalliah, C.J.; Lee, G.; Voskoboinik, A.; Chieng, D.; Prabhu, S.; Parameswaran, R.; Sugumar, H.; Al-Kaisey, A.; McLellan, A.; et al. Sex-Related Differences in Atrial Remodeling in Patients With Atrial Fibrillation: Relationship to Ablation Outcomes. Circ. Arrhythmia Electrophysiol. 2022, 15, e009925. [Google Scholar] [CrossRef]
- Lu, Z.; Aribas, E.; Geurts, S.; Roeters van Lennep, J.E.; Ikram, M.A.; Bos, M.M.; de Groot, N.M.S.; Kavousi, M. Association between Sex-Specific Risk Factors and Risk of New-Onset Atrial Fibrillation Among Women. JAMA Netw. Open 2022, 5, e2229716. [Google Scholar] [CrossRef]
- Hu, P.; Huang, J.; Lu, Y.; Zheng, M.; Li, H.; Duan, X.; Deng, H.; Zhao, W.; Liu, X. Circulating sex hormones and risk of atrial fibrillation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 952430. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Grimaldi, T.; Origliani, G.; Fantini, G.; Coppi, F.; Modena, M.G. Menopause and cardiovascular risk. Pathophysiol. Haemost. Thromb. 2002, 32, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Bretler, D.M.; Hansen, P.R.; Lindhardsen, J.; Ahlehoff, O.; Andersson, C.; Jensen, T.B.; Raunso, J.; Torp-Pedersen, C.; Gislason, G.H. Hormone Replacement Therapy and Risk of New-Onset Atrial Fibrillation after Myocardial Infarction—A Nationwide Cohort Study. PLoS ONE 2012, 7, e51580. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Haung, Y.B.; Kuo, H.F.; Tang, W.H.; Hsu, P.C.; Su, H.M.; Lin, T.H.; Chu, C.S.; Jhuo, S.J.; Lee, K.T.; et al. Hormone replacement therapy and risk of atrial fibrillation in Taiwanese menopause women: A nationwide cohort study. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.A.; Rexrode, K.M.; Sandhu, R.K.; Moorthy, M.V.; Conen, D.; Albert, C.M. Menopausal age, postmenopausal hormone therapy and incident atrial fibrillation. Heart 2017, 103, 1954–1961. [Google Scholar] [CrossRef]
- Berger, W.R.; Meulendijks, E.R.; Limpens, J.; van den Berg, N.W.E.; Neefs, J.; Driessen, A.H.G.; Krul, S.P.J.; van Boven, W.J.P.; de Groot, J.R. Persistent atrial fibrillation: A systematic review and meta-analysis of invasive strategies. Int. J. Cardiol. 2019, 278, 137–143. [Google Scholar] [CrossRef]
- Vos, L.M.; Kotecha, D.; Geuzebroek, G.S.C.; Hofman, F.N.; van Boven, W.J.P.; Kelder, J.; de Mol, B.; van Putte, B.P. Totally thoracoscopic ablation for atrial fibrillation: A systematic safety analysis. Europace 2018, 20, 1790–1797. [Google Scholar] [CrossRef]
- Russo, A.M.; Zeitler, E.P.; Giczewska, A.; Silverstein, A.P.; Al-Khalidi, H.R.; Cha, Y.M.; Monahan, K.H.; Bahnson, T.D.; Mark, D.B.; Packer, D.L.; et al. Association Between Sex and Treatment Outcomes of Atrial Fibrillation Ablation Versus Drug Therapy: Results From the CABANA Trial. Circulation 2021, 143, 661–672. [Google Scholar] [CrossRef]
- Lip, G.Y.; Laroche, C.; Boriani, G.; Cimaglia, P.; Dan, G.A.; Santini, M.; Kalarus, Z.; Rasmussen, L.H.; Popescu, M.I.; Tica, O.; et al. Sex-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: A report from the Euro Observational Research Programme Pilot survey on Atrial Fibrillation. Europace 2015, 17, 24–31. [Google Scholar] [CrossRef]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Sanchez-Somonte, P.; Gul, E.E.; Verma, A. The Importance of Arrhythmia Burden for Outcomes and Management Related to Catheter Ablation of Atrial Fibrillation. Korean Circ. J. 2021, 51, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.; McGuire, D.K.; Murphy, S.A.; Stanek, H.G.; Das, S.R.; Vongpatanasin, W.; Wians, F.H., Jr.; Grundy, S.M.; de Lemos, J.A. Race and gender differences in C-reactive protein levels. J. Am. Coll. Cardiol. 2005, 46, 464–469. [Google Scholar] [CrossRef] [PubMed]


| Women | Men | p-Value | Missing | |
|---|---|---|---|---|
| N | 143 | 428 | ||
| Age (y) | 63.3 (8.3) | 59.3 (8.5) | <0.001 | |
| BMI (kg/m2) | 27.3 (4.5) | 27.7 (3.6) | 0.243 | |
| Height (m) | 1.70 (0.07) | 1.84 (0.07) | <0.001 | |
| Weight (kg) | 79.0 (14.0) | 93.7 (13.3) | <0.001 | |
| Total duration AF (y) | 4 [2, 8] | 4 [2, 8] | 0.681 | 1.1 |
| Previous catheter ablation n (%) | 29 (20.3) | 79 (18.5) | 0.72 | |
| Myocardial infarction n (%) | 2 (1.4) | 28 (6.5) | 0.03 | |
| AF Type n (%) | 0.717 | |||
| Paroxysmal AF | 52 (36.4) | 155 (36.2) | ||
| Persistent AF | 91 (63.6) | 273 (63.8) | ||
| Serum biomarkers | ||||
| CRP (mg/L) | 2.00 [0.90, 4.28] | 1.30 [0.65, 2.90] | 0.006 | 17.9 |
| Troponin T (ng/L) | 6.0 [5.0, 10.0] | 9.0 [6.0, 19.75] | <0.001 | 22.6 |
| NT-proBNP (ng/L) | 425 [180, 916] | 256 [101, 618] | <0.001 | 6.8 |
| CHADSVASc | ||||
| Congestive HD n (%) | 5 (3.5) | 34 (7.9) | 0.102 | |
| Hypertension n (%) | 68 (47.6) | 182 (42.5) | 0.341 | |
| Age ≥ 65 n (%) | 69 (48.3) | 127 (29.7) | <0.001 | |
| Age ≥ 75 n (%) | 9 (6.3) | 8 (1.9) | 0.016 | |
| Diabetes mellitus n (%) | 6 (4.2) | 29 (6.8) | 0.362 | |
| Stroke n (%) | 17 (11.9) | 28 (6.5) | 0.061 | |
| Vascular Disease n (%) | 10 (7.0) | 69 (16.1) | 0.009 | |
| Female sex n (%) | 143 (100) | 0 (0) | ||
| CHA2DSVASc score | 2 [1, 3] | 1 [0, 2] | <0.001 | |
| Echocardiographic characteristics | ||||
| LVEF (%) | 53.0 (10.3) | 52.0 (10.2) | 0.361 | 14.5 |
| LAVI (mL/m2) | 42.6 (13.5) | 42.0 (12.3) | 0.649 | 9.1 |
| Mitral valve insufficiency n (%) | 0.001 | 1.1 | ||
| None | 13 (9.2) | 99 (23.4) | ||
| Mild | 85 (59.9) | 241 (57.0) | ||
| Moderate | 41 (28.9) | 77 (18.2) | ||
| Severe | 3 (2.1) | 6 (1.4) | ||
| Aorta valve insufficiency n (%) | 0.52 | 1.1 | ||
| None | 104 (73.2) | 328 (77.5) | ||
| Poor | 6 (4.2) | 15 (3.5) | ||
| Mild | 31 (21.8) | 73 (17.3) | ||
| Moderate | 1 (0.7) | 7 (1.7) | ||
| Antiarrhythmic medication | ||||
| Class IA n (%) | 8 (5.6) | 6 (1.4) | 0.013 | |
| Class IC n (%) | 41 (28.7) | 130 (30.4) | 0.78 | |
| Class II n (%) | 73 (51.0) | 199 (46.5) | 0.397 | |
| Class III n (%) | 56 (39.2) | 191 (44.6) | 0.296 | |
| Class IV n (%) | 20 (14.0) | 54 (12.6) | 0.781 | |
| Other medication | ||||
| ACE inhibitor n (%) | 27 (18.9) | 107 (25.0) | 0.167 | |
| Loop diuretics n (%) | 22 (15.4) | 36 (8.4) | 0.026 | |
| Thiazide diuretic n (%) | 19 (13.3) | 47 (11.0) | 1 | |
| Angiotensin II antagonist n (%) | 30 (21.0) | 74 (17.3) | 0.387 | |
| Calcium antagonist n (%) | 13 (9.1) | 42 (9.8) | 0.929 | |
| Cholesterol inhibitor n (%) | 34 (23.8) | 116 (27.1) | 0.501 | |
| Nitrates n (%) | 3 (2.1) | 9 (2.1) | 1 | |
| Potassium diuretics n (%) | 6 (4.2) | 19 (4.4) | 1 | |
| OAC n (%) | 142 (99.3) | 416 (97.2) | 0.256 | |
| Women (n = 143) | Men (n = 428) | |
|---|---|---|
| Completed 2Y FU | 140 | 403 |
| Freedom of AF | 61 (43.6%) | 241 (59.8%) |
| Atrial fibrillation recurrence | 26 (18.6%) | 76 (18.9%) |
| Atrial tachycardia/atrial flutter recurrence | 53 (37.9 %) | 86 (21.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wesselink, R.; Mossink, B.; Meulendijks, E.R.; van den Berg, N.W.E.; Neefs, J.; Kawasaki, M.; Fabrizi, B.; Piersma, F.R.; Al-Shama, R.F.M.; de Vries, T.A.C.; et al. Women Have More Recurrences of Atrial Fibrillation than Men after Thoracoscopic Ablation and Suffer More from Established Risk Factors. J. Clin. Med. 2023, 12, 2650. https://doi.org/10.3390/jcm12072650
Wesselink R, Mossink B, Meulendijks ER, van den Berg NWE, Neefs J, Kawasaki M, Fabrizi B, Piersma FR, Al-Shama RFM, de Vries TAC, et al. Women Have More Recurrences of Atrial Fibrillation than Men after Thoracoscopic Ablation and Suffer More from Established Risk Factors. Journal of Clinical Medicine. 2023; 12(7):2650. https://doi.org/10.3390/jcm12072650
Chicago/Turabian StyleWesselink, Robin, Bente Mossink, Eva R. Meulendijks, Nicoline W. E. van den Berg, Jolien Neefs, Makiri Kawasaki, Benedetta Fabrizi, Femke R. Piersma, Rushd F. M. Al-Shama, Tim A. C. de Vries, and et al. 2023. "Women Have More Recurrences of Atrial Fibrillation than Men after Thoracoscopic Ablation and Suffer More from Established Risk Factors" Journal of Clinical Medicine 12, no. 7: 2650. https://doi.org/10.3390/jcm12072650
APA StyleWesselink, R., Mossink, B., Meulendijks, E. R., van den Berg, N. W. E., Neefs, J., Kawasaki, M., Fabrizi, B., Piersma, F. R., Al-Shama, R. F. M., de Vries, T. A. C., de Jong, J. S. S. G., van Boven, W. J. P., Driessen, A. H. G., & de Groot, J. R. (2023). Women Have More Recurrences of Atrial Fibrillation than Men after Thoracoscopic Ablation and Suffer More from Established Risk Factors. Journal of Clinical Medicine, 12(7), 2650. https://doi.org/10.3390/jcm12072650





