Mortality, Intensive Care Unit Admission, and Intubation among Hospitalized Patients with COVID-19: A One-Year Retrospective Study in Jordan
Abstract
1. Introduction
2. Material and Methods
2.1. Design, Aim, and Setting
2.2. Operational Definitions
2.3. Characteristics of the Study Population
2.4. Data Collection
2.5. Ethical Statement
2.6. Statistical Analysis
3. Results
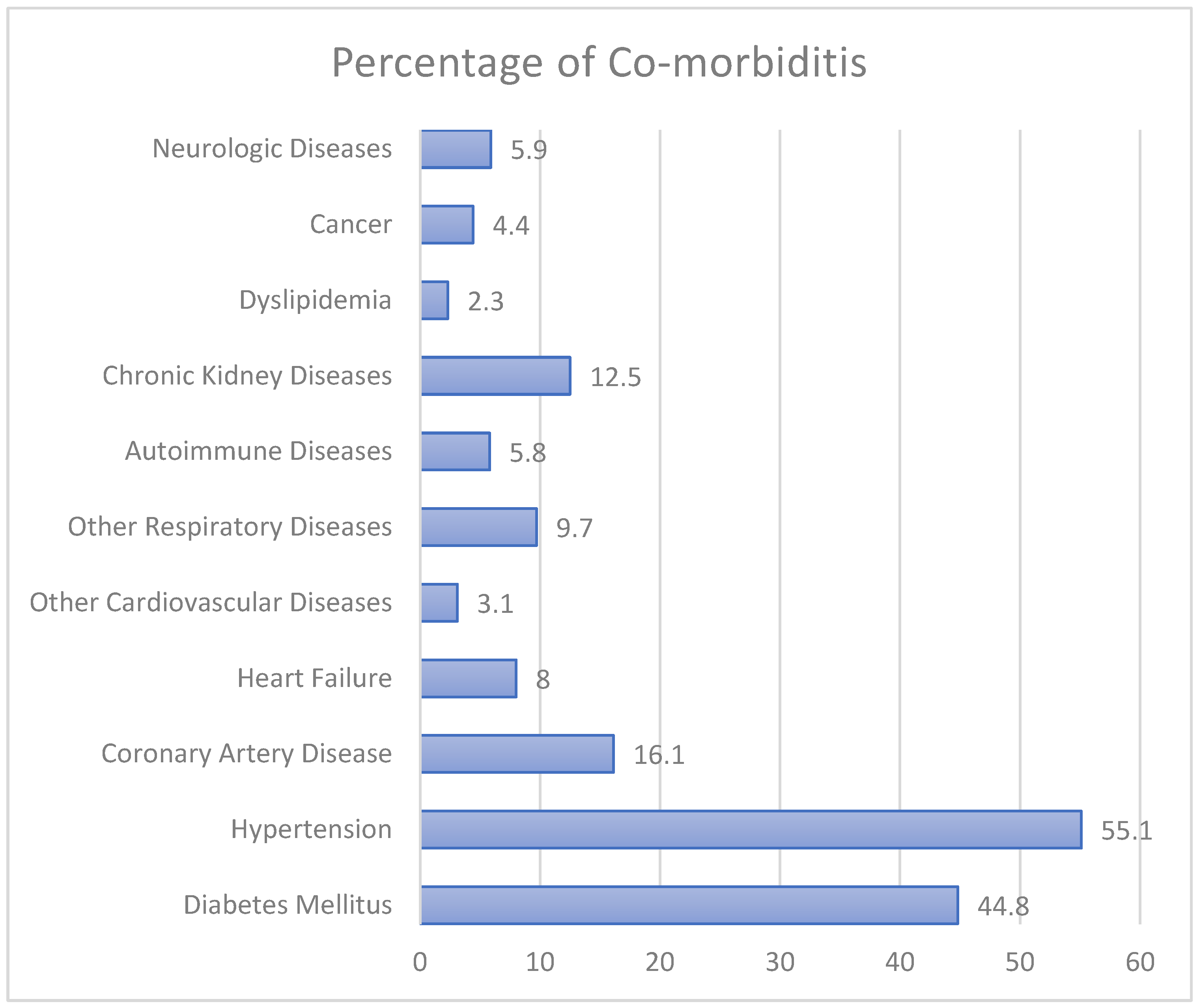
3.1. Demographic and Clinical Characteristics and Co-Morbid Conditions of Jordanian Patients with COVID-19
3.2. Clinical, Laboratory, and Imaging Parameters of Jordanian COVID-19 Patients (n = 745)
3.3. Predictors of COVID-19-Related Outcomes (Mortality, Intensive Care Unit Admission, and Intubation)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, C. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19). Braz. J. Implantol. Health Sci. 2020, 2. Available online: https://bjihs.emnuvens.com.br/bjihs/article/view/172 (accessed on 3 March 2023).
- Are, E.B.; Song, Y.; Stockdale, J.E.; Tupper, P.; Colijn, C. COVID-19 endgame: From pandemic to endemic? Vaccination, reopening and evolution in low-and high-vaccinated populations. J. Theor. Biol. 2023, 559, 111368. [Google Scholar] [CrossRef] [PubMed]
- Negru, P.A.; Radu, A.-F.; Vesa, C.M.; Behl, T.; Abdel-Daim, M.M.; Nechifor, A.C.; Endres, L.; Stoicescu, M.; Pasca, B.; Tit, D.M. Therapeutic dilemmas in addressing SARS-CoV-2 infection: Favipiravir versus Remdesivir. Biomed. Pharmacother. 2022, 147, 112700. [Google Scholar] [CrossRef] [PubMed]
- Isgrò, C.; Sardanelli, A.M.; Palese, L.L. Systematic search for SARS-CoV-2 main protease inhibitors for drug repurposing: Ethacrynic acid as a potential drug. Viruses 2021, 13, 106. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health (NIH). Clinical Spectrum of SARS-CoV-2 Infection. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 28 January 2021).
- Fitero, A.; Bungau, S.G.; Tit, D.M.; Endres, L.; Khan, S.A.; Bungau, A.F.; Romanul, I.; Vesa, C.M.; Radu, A.-F.; Tarce, A.G. Comorbidities, Associated Diseases, and Risk Assessment in COVID-19—A Systematic Review. Int. J. Clin. Pract. 2022, 2022, 1571826. [Google Scholar] [CrossRef]
- Li, X.; Xu, S.; Yu, M.; Wang, K.; Tao, Y.; Zhou, Y.; Shi, J.; Zhou, M.; Wu, B.; Yang, Z.; et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020, 146, 110–118. [Google Scholar] [CrossRef]
- Palaiodimos, L.; Kokkinidis, D.G.; Li, W.; Karamanis, D.; Ognibene, J.; Arora, S.; Southern, W.N.; Mantzoros, C.S. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metab. Clin. Exp. 2020, 108, 154262. [Google Scholar] [CrossRef]
- Parohan, M.; Yaghoubi, S.; Seraji, A.; Javanbakht, M.H.; Sarraf, P.; Djalali, M. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of observational studies. Aging Male Off. J. Int. Soc. Study Aging Male 2020, 23, 1416–1424. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Cecconi, M.; Piovani, D.; Brunetta, E.; Aghemo, A.; Greco, M.; Ciccarelli, M.; Angelini, C.; Voza, A.; Omodei, P.; Vespa, E.; et al. Early Predictors of Clinical Deterioration in a Cohort of 239 Patients Hospitalized for Covid-19 Infection in Lombardy, Italy. J. Clin. Med. 2020, 9, 1548. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef]
- Huang, S.; Wang, J.; Liu, F.; Liu, J.; Cao, G.; Yang, C.; Liu, W.; Tu, C.; Zhu, M.; Xiong, B. COVID-19 patients with hypertension have more severe disease: A multicenter retrospective observational study. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2020, 43, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, M.; Dong, Y.; Zhou, H.; Zhang, Z.; Tian, C.; Qin, R.; Wang, H.; Shen, Y.; Du, K.; et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes/Metab. Res. Rev. 2020, 36, e3319. [Google Scholar] [CrossRef]
- Alshukry, A.; Ali, H.; Ali, Y.; Al-Taweel, T.; Abu-Farha, M.; AbuBaker, J.; Devarajan, S.; Dashti, A.A.; Bandar, A.; Taleb, H.; et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) patients in Kuwait. PLoS ONE 2020, 15, e0242768. [Google Scholar] [CrossRef]
- Nassar, Y.; Mokhtar, A.; Elhadidy, A.; Elsayed, M.; Mostafa, F.; Rady, A.; Eladawy, A.; Elshazly, M.; Saeed, M.; Mokhtar, S.; et al. Outcomes and risk factors for death in patients with coronavirus disease-2019 (COVID-19) pneumonia admitted to the intensive care units of an Egyptian University Hospital. A retrospective cohort study. J. Infect. Public Health 2021, 14, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Al Awaidy, S.T.; Khamis, F.; Al Rashidi, B.; Al Wahaibi, A.H.; Albahri, A.; Mahomed, O. Epidemiological Characteristics of 69,382 COVID-19 Patients in Oman. J. Epidemiol. Glob. Health 2021, 11, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Alsayer, R.M.; Alsharif, H.M.; Al Baadani, A.M.; Kalam, K.A. Clinical and epidemiological characteristics of COVID-19 mortality in Saudi Arabia. Saudi Med. J. 2021, 42, 1083–1094. [Google Scholar] [CrossRef]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2023. Available online: https://covid19.who.int/ (accessed on 28 February 2023).
- Samrah, S.M.; Al-Mistarehi, A.-H.W.; Ibnian, A.M.; Raffee, L.A.; Momany, S.M.; Al-Ali, M.; Hayajneh, W.A.; Yusef, D.H.; Awad, S.M.; Khassawneh, B.Y. COVID-19 outbreak in Jordan: Epidemiological features, clinical characteristics, and laboratory findings. Ann. Med. Surg. 2020, 57, 103–108. [Google Scholar] [CrossRef]
- Velavan, T.P.; Pallerla, S.R.; Rüter, J.; Augustin, Y.; Kremsner, P.G.; Krishna, S.; Meyer, C.G. Host genetic factors determining COVID-19 susceptibility and severity. EBioMedicine 2021, 72, 103629. [Google Scholar] [CrossRef] [PubMed]
- Marois, G.; Muttarak, R.; Scherbov, S. Assessing the potential impact of COVID-19 on life expectancy. PLoS ONE 2020, 15, e0238678. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, Z.; Samra, J.K.; Mullainathan, S. Individual differences in normal body temperature: Longitudinal big data analysis of patient records. BMJ 2017, 359, j5468. [Google Scholar] [CrossRef]
- Marathe, P.H.; Gao, H.X.; Close, K.L. American Diabetes Association Standards o f Medical Care in Diabetes 2017. J. Diabetes 2017, 9, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.; Joannidis, M. Acute kidney injury 2016: Diagnosis and diagnostic workup. Crit. Care 2016, 20, 299. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Controlling and Monitoring the Tobacco Epidemic; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Findley, T.W.; Daum, M.C. Research in physical medicine and rehabilitation: III. The Chart Review or How to Use Clinical Data for Exploratory Retrospective Studies. Am. J. Phys. Med. Rehabil. 1991, 70, S23–S30. [Google Scholar] [CrossRef]
- Soares, R.C.M.; Mattos, L.R.; Raposo, L.M. Risk Factors for Hospitalization and Mortality due to COVID-19 in Espírito Santo State, Brazil. Am. J. Trop. Med. Hyg. 2020, 103, 1184–1190. [Google Scholar] [CrossRef]
- Alsofayan, Y.M.; Althunayyan, S.M.; Khan, A.A.; Hakawi, A.M.; Assiri, A.M. Clinical characteristics of COVID-19 in Saudi Arabia: A national retrospective study. J. Infect. Public Health 2020, 13, 920–925. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Byambasuren, O.; Cardona, M.; Bell, K.; Clark, J.; McLaws, M.-L.; Glasziou, P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: Systematic review and meta-analysis. Off. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2020, 5, 223–234. [Google Scholar]
- Yang, R.; Gui, X.; Xiong, Y. Comparison of Clinical Characteristics of Patients with Asymptomatic vs Symptomatic Coronavirus Disease 2019 in Wuhan, China. JAMA Netw. Open 2020, 3, e2010182. [Google Scholar] [CrossRef]
- Cheng, D.; Calderwood, C.; Skyllberg, E.; Ainley, A. Clinical characteristics and outcomes of adult patients admitted with COVID-19 in East London: A retrospective cohort analysis. BMJ Open Respir. Res. 2021, 8, e000813. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.; Ryan, P.; Rodríguez-Baño, J.; Jarrín, I.; Carratalà, J.; Pachón, J.; Yllescas, M.; Arriba, J.R. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 1525–1536. [Google Scholar] [CrossRef]
- Nachtigall, I.; Lenga, P.; Jóźwiak, K.; Thürmann, P.; Meier-Hellmann, A.; Kuhlen, R.; Brederlau, J.; Bauer, T.; Tebbenjohanns, J.; Schwegmann, K.; et al. Clinical course and factors associated with outcomes among 1904 patients hospitalized with COVID-19 in Germany: An observational study. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B.; Hu, L.; Ming, Q.; Wei, X.J.; Zhang, Z.Y.; Chen, L.D.; Wang, M.H.; Yao, W.Z.; Huang, Q.F.; Ye, Z.Q.; et al. Risk factors for mortality of coronavirus disease-2019 (COVID-19) patients in two centers of Hubei province, China: A retrospective analysis. PLoS ONE 2021, 16, e0246030. [Google Scholar] [CrossRef]
- Shi, C.; Wang, L.; Ye, J.; Gu, Z.; Wang, S.; Xia, J.; Xie, Y.; Li, Q.; Xu, R.; Lin, N. Predictors of mortality in patients with coronavirus disease 2019: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 663. [Google Scholar] [CrossRef]
- Henry, B.M.; Aggarwal, G.; Wong, J.; Benoit, S.; Vikse, J.; Plebani, M.; Lippi, G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am. J. Emerg. Med. 2020, 38, 1722–1726. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Bungau, S.G.; Radu, A.-F.; Batiha, G.E.-S. The potential molecular implications of adiponectin in the evolution of SARS-CoV-2: Inbuilt tendency. J. King Saud Univ.-Sci. 2022, 34, 102347. [Google Scholar] [CrossRef]
- Mejía, F.; Medina, C.; Cornejo, E.; Morello, E.; Vásquez, S.; Alave, J.; Schwalb, A.; Málaga, G. Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru. PLoS ONE 2020, 15, e0244171. [Google Scholar] [CrossRef]
- Sulaiman, T.; Algharawi, A.A.; Idrees, M.; Alzaidy, R.H.; Faris, K.; Cullingford, G.; Rasheed, J. The prevalence of gastrointestinal symptoms among patients with COVID-19 and the effect on the severity of the disease. JGH Open Open Access J. Gastroenterol. Hepatol. 2020, 4, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Tariq, R.; Saha, S.; Furqan, F.; Hassett, L.; Pardi, D.; Khanna, S. Prevalence and Mortality of COVID-19 Patients With Gastrointestinal Symptoms: A Systematic Review and Meta-analysis. Mayo Clin. Proc. 2020, 95, 1632–1648. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Jering, K.S.; Vaduganathan, M.; Claggett, B.L.; Cunningham, J.W.; Rosenthal, N.; Signorovitch, J.; Thune, J.J.; Vardeny, O.; Solomon, S.D. Clinical Outcomes in Patients With Heart Failure Hospitalized With COVID-19. JACC Heart Fail. 2021, 9, 65–73. [Google Scholar] [CrossRef]
- Hussain Alsayed, H.A.; Saheb Sharif-Askari, F.; Saheb Sharif-Askari, N.; Hussain, A.A.S.; Hamid, Q.; Halwani, R. Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: A retrospective cohort study. PLoS ONE 2021, 16, e0258643. [Google Scholar] [CrossRef]
- Lai, C.C.; Chen, C.H.; Wang, C.Y.; Chen, K.H.; Wang, Y.H.; Hsueh, P.R. Clinical efficacy and safety of remdesivir in patients with COVID-19: A systematic review and network meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 2021, 76, 1962–1968. [Google Scholar] [CrossRef]
- Dessie, Z.G.; Zewotir, T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef]
- Parra-Bracamonte, G.M.; Lopez-Villalobos, N.; Parra-Bracamonte, F.E. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large data set from Mexico. Ann. Epidemiol. 2020, 52, 93–98.e92. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Izquierdo, M.; Del Carmen Valero-Ubierna, M.; R-delAmo, J.L.; Fernández-García, M.; Martínez-Diz, S.; Tahery-Mahmoud, A.; Rodríguez-Camacho, M.; Gámiz-Molina, A.B.; Barba-Gyengo, N.; Gámez-Baeza, P.; et al. Sociodemographic, clinical and laboratory factors on admission associated with COVID-19 mortality in hospitalized patients: A retrospective observational study. PLoS ONE 2020, 15, e0235107. [Google Scholar] [CrossRef]
- Camelo, S.; Latil, M.; Agus, S.; Dioh, W.; Veillet, S.; Lafont, R.; Dilda, P.J. A comparison between virus-versus patients-centred therapeutic attempts to reduce COVID-19 mortality. Emerg. Microbes Infect. 2021, 10, 2256–2263. [Google Scholar] [CrossRef] [PubMed]
| Variable | |
|---|---|
| Age, mean (SD), years | 63.15 ± 15.99 |
| Sex | |
| Male, n (%) | 382 (51.3) |
| Female, n (%) | 363 (48.7) |
| Smoking status | |
| Non-smoker, n (%) | 645 (86.6) |
| Smoker, n (%) | 60 (8.1) |
| Ex-smoker, n (%) | 40 (5.4) |
| Admission | |
| Floor, n (%) | 524 (71.7) |
| Intensive care unit, n (%) | 207 (28.3) |
| Discharge, n (%) | 547 (74.7) |
| Vaccination status | |
| Not vaccinated, n (%) | 721 (96.8) |
| First shot, n (%) | 265 (35.6) |
| Second shot, n (%) | 480 (64.4) |
| Chief Complaints | |
| Generalized weakness, n (%) | 110 (15.0) |
| Shortness of breath, n (%) | 256 (35.0) |
| Cough, n (%) | 113 (15.5) |
| Fever, n (%) | 89 (12.2) |
| GI symptoms, n (%) | 50 (6.8) |
| Chest pain | 27 (3.7) |
| Asymptomatic, n (%) | 4 (0.5) |
| Others, n (%) | 71 (9.7) |
| Fatigue | |
| Yes, n (%) | 366 (49.6) |
| Fever reported/prior to admission | |
| Yes, n (%) | 348 (47.2) |
| Chills | |
| Yes, n (%) | 265 (35.9) |
| GI Symptoms | |
| Yes, n (%) | 226 (30.4) |
| Headache | |
| Yes, n (%) | 82 (11.0) |
| Nasal Discharge | |
| Yes, n (%) | 34 (4.6) |
| Sore throat | |
| Yes, n (%) | 60 (8.1) |
| Chest pain | |
| Yes, n (%) | 207(27.8) |
| Shortness of Breath | |
| Yes, n (%) | 455(61.2) |
| Cough | |
| Yes, n (%) | 492(66.6) |
| COVID-19-related health outcomes | |
| COVID-19-related death, n (%) | 171 (23.0) |
| Non-COVID-19-related death, n (%) | 14 (2.3) |
| Discharge, n (%) | 97 (46.9) |
| Outcomes among ICU patients | |
| COVID-19-related death, n (%) | 110 (53.1) |
| Variable | |
|---|---|
| Chest X-ray | |
| Clear, n (%) | 82 (11.7) |
| Chest X-ray, n (%) | 122 (17.4) |
| Bilateral infiltrate, n (%) | 496 (70.9) |
| High-Resolution Computed Tomography | |
| Clear, n (%) | 3 (5.0) |
| Ground glass, n (%) | 70 (70.0) |
| Fibrotic changes, n (%) | 25 (25.0) |
| Computed Tomography Pulmonary Angiography | |
| Negative, n (%) | 37 (86.0) |
| Positive, n (%) | 6 (14.0) |
| Troponin | |
| Negative, n (%) | 377 (78.9) |
| Positive, n (%) | 101 (21.1) |
| D-Dimer | |
| Negative, n (%) | 91 (14.3) |
| Positive, n (%) | 546 (85.7) |
| Acute Kidney Injury (Creatinine) | |
| Negative, n (%) | 511 (69.5) |
| Positive, n (%) | 224 (30.5) |
| Last HBA1C | |
| Controlled, n (%) | 190 (87.6) |
| Uncontrolled, n (%) | 27 (12.4) |
| O2 Saturation | |
| ≥94, n (%) | 239 (33.7) |
| 90–94, n (%) | 173 (24.4) |
| <90, n (%) | 297 (419) |
| Documented Fever | |
| Yes, n (%) | 578 (77.5) |
| No, n (%) | 168 (22.5) |
| Treatments Used | |
| Steroids, n (%) | 656 (88.6) |
| Tocilizumab, n (%) | 68 (9.1) |
| Remdesivir, n (%) | 183 (24.6) |
| Variables | |
|---|---|
| IL-6 (pg/mL), median (IQR) | 13.00 (22) |
| Brain natriuretic peptide (pg/mL) | 74.75 (75) |
| Pro-calcitonin (ng/mL) | 0.10 (0) |
| Hemoglobin (g/dL) | 13.05 (1.85) |
| White blood cell count | 9.2 × 103 (7.58 × 103) |
| Neutrophil absolute count | 7.97× 103 (6.63 × 103) |
| Lymphocyte absolute count | 0.85 × 103 (0.42 × 103) |
| Platelet count | 189.00 × 103 (190.00 × 103) |
| C-Reactive protein (mg/L) | 99.95 (138.35) |
| Ferritin (ng/mL) | 330.95 (367.00) |
| Lactate dehydrogenase (u/L) | 768.00 (406.00) |
| Potassium (mg/dL) | 4.30 (0.98) |
| Sodium (mg/dL) | 139.50 (8.80) |
| Urea (mg/dL) | 61.55 (40.50) |
| Alanine transaminase (u/L) | 31.00 (22.00) |
| Aspartate transaminase (u/L) | 40.00(53.00) |
| Total bilirubin mg/dL | 0.50 (0.00) |
| Direct bilirubin mg/dL | 0.20 (0.00) |
| COVID-19-Related Mortality | |||||
|---|---|---|---|---|---|
| Variable | Response | Correlation Coefficient | (95% CI) | Adjusted Odds Ratio (OR) | (95% CI) |
| Age | 1.047 | (1.033–1.061) * | 1.013 | (0.984–1.042) | |
| Gender | Male | 1.067 | (0.764–1.489) | ||
| Female | |||||
| Smoking status | Smoker | 0.523 | (0.252–1.088) | ||
| Ex-smoker | 1.566 | (0.798–3.071) | |||
| Chief complaints | Fatigue | R | R | R | R |
| SOB | 0.772 | (0.471–1.265) | 0.572 | (0.201–1.633) | |
| Cough | 0.544 | (0.293–1.010) | 0.433 | (0.112–1.671) | |
| Fever | 0.372 | (0.182–0.761) | 0.824 | (0.231–2.937) | |
| Atypical | 1.183 | (0.627–2.230) | 1.013 | (0.220–4.670) | |
| GI symptoms | 0.558 | (0.250–1.248) | 0.484 | (0.111–2.120) | |
| Chest pain | 0.622 | (0.230–1.680) | 0.928 | (0.163–5.280) | |
| Asymptomatic | - | - | - | - | |
| Headache | 0.544 | (0.059–5.053) | |||
| Runny nose | |||||
| GI symptoms | Yes | 0.722 | (0.495–1.052) | ||
| Diabetes mellitus | - | 1.497 | (1.071–2.092) | 1.220 | (0.567–2.623) |
| Hypertension | - | 1.738 | (1.229–2.457) | 1.123 | (0.484–2.606) |
| Coronary artery disease | - | 1.582 | (1.033–2.423) | 0.734 | (0.308–1.752) * |
| Chronic kidney disease | - | 3.501 | (2.229–5.499) | 3.831 | (1.179–12.446) |
| Asthma | - | 0.851 | (0.397–1.823) | - | - |
| COPD | - | 0.434 | (0.128–1.479) | - | - |
| Dyslipidemia | - | 0.628 | (0.178–2.209) | - | - |
| Cancer | - | 0.985 | (0.435–2.232) | - | - |
| Neurologic diseases | - | 3.056 | (1.639–5.698) * | 0.843 | (0.197–3.617) |
| Heart failure | - | 4.191 | (2.423–7.249) | 0.956 | (0.298–3.065) |
| Autoimmune diseases | - | 1.462 | (0.755–2.832) | - | - |
| Other respiratory disease | - | 1.462 | (0.755–2.832) | - | - |
| Other cardiovascular diseases | - | 1.281 | (0.576–2.849) | ||
| Documented fever | 1.132 | (0.765–1.675) | |||
| Chest X-ray | |||||
| Unilateral changes | 3.422 | (1.621–7.360) | 3.995 | (0.744–21.456) | |
| Bilateral changes | 3.454 | (1.621–7.360) * | 2.187 | (0.488–9.797) | |
| Computerized tomographic pulmonary angiography | Positive | 0.540 | (0.056–5.208) | ||
| IL-6 | - | 1.004 | (0.993–1.014) | ||
| BNP | - | 1.001 | (1.000–1.001) | ||
| Troponin | - | 4.641 | (2.917–7.383) | 3.060 | (1.156–8.102) * |
| Procalcitonin | - | 0.788 | (0.728–0.852) * | 0.896 | (0.759–1.058) |
| Hemoglobin | - | 0.788 | (0.728–0.852) * | 0.896 | (0.759–1.058) |
| White blood cells | - | 1.035 | (1.009–1.062) * | 0.835 | (0.613–1.138) |
| Neutrophils | - | 1.125 | (1.084–1.167) * | 1.306 | (0.933–1.828) |
| Lymphocytes | 1.013 | (0.971–1.058) | |||
| Platelets | 1.000 | (1.000–1.001) | |||
| CRP | 1.000 | (1.000–1.000) | |||
| Ferritin | 1.001 | (1.000–1.001) | |||
| D-dimer | 3.680 | (1.803–7.509) * | 0.945 | (0.270–3.305) | |
| LDH | 1.001 | (1.001–1.002) * | 1.002 | (1.001–1.003) * | |
| Creatinine | 3.756 | (2.640–5.343) * | 1.128 | (0.448–2.840) | |
| Potassium | 1.008 | (1.002–1.014) * | 0.986 | (0.922–1.056) | |
| Sodium | 1.001 | (0.995–1.006) | |||
| Urea | 1.000 | (1.000–1.000) | |||
| ALT | 1.000 | (0.999–1.002) | |||
| AST | 1.002 | (1.000–1.003) | |||
| Bilirubin | 1.222 | (1.058–1.410) * | 0.837 | (0.450–1.555) | |
| Direct bilirubin | 1.001 | (0.995–1.008) | |||
| Last HBA1C | 2.146 | (0.886–5.197) | |||
| O2 saturation | |||||
| ≥94 | R | R | R | ||
| 90–94 | 1.186 | (0.708–1.986) | 1.558 | (0.515–4.711) | |
| <90 | 2.630 | (1.729–3.998) * | 2.761 | (1.066–7.155) * | |
| Steroids | Yes | 1.609 | (0.896–2.890) | ||
| Tocilizumab | Yes | 1.584 | (0.930–2.698) | ||
| Remedisvir | Yes | 0.916 | (0.621–1.352) | ||
| COVID Related ICU Admission | |||||
|---|---|---|---|---|---|
| Variable | Response | Correlation Coefficient | (95% CI) | Adjusted Odds Ratio (OR) | (95% CI) |
| Age | 1.034 | (1.021–1.046) * | 0.983 | (0.936–1.033) | |
| Gender | Male | 0.968 | (0.702–1.336) | ||
| Female | |||||
| Smoking status | Smoker | 0.643 | (0.333–1.238) | 0.796 | (0.088–7.169) |
| Ex-smoker | 2.203 | (1.147–4.234) * | 1.174 | (0.077–17.827) | |
| Chief complaints | Fatigue | R | R | R | R |
| SOB | 1.115 | (0.680–1.828) | |||
| Cough | 0.762 | (0.416–1.393) | |||
| Fever | 0.888 | (0.473–1.669) | |||
| Atypical | 1.216 | (0.625–2.366) | |||
| GI symptoms | 0.920 | (0.431–1.964) | |||
| Chest pain | 0.765 | (0.281–2.083) | |||
| Asymptomatic | - | - | |||
| Headache | 1.699 | (0.271–10.663) | |||
| Runny nose | - | - | |||
| GI symptoms | Yes | 0.584 | (0.402–0.848) | 0.316 | (0.056–1.791) |
| Diabetes mellitus | - | 1.967 | (1.420–2.724) * | 1.136 | (0.299–4.310) |
| Hypertension | - | 1.675 | (1.201–2.336) * | 0.638 | (0.126–3.227) |
| Coronary artery disease | - | 1.066 | (0.692–1.642) | ||
| Chronic kidney disease | - | 2.542 | (1.627–3.972) * | 0.637 | (0.077–5.306) |
| Asthma | - | 0.748 | (0.349–1.605) | ||
| COPD | - | 0.890 | (0.346–2.290) | ||
| Dyslipidemia | - | 1.799 | (0.675–4.791) | ||
| Cancer | - | 0.670 | (0.286–1.569) | ||
| Neurologic diseases | - | 1.714 | (0.909–3.230) | ||
| Heart failure | - | 4.239 | (2.452–7.330) * | 7.894 | (1.391–44.818) |
| Autoimmune diseases | - | 0.755 | (0.365–1.562) | ||
| Other respiratory disease | - | 1.127 | (0.664–1.914) | ||
| Other cardiovascular diseases | - | 3.052 | (1.461–6.372) * | 0.340 | (0.018–6.556) |
| Documented fever | 1.096 | (0.750–1.600) | |||
| Chest X-ray | |||||
| Unilateral changes | 2.884 | (1.295–6.423) * | 2.245 | (0.165–30.617) | |
| Bilateral changes | 3.554 | (1.730–7.300) * | 1.465 | (0.126–16.988) | |
| Computerized tomographic pulmonary angiography | Positive | 1.350 | (0.213–8.551) | ||
| IL-6 | - | 1.000 | (0.989–1.010) | ||
| BNP | - | 1.000 | (1.000–1.001) | ||
| Troponin | - | 3.384 | (2.139–5.352) * | 0.655 | (0.118–3.620) |
| Procalcitonin | - | 1.013 | (0.971–1.058) | ||
| Hemoglobin | - | 0.853 | (0.792–0.918) * | 0.771 | (0.554–1.072) |
| White blood cells | - | 1.013 | (0.996–1.030) | ||
| Neutrophils | - | 1.058 | (1.025–1.092) * | 1.003 | (0.984–1.023) |
| Lymphocytes | 1.011 | (0.969–1.055) | |||
| Platelets | 1.000 | (1.000–1.001) | |||
| CRP | 1.000 | (0.999–1.000) | |||
| Ferritin | 1.001 | (1.000–1.001) | |||
| D-dimer | 2.274 | (1.268–4.077) * | 1.907 | (0.087–41.862) | |
| LDH | 1.001 | (1.000–1.001) | |||
| Creatinine | 2.327 | (1.657–3.268) * | 0.901 | (0.176–4.608) | |
| Potassium | 1.013 | (1.006–1.019) * | 1.051 | (0.951–1.161) | |
| Sodium | 0.992 | (0.987–0.997) * | 1.024 | (0.932–1.125) | |
| Urea | 1.001 | (1.000–1.000) | |||
| ALT | 1.001 | (0.999–1.003) | |||
| AST | 1.000 | (0.999–1.001) | |||
| Bilirubin | 1.093 | (0.959–1.245) | |||
| Direct bilirubin | 1.006 | (1.000–1.013) | |||
| Last HBA1C | 0.987 | (0.349–2.788) | |||
| O2 saturation | |||||
| ≥94 | R | R | R | R | |
| 90–94 | 1.023 | (0.616–1.698) | 1.020 | (0.141–7.397) | |
| <90 | 2.945 | (1.965–4.412) * | 0.938 | (0.162–5.417) | |
| Steroids | Yes | 0.782 | (0.471–1.300) | ||
| Tocilizumab | Yes | 2.479 | (1.494–4.114) * | 1.675 | (0.294–9.524) |
| Remedisvir | Yes | 0.591 | (0.414–0.845) * | 0.192 | (0.044–0.835) * |
| COVID-19-Related Mortality | |||||
|---|---|---|---|---|---|
| Variable | Response | Correlation Coefficient | (95% CI) | Adjusted Odds Ratio (OR) | (95% CI) |
| Age | 1.012 | (0.997–1.028) | |||
| Gender | Male | 1.121 | (0.704–1.785) | ||
| Female | |||||
| Smoking status | Smoker | 1.168 | (0.510–2.676) | ||
| Ex-smoker | 2.212 | (0.978–5.002) | |||
| Chief complaints | Fatigue | R | R | ||
| SOB | 1.143 | (0.578–2.261) | |||
| Cough | 0.658 | (0.269–1.610) | |||
| Fever | 0.539 | (0.196–1.482) | |||
| Atypical | 1.368 | (0.576–3.249) | |||
| GI symptoms | 0.318 | (0.069–1.465) | |||
| Chest pain | 0.597 | (0.126–2.819) | |||
| Asymptomatic | |||||
| Headache | 1.865 | (0.193–17.992) | |||
| Runny nose | |||||
| GI symptoms | Yes | 0.538 | (0.304–0.954) * | 0.323 | (0.105–0.993) * |
| Diabetes mellitus | - | 1.177 | (0.740–1.873) | ||
| Hypertension | - | 1.093 | (0.684–1.748) | ||
| Coronary artery disease | - | 0.634 | (0.308–1.306) | ||
| Chronic kidney disease | - | 2.074 | (1.153–3.728) * | 3.640 | (0.895–14.802) |
| Asthma | - | 0.656 | (0.198–2.178) | ||
| COPD | - | 1.776 | (0.589–5.356) | ||
| Dyslipidemia | - | ||||
| Cancer | - | 1.188 | (0.406–3.478) | ||
| Neurologic diseases | - | 4.075 | (2.028–8.188) * | 0.733 | (0.144–3.732) |
| Heart failure | - | 0.754 | (0.344–1.651) | ||
| Autoimmune diseases | - | 0.604 | (0.182–1.999) | ||
| Other respiratory disease | - | 1.035 | (0.477–2.245) | ||
| Other cardiovascular diseases | - | 1.283 | (0.436–3.776) | ||
| Documented fever | 1.163 | (0.679–1.993) | |||
| Chest X-ray | |||||
| Unilateral changes | 4.558 | (1.297–16.016) * | 6.307 | (0.766–51.925) | |
| Bilateral changes | 3.375 | (1.031–11.048) * | 1.820 | (0.259–12.790) | |
| Computerized tomographic pulmonary angiography | Positive | ||||
| IL-6 | - | 1.006 | (0.995–1.017) | ||
| BNP | - | 1.000 | (1.000–1.001) | ||
| Troponin | - | 2.974 | (1.693–5.223) * | 1.544 | (0.563–4.234) |
| Procalcitonin | - | 1.034 | (0.989–1.081) | ||
| Hemoglobin | - | 0.812 | (0.733–0.899) | 0.917 | (0.752–1.118) |
| White blood cells | - | 1.001 | (0.997–1.005) | ||
| Neutrophils | - | 1.002 | (0.997–1.006) | ||
| Lymphocytes | 0.931 | (0.769–1.128) | |||
| Platelets | 0.999 | (0.997–1.000) | |||
| CRP | 1.000 | (1.000–1.000) | |||
| Ferritin | 1.001 | (1.000–1.001) | |||
| D-dimer | 2.951 | (1.048–8.310) * | 2.766 | (0.310–24.703) | |
| LDH | 1.001 | (1.001–1.002) * | 1.001 | (1.000–1.002) | |
| Creatinine | 2.306 | (1.440–3.693) * | 1.856 | (0.643–5.357) | |
| Potassium | 0.926 | (0.843–1.017) | |||
| Sodium | 1.027 | (1.007–1.047) * | 1.046 | (0.992–1.103) | |
| Urea | 1.000 | (1.000–1.000) | |||
| ALT | 1.004 | (1.002–1.007) * | 1.005 | (0.996–1.014) | |
| AST | 1.003 | (1.001–1.005) * | 1.001 | (0.999–1.003) | |
| Bilirubin | 1.090 | (0.939–1.264) | |||
| Direct bilirubin | 0.954 | (0.900–1.011) | |||
| Last HBA1C | 0.764 | (0.167–3.495) | |||
| O2 saturation | - | - | - | - | |
| ≥94 | R | R | R | R | |
| 90–94 | 1.666 | (0.750–3.697) | 5.536 | (0.771–39.750) | |
| <90 | 4.015 | (2.089–7.715) * | 16.585 | (2.892–95.118) * | |
| Steroids | Yes | 5.533 | (1.334–22.947) * | 0.551 | (0.077–3.940) |
| Tocilizumab | Yes | 1.483 | (0.725–3.032) | ||
| Remedisvir | Yes | 1.094 | (0.645–1.856) | ||
| COVID-19 Related Length of Hospital Stay | |||||
|---|---|---|---|---|---|
| Variable | Response | (CB (95% CI) | (95% CI) | AB (95% CI) | (95% CI) |
| Age | 0.015 | (−0.027–0.057) | |||
| Gender | Male | −1.158 | (−2.478–0.162) | ||
| Female | |||||
| Smoking status | Smoker | 0.945 | 0.945 | ||
| Ex-smoker | 0.730 | (−0.924–1.109) | |||
| Chief complaints | Fatigue | −0.500 | (−1.077–0.177) | ||
| SOB | 1.062 | (2.358–−0.299) | |||
| Cough | −0.652 | (−1.370–0.120) | |||
| Fever | 0.334 | (−0.344–1.012) | |||
| Atypical | 0.211 | (−0.930–1.945) | |||
| GI symptoms | −1.260 | (−1.992–0.023) | |||
| Chest pain | 0.444 | (−1.203–1.975) | |||
| Asymptomatic | −0.399 | (−1.485–0.310 | |||
| Headache | −1.205 | (−2.354–0.132) | |||
| Runny nose | −1.762 | (−2.214–0.256) | |||
| GI symptoms | Yes | −1.260 | (−2.697–0.178) | ||
| Diabetes mellitus | - | 0.766 | (−0.562–2.094) | ||
| Hypertension | - | 0.994 | (−0.335–2.323) | ||
| Coronary artery disease | - | 0.781 | (−1.006–2.568) | ||
| Chronic kidney disease | - | −0.395 | (−2.384–1.595) | ||
| Asthma | - | 0.011 | (−2.961–2.984) | ||
| COPD | - | −2.163 | (−5.940–1.613) | ||
| Dyslipidemia | - | 0.455 | (−3.933–4.823) | ||
| Cancer | - | −1.052 | (−4.229–2.126) | ||
| Neurologic diseases | - | 2.074 | (−0.726–4.875) | ||
| Heart failure | - | 1.372 | (−1.048–3.793) | ||
| Autoimmune diseases | - | 1.491 | (−1.342–4.325) | ||
| Other respiratory disease | - | −0.681 | (−3.877–2.775) | ||
| Other cardiovascular diseases | - | −0.551 | (−3.877–2.775) | ||
| Documented fever | 0.724 | (−0.848–2.296) | |||
| Chest X-ray | |||||
| Unilateral changes | 0.856 | (−0.226–1.976) | |||
| Bilateral changes | 1.129 | (0.140–2.118) * | 0.631 | (−1.075–2.337) | |
| Computerized tomographic pulmonary angiography | Positive | −10.207 | (−21.663–1.248) | ||
| IL-6 | - | −0.023 | (−0.063–0.016) | ||
| BNP | - | ||||
| Troponin | - | −0.607 | (−2.708–1.494) | ||
| Procalcitonin | - | 0.198 | (−0.004–0.400) | ||
| Hemoglobin | - | −0.318 | (−0.618–−0.019) | 0.164 | (−0.323–0.651) |
| White blood cells | - | 0.002 | (−0.014–0.019) | ||
| Neutrophils | - | 0.004 | (−0.014–0.023) | ||
| Lymphocytes | −0.051 | (−0.241–0.139) | |||
| Platelets | 0.003 | (0.000–0.005) | |||
| CRP | 0.001 | (0.000–0.002) | |||
| Ferritin | 0.002 | (0.001–0.004) | |||
| D-dimer | 2.398 | (0.543–4.254) * | |||
| LDH | 9.588 × 10−5 | (−0.001–0.002) | |||
| Creatinine | −0.710 | (−2.149–0.730) | |||
| Potassium | 0.023 | (−0.001–0.048) | |||
| Sodium | −0.022 | (−0.044–0.000) | |||
| Urea | 0.000 | (−0.001–0.000) | |||
| ALT | −0.005 | (−0.011–0.002) | |||
| AST | −0.001 | (−0.006–0.003) | |||
| Bilirubin | 0.662 | (0.069–1.254) * | 0.138 | (−0.752–1.029) | |
| Direct bilirubin | −0.006 | (−0.035–0.022) | |||
| Last HBA1C | 0.692 | (−3.749–5.134) | |||
| O2 saturation | |||||
| =>94 | R | R | |||
| 90–94 | 0.943 | (−0.841–1.321) | |||
| <90 | 1.471 | (0.705–2.236) * | 0.215 | (−1.037–1.466) | |
| Steroids | Yes | 1.837 | (−0.335–4.010) | ||
| Tocilizumab | Yes | 2.815 | (0.553–5.078) * | 2.689 | (−0.757–6.134) |
| Remedisvir | Yes | 1.564 | 0.043–3.085) * | 0.054 | (−2.380–2.488) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Oweidat, K.; Al-Amer, R.; Saleh, M.Y.; Albtoosh, A.S.; Toubasi, A.A.; Ribie, M.K.; Hasuneh, M.M.; Alfaqheri, D.L.; Alshurafa, A.H.; Ribie, M.; et al. Mortality, Intensive Care Unit Admission, and Intubation among Hospitalized Patients with COVID-19: A One-Year Retrospective Study in Jordan. J. Clin. Med. 2023, 12, 2651. https://doi.org/10.3390/jcm12072651
Al Oweidat K, Al-Amer R, Saleh MY, Albtoosh AS, Toubasi AA, Ribie MK, Hasuneh MM, Alfaqheri DL, Alshurafa AH, Ribie M, et al. Mortality, Intensive Care Unit Admission, and Intubation among Hospitalized Patients with COVID-19: A One-Year Retrospective Study in Jordan. Journal of Clinical Medicine. 2023; 12(7):2651. https://doi.org/10.3390/jcm12072651
Chicago/Turabian StyleAl Oweidat, Khaled, Rasmieh Al-Amer, Mohammad Y. Saleh, Asma S. Albtoosh, Ahmad A. Toubasi, Mona Khaled Ribie, Manar M. Hasuneh, Daniah L. Alfaqheri, Abdullah H. Alshurafa, Mohammad Ribie, and et al. 2023. "Mortality, Intensive Care Unit Admission, and Intubation among Hospitalized Patients with COVID-19: A One-Year Retrospective Study in Jordan" Journal of Clinical Medicine 12, no. 7: 2651. https://doi.org/10.3390/jcm12072651
APA StyleAl Oweidat, K., Al-Amer, R., Saleh, M. Y., Albtoosh, A. S., Toubasi, A. A., Ribie, M. K., Hasuneh, M. M., Alfaqheri, D. L., Alshurafa, A. H., Ribie, M., Ali, A. M., & Obeidat, N. (2023). Mortality, Intensive Care Unit Admission, and Intubation among Hospitalized Patients with COVID-19: A One-Year Retrospective Study in Jordan. Journal of Clinical Medicine, 12(7), 2651. https://doi.org/10.3390/jcm12072651








